Abstract
Background.
Myeloablative therapy for high-risk neuroblastoma commonly includes melphalan. Increased cellular glutathione (GSH) can mediate melphalan resistance. Buthionine sulfoximine (BSO), a GSH synthesis inhibitor, enhances melphalan activity against neuroblastoma cell lines, providing the rationale for a Phase 1 trial of BSO-melphalan.
Procedures.
Patients with recurrent/resistant high-risk neuroblastoma received BSO (3 gram/m2 bolus, then 24 grams/m2/day infusion days −4 to −2), with escalating doses of intravenous melphalan (20–125 mg/m2) days −3 and −2, and autologous stem cells day 0 using 3 + 3 dose escalation.
Results.
Among 28 patients evaluable for dose escalation, one dose-limiting toxicity occurred at 20 mg/m2 melphalan (grade 3 aspartate aminotransferase/alanine aminotransferase) and one at 80 mg/m2 (streptococcal bacteremia, grade 4 hypotension/pulmonary/hypocalcemia) without sequelae. Among 25 patients evaluable for response, there was one partial response (PR) and two mixed responses (MRs) among eight patients with prior melphalan exposure; one PR and three MRs among 16 patients without prior melphalan; one stable disease with unknown melphalan history. Melphalan pharmacokinetics with BSO were similar to reports for melphalan alone. Melphalan Cmax for most patients was below the 10 μM concentration that showed neuroblastoma preclinical activity with BSO.
Conclusions.
BSO (75 gram/m2) with melphalan (125 mg/m2) is tolerable with stem cell support and active in recurrent/refractory neuroblastoma. Further dose escalation is feasible and may increase responses.
Keywords: buthionine sulfoximine, melphalan, neuroblastoma, Phase 1, transplant
INTRODUCTION
Survival for children with high-risk neuroblastoma is only 45% despite advances with aggressive treatment including chemotherapy induction, myeloablative chemotherapy consolidation, radiotherapy, isotretinoin, and immunotherapy.[1,2] Current myeloablative regimens for high-risk neuroblastoma often include melphalan (l-phenylalanine mustard).[3–5] Melphalan resistance can occur through increased glutathione (GSH) production, which binds to melphalan and inactivates it.[4–7] The synthesis of GSH, a cellular antioxidant, requires glutamate-cysteine ligase (GCL) (γ-glutamylcysteine synthetase).[8] Buthionine sulfoximine (BSO), a selective GCL inhibitor, decreases intracellular GSH [9–11] and enhanced melphalan sensitivity in cancer cell lines,[7,12–16] including ovarian cancer, melanoma, multiple myeloma, and neuroblastoma.[17–21] BSO alone has variable but minimal cytotoxic effects,[13,16, 22,23] a notable exception being melanoma [24,25] and neuroblastoma [20,21] cell lines, where BSO has significant cytotoxicity. However, BSO cytotoxicity for neuroblastoma cell lines is not observed in physiological hypoxia [26] and is likely due to non-physiological (atmospheric) oxygen levels employed for most cell cultures.[27]
GSH depletion by BSO preceding melphalan administration to mice with intracranial human glioma xenografts increased survival over melphalan alone.[28] In neuroblastoma, BSO significantly enhanced cytotoxicity at melphalan concentrations of 10 μM or greater, even in highly melphalan-resistant cell lines.[18,19] The toxicities of BSO with melphalan in mice are similar to melphalan alone.[29] Clinical trials of BSO with low dose (15 mg/m2) melphalan in adult solid tumors demonstrated tolerability with side effects of myelosuppression and mild nausea/vomiting, but only modest activity despite GSH depletion to less than 10% of baseline in tumor sections.[30–32] Continuous infusion BSO was optimal for GSH depletion.[32]
A previous pilot study in 32 children with neuroblastoma utilized BSO (3 g/m2 bolus and either 0.75 or 1 g/m2/hr 72 hr continuous infusion) followed by melphalan (15 mg/m2) without stem cell support for one to four courses. Both BSO dose levels were tolerable.[33] GSH depletion was demonstrated in peripheral blood monocytes. Higher dose BSO achieved higher steady state plasma concentrations and area under the curve (AUC). The major toxicity was myelosuppression, with one case of grade 3 bilirubin/transaminases and five grades 3–4 infections. Two deaths from neurologic toxicity associated with acute renal tubular necrosis were attributed to concomitant cephalosporins. Responses were encouraging, with four partial responses (PRs) and one mixed response (MR) among 31 evaluable patients. Three of five responders had received prior myeloablative doses of melphalan.
We hypothesized that BSO given with melphalan doses greater than 15 mg/m2 would enhance activity against neuroblastoma and be tolerable by adding hematopoietic stem cell support to abrogate myelotoxicity, and prohibition of concomitant cephalosporins to minimize toxicity. Based on this rationale, we conducted a Phase I study (NCT00005835) in the New Approaches to Neuroblastoma Therapy (NANT) (www.NANT.org) consortium. The study aims were to determine the maximum tolerated dose (MTD) and toxicities of melphalan given with fixed doses of BSO and autologous hematopoietic stem cell support in children with recurrent/refractory neuroblastoma, the pharmacokinetics (PK) of melphalan in this regimen, and the response rate within the confines of a Phase 1 trial.
METHODS
Eligibility
Eligible patients were 9 months–30 years of age with recurrent/refractory high-risk neuroblastoma (Table I) who either had progressive disease (PD) any time before enrollment, or had no response, MR, or PR after at least four cycles of induction chemotherapy. Patients with PR to induction required a biopsy demonstrating neuroblastoma. Other requirements included cardiac shortening fraction greater than 30%, glomerular filtration rate greater than 100 cc/min/1.73 m2, creatinine less than 1.5 times age normals, no neurologic toxicity, absolute neutrophil count (ANC) greater than 500/m3, platelets greater than 20,000/m3, aspartate aminotransferase less than 2.5 times normal, alanine aminotransferase less than 113, and hemoglobin greater than 10 gm/dl (transfusion allowed). Prior therapy exclusions included myeloablative therapy within 3 months of enrollment. Prior metaiodobenzylguanidine (MIBG) cumulative dose was limited to 24 mCi/kg completed at least 8 weeks prior to therapy start. Initially, eligibility included no radiation therapy to kidneys, liver, heart, skull, or face for 6 months; less than 25% of liver receiving more than 1,800 cGy, less than 20% of one kidney receiving more than 1,200 cGy, and less than 10 cc3 brain receiving more than 1,000 cGy. After the 17th patient, the prior brain radiation restriction was amended to exclude only prior radiation to the brain parenchyma. Available autologous hematopoietic stem cells were required to be either ≥1.5 × 106 CD34+/kg unpurged peripheral blood stem cell (PBSC), ≥ 1 × 106 viable CD34+ cells/kg immunomagnetic purged PBSC,[4] or ≥ 1 ×108 mononuclear cells/kg purged bone marrow (BM) with no tumor cells detected by immunocytology.[4] Protocol approval from each local institutional review board and written informed assent/consent from all patients and/or legal guardians were obtained.
TABLE I.
Patient Characteristics of 28 Patients Evaluable for Dose Escalation
| Number of patients (%) | |
|---|---|
| Male:Female | 21 (75%):7 (25%) |
| Median age at diagnosis (years) | 5 (2–17) |
| Median months from diagnosis to enrollment | 18 (5–75) |
| Stage at diagnosis | |
| 2b | 1 (4%) |
| 3 | 3 (11%) |
| 4 | 24 (86%) |
| MYCN status at diagnosis | |
| Amplified | 4 (14%) |
| Nonamplified | 18 (64%) |
| Unknown | 6 (21%) |
| Histopathology at diagnosis | |
| Unfavorable | 17 (61%) |
| Favorable | 0 (0%) |
| Unknown | 11 (39%) |
| History of relapse at any time prior to enrollment | |
| Yes | 22 (79%) |
| No | 6 (21%) |
| Therapy received prior to enrollment | |
| Chemotherapy | 28 (100%) |
| Prior melphalana | 10 (36%) |
| No prior melphalan | 17 (61%) |
| Prior melphalan history unknown | 1 (3%) |
| Prior myeloablative stem cell transplant | 11 (39%) |
| No prior myeloablative stem cell transplant | 17 (61%) |
| Prior radiation therapy | 16 (57%) |
| No prior radiation therapy | 12 (43%) |
| Median baseline GFRb(range) | 125 (102–181) |
| Sites of tumor at enrollment | |
| CT/MRI | 20 (71%) |
| MIBG | 25 (89%) |
| Bone marrow | 19 (68%) |
| Type stem cells infused | |
| Unpurged PBSC | 26 (93%) |
| Purged PBSC | 1 (4%) |
| No stem cells infused | 1(4%) |
Nine of 10 patients with prior melphalan received myeloablative dose;
glomerular filtration rate.
Patients were required to have at least one of the following tumor sites at enrollment: BM metastases by routine morphology, a soft tissue mass at least 20 mm in size on magnetic resonance imaging (MRI) or computed tomography (CT) scan, or at least 10 mm on spiral CT scan, and/or at least one MIBG-avid site. If all tumor sites had received prior radiation, a biopsy of one site was required to document neuroblastoma. Patients with intraparenchymal brain lesions or meningeal/parameningeal lesions with intracranial extension were ineligible. The study was amended after 20 patients enrolled to allow prior febrile seizures, if the patient was seizure free for 1 year before enrollment.
Study Design
BSO was given in fixed doses as an intravenous (IV) bolus of 3 g/m2 Day −4 over 30 min, followed by a 72 hr continuous IV infusion of 1 g/m2/hr (total dosage BSO 75 g/m2). Melphalan dose was escalated (Table II) using a standard 3 + 3 design [34] on days −3 and −2. BSO (NSC #326321) was provided by the National Cancer Institute from April 2000 until June 2004; subsequently it was provided under investigational new drug (IND)# 69,112. Study enrollment was suspended for 14 months for the IND transfer. Stem cells were infused Day 0, and filgrastim (G-CSF) was initiated and continued until ANC was greater than 1,500/m3. Only one course of therapy was allowed. The study was amended after 20 patients enrolled to require hospitalization until neutrophil engraftment and standard supportive care for myeloablative therapy.
TABLE II.
Dose-Limiting Toxicity in 28 Patients Evaluable for Dose Escalation
| Dose Level | Melphalan dose (mg/m2 total) | Number enrolled | Number evaluable for dose escalation | Number with dose limiting toxicity | Type of dose limiting toxicity |
|---|---|---|---|---|---|
| 1a | 40 | 1 | 1 | 0 | |
| Study amended to dose levels below | |||||
| 1b | 20 | 5 | 5 | 1 | Grade 3 AST/ALT during BSO requiring stopping therapy |
| 2 | 25 | 1 | 1 | 0 | |
| Study amended to dose levels below | |||||
| 1a | 40 | 4c | 3 | 0 | |
| 2a | 50 | 3d | 3 | 0 | |
| 3a | 64 | 4c | 3 | 0 | |
| 4a | 80 | 6 | 6 | 1 | Alpha streptococcal sepsis with grade 4 cardiac, pulmonary (mechanical ventilation), and hypocalcemia toxicities resolved without sequelae |
| 5a | 100 | 3 | 3 | 0 | |
| 6a | 125 | 4c | 3 | 0 | |
Original dose level 1;
amended dose level 1;
one patient withdrew at each of these dose levels prior to receiving any protocol therapy;
one patient found ineligible on routine audit due to single prior febrile seizure; considered evaluable since study subsequently amended to include such patients. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Antibiotics, antifungals, and antiviral agents were prohibited for 7 days prior to BSO. Cephalosporins and acetaminophen were prohibited from 7 days before BSO until 14 days after BSO completion. Nystatin and trimethoprim/sulfamethoxazole were prohibited from 7 days prior to BSO until 7 days after BSO completion.
Toxicity was graded using the Common Terminology Criteria for Adverse Events version 3.0. Patients were evaluable for dose escalation if they completed assigned doses of BSO and melphalan, received stem cells, and completed 28 days of follow-up or experienced dose-limiting toxicity (DLT) earlier. Engraftment was defined as three consecutive ANCs ≥ 500 and platelets ≥ 20,000. Response was evaluated 28 days after stem cell infusion, or after engraftment and resolution of grades 3–4 toxicities (whichever occurred later). Patients were evaluable for response if they completed BSO and the assigned melphalan dose. Overall response was graded utilizing the NANT Response Criteria,[35] which integrates three components: Curie scoring [36] for MIBG-avid sites, Response Evaluation Criteria in Solid Tumors (RECIST 1.0) [37] for soft tissue sites, and standard morphology for BM. An overall complete response (CR) or PR required a CR or PR for one component, with noninvolvement or a CR or PR, respectively, for each of the other two components. An overall MR was defined as CR or PR for at least one response component, with stable disease (SD) for at least one component, and no PD for any component. Soft tissue lesions with stable size were considered SD, even if MIBG uptake resolved. BM responses were graded as follows: CR defined as no tumor cells on two bilateral BM aspirates and biopsies at least 3 weeks apart; PD defined as either (i) tumor seen on two consecutive BMs done at least 3 weeks apart if BM negative at enrollment or (ii) BM metastases at enrollment with an increase to at least 25% tumor and doubling of baseline amount; or SD defined as any residual BM tumor not qualifying as CR or PD. If the overall response was CR or PR, then MIBG scans, CT/MRI scans, and BM biopsy slides were centrally reviewed. Patients with an overall response of SD had central review of scans and BM slides only if they demonstrated tumor at enrollment per treating site assessment. Progression free survival was defined as time from treatment start to PD or death (whichever came first), or last follow-up. Event-free survival (EFS) was defined as time from treatment start to PD, second malignancy, or death (whichever came first), or last follow-up. Overall survival (OS) was defined as time from treatment start to death or last follow-up.
Pharmacokinetics
Peripheral blood samples for melphalan PK were obtained day −3: prior to start and at end of melphalan; and at 5 min, 10 min, 15 min, 1 hr, 2 hr, and 4 hr after melphalan completion into heparinized tubes placed on ice, then centrifuged for 10 min at 2,500 revolutions per minute. Plasma was stored in polyethylene tubes at ≤ −70°C until analyzed.
Plasma levels of melphalan and its spontaneous degradation products, mono- and dihydroxymelphalan (MHM and DHM), were determined using a liquid chromatography/tandem mass spectrometry assay. The analytes and spiked internal standard (propylparaben [PPB], 2 μl of 10 μg/ml solution) were extracted from plasma (0.1 ml) by protein precipitation with methanol (0.2 ml). After vortexing for 1 min and centrifuging for 15 min at 15,700 × g at 4°C, 10 μl of the supernatant was injected into a high-performance liquid chromatography instrument (Agilent 1200; Agilent Technologies, Santa Clara, CA) operated by Analyst™ software (version 1.5). The separation column was an Agilent ZORBAX Eclipse XDB-Phenyl (Agilent Technologies) (3.0 × 100 mm, 3.5 μm). Gradient elution with methanol (0.1% formic acid) and 2 mM ammonium formate in water (0.1% formic acid) was used at a flow rate of 0.3 ml/min. The column effluent was connected to an electrospray ionization interface operated in the positive mode (500°C and 4,000 V ionization voltage); nebulizer gas = 50 pounds per square inch (psi) and turbo gas = 30 psi, collision-activated dissociation gas was set at medium and curtain gas at 25 psi of the tandem mass spectrometer (Applied Biosystems 4000 QTRAP; Life Technologies; Grand Island, NY). The collision energy for melphalan, MHM, DHM, and PPB were 47, 55, 41, and 29 eV, respectively. Analyte detection used multiple reaction monitoring, and the transitions were m/z 306.0 → m/z 169.2 for melphalan, m/z 288.0 → m/z 198.7 for MHM, m/z 269.1 → m/z 176.2 for DHM, and m/z 180.9 → m/z 95 for PPB. Melphalan, MHM, and DHM calibration standards were prepared at 30–6,000 ng/ml (melphalan) and 7.5–1,500 ng/ml (MHM and DHM). Regression parameters of slope, intercept, and correlation coefficient were calculated by least-squares linear regression analysis using a weight factor of 1/x2.
Plasma concentrations (C) versus time (t) data were fitted to a biexponential equation and pharmacokinetic parameters were calculated assuming an open two-compartment model for melphalan disposition as follows: ln C = ln (A·e−α·t + B·e−β·t), Kel = elimination constant = α·β/k21, AUC = area under the C versus t curve = (A/α) + (B/β), CL = systemic clearance = dose/AUC, Vd = apparent volume of distribution = CL/kel.
RESULTS
Patient Characteristics
Thirty-one patients enrolled from April 2000 to January 2013. One patient was declared ineligible 2 years off therapy due to an audit finding that the patient had a febrile seizure prior to enrollment (an eligibility exclusion). The study was subsequently amended to allow prior febrile seizures occurring more than 1 year prior to enrollment. This ineligible patient had no DLT and was included in dose escalation and response analyses. Including this patient, 28 patients were evaluable for dose escalation (Table I). Three patients were enrolled but taken off protocol prior to any therapy due to bacteremia that required protocol-excluded antibiotics (n = 1) or tumor progression (n = 2) and were considered inevaluable for dose escalation, response, and engraftment.
Engraftment
One patient stopped protocol therapy after BSO and did not receive melphalan or stem cells, thus 27 patients were evaluable for engraftment. Median stem cell dose was 3.6 × 106 (range 1.2–19.6 × 106) CD34+/kg. Median time to neutrophil engraftment (n = 24) was 10 days (range 9–14 days). Three patients never had an ANC < 500 (at 20 mg/m2 melphalan). Median time to platelet engraftment (n = 21) was 12 days (range 7–49). Six patients never had < 20,000 platelets [at dose levels of 20 (n = 3), 50 (n = 1), and 125 (n = 2) mg/m2 melphalan].
Toxicity/MTD
Toxicity details are in Tables II and III. Study accrual was suspended for 16 months after the first patient (treated at 40 mg/m2 melphalan) due to review by the National Cancer Institute of two fatal toxicities on a prior pilot of BSO-melphalan using the same dose of BSO and 15 mg/m2 melphalan [33]. The study was amended before reactivation to include modified eligibility, more stringent toxicity monitoring, and a lower dose escalation schedule. The first patient treated was included in evaluation of the new Dose Level 1 (20 mg/m2 melphalan). After the first seven patients were accrued, the dose escalation schema was amended to accrue at Dose Level 1a (40 mg/m2 melphalan). No MTD was determined at melphalan dose levels ranging from 20 to 125 mg/m2 (Table II), with only 2 DLTs among 28 evaluable patients. Twenty patients had grade 3 or 4 infections. Three patients were treated without DLT at 125 mg/m2 melphalan, the highest dose evaluated. Grade 3–4 toxicities (Table III) were as expected for a myeloablative regimen. The study was stopped prior to reaching a MTD due to slow accrual.
TABLE III.
Grades 3–4 Toxicities (Excluding Those Unrelated to Protocol Therapy) in 28 Patients Evaluable for Dose Escalation
| Dose level | Toxicity | Number of patients experiencing maximum grade toxicity |
|
|---|---|---|---|
| Grade 3 | Grade 4 | ||
| 1 (4/00) (n = 1) | Infection-blood | 1 | 0 |
| 1 (8/01) (n =5) | ALT | 1 | 0 |
| AST | 1 | 0 | |
| GGT | 1 | 0 | |
| Infection + normal absolute neutrophil count; skin/cellulitis | 1 | 0 | |
| Infection-blood | 1 | 0 | |
| Infection-cathether related | 1 | 0 | |
| PTT | 1 | 0 | |
| Anorexia | 0 | 1 | |
| Nausea | 1 | 0 | |
| Vomiting | 0 | 1 | |
| Hypokalemia | 1 | 0 | |
| 1a (n = 3) | ALT | 1 | 0 |
| Febrile neutropenia | 2 | 0 | |
| Infection/lip perioral HSV-1 | 1 | 0 | |
| Sinus infection | 1 | 0 | |
| Anorexia | 2 | 0 | |
| PTT | 1 | 0 | |
| Hypokalemia | 1 | 0 | |
| 2a (n = 3) | Febrile neutropenia | 2 | 0 |
| Infection—blood | 1 | 0 | |
| Hypokalemia | 1 | 0 | |
| 3a (n = 3) | GGT | 1 | 0 |
| Fever | 1 | 0 | |
| Febrile neutropenia | 1 | 0 | |
| Infection—catheter related | 0 | 1 | |
| Infection—respiratory | 1 | 0 | |
| Anorexia | 1 | 0 | |
| 4a (n = 6) | ALT | 1 | 0 |
| Hypoxia | 0 | 1 | |
| Cardiac left ventricular function | 1 | 0 | |
| Cardiac troponin | 0 | 1 | |
| Cardiopulmonary arrest | 0 | 1 | |
| Hypotension | 0 | 1 | |
| Febrile neutropenia | 3 | 0 | |
| Infection—blood | 1 | 1 | |
| Infection—catheter related | 1 | 0 | |
| Anorexia | 4 | 0 | |
| Mucositis/pharyngitis/stomatitis | 1 | 0 | |
| Nausea | 2 | 0 | |
| Vomiting | 1 | 0 | |
| Hypocalcemia | 0 | 1 | |
| 5a (n = 3) | Fever | 1 | 0 |
| Febrile neutropenia | 2 | 0 | |
| Infection—blood | 2 | 0 | |
| Infection—respiratory | 1 | 0 | |
| Infection—urinary tract | 1 | 0 | |
| Pain—abdominal | 1 | 0 | |
| Anorexia | 3 | 0 | |
| Mucositis/pharyngitis/stomatitis | 2 | 0 | |
| Nausea | 2 | 0 | |
| Vomiting | 1 | 0 | |
| 6a (n = 3) | Hypoxia | 1 | 0 |
| Febrile neutropenia | 3 | 0 | |
| Infection—urinary tract | 1 | 0 | |
| Pain—abdominal | 1 | 0 | |
| Pain—oral cavity | 1 | 0 | |
| Anorexia | 2 | 0 | |
| Mucositis/pharyngitis/stomatitis | 1 | 0 | |
| Nausea | 1 | 0 | |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Response
Overall responses included two PRs and five MRs among 25 evaluable patients (Table IV). Patient #15 had a CR for MIBG lesions (baseline Curie score was 5), but was a MR due to minimal (< 5%) persistent BM tumor. The remaining patients had SD (n = 10) and PD (n = 8). Six of 31 enrolled patients were inevaluable for response: three were withdrawn prior to any protocol therapy, one did not have a complete disease evaluation at therapy completion, one received only BSO due to hepatic DLT, and one was found on central review to have no tumor sites at enrollment. This patient remained eligible and evaluable for dose escalation, since eligibility was based on the treating institution’s assessment of tumor sites. Response (PR + MR) rates among patients with history of prior melphalan (3/8 = 37.5%) were not significantly different than for patients without prior melphalan history (4/16 = 25%) (P = 0.65). One of 25 patients with response of SD had an unknown prior melphalan history. The two overall PRs occurred in patients with a history of tumor relapse prior to enrollment. Among the 28 patients evaluable for dose escalation, the median PFS and EFS was 5.2 months (95% CI 3.2–9.1). There were no second malignancies. Median OS was 20.6 months (95% CI 9.2–36.8).
TABLE IV.
Overall Responses of Partial or Mixed Response Among 25 Patients Evaluable for Response
| Patient study # | Overall response | MIBG response | Baseline Curie score | CT-MRI response | Baseline sum of longest diameters (cm) | Bone marrow response (% tumor at baseline and end of therapy) | Prior melphalan therapy | Melphalan dose level (mg/m2) |
|---|---|---|---|---|---|---|---|---|
| 9 | PR | PR | 3 | PR | 8.4 | CR (10% to 0%) | No | 40 |
| 22 | PR | CR | 2 | PR | 6.9 | NI | Yes (CEM) | 80 |
| 15 | MR | CR | 5 | NI | 0 | SD (<5% to <5%) | No | 64 |
| 18 | MR | SD | 1 | PR | 2.5 | SD (<1% to >1–<5%) | Yes (CEM) | 64 |
| 19 | MR | SD | ND | SD | 4.9 | CRa (10% to 0%) | No | 80 |
| 26 | MR | CR | 1 | SD | 4.5 | SDb (50% to <1%) | No | 100 |
| 27 | MR | PR | 20 | NI | 0 | SD (5% to 5%) | Yes (CEM) | 100 |
MIBG scan at baseline and bone marrrow slides not submitted for review. Response graded per review of treating site reports. CT response confirmed on central review as SD;
bone marrow slides submitted for review from end of protocol therapy only; reviewed local reports for baseline. PR, partial response; CR, complete response; MR, mixed response; NI, not involved; SD, stable disease; CEM, carboplatin/etoposide/melphalan myeloablative regimen.
Pharmacokinetics
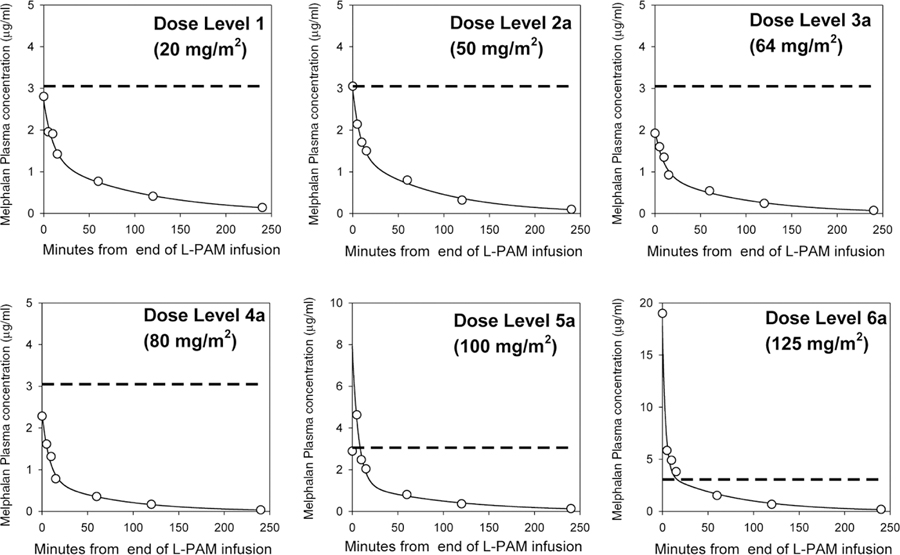
PK sampling was performed in 17 patients, with analyses done in 15 patients, and two patients with three or more samples missing excluded (Table V). Melphalan plasma disposition was well described by a first-order, two-compartment open pharmacokinetic model. Figure 1 shows concentration versus time curves of melphalan generated using this model and data from a representative patient at each dose level. Monohydroxymelphalan or dihydroxymelphalan was not detected in any samples.
TABLE V.
Pharmacokinetic Parameters of Melphalan in 15 Patients Treated With BSO-Melphalan
| Dose level (no. of patients) | Day –3 (mg/m2 melphalan) | Day –2 (mg/m2 melphalan) | Total dose melphalan (mg/m2) | Cmax (μg/ml) | AUC0→infa (μg/ml·hr) | CLb (l/hr/m2) | Vdc (l/m2) |
|---|---|---|---|---|---|---|---|
| 1/1a (n = 4)d | 0/20 | 20 | 20/40 | 2.44 ± 0.68 | 1.6 ± 0.9 | 16.6 ± 9.2 | 9.2 ± 3.9 |
| 2a (n = 3) | 25 | 25 | 50 | 2.52 ± 0.69 | 1.7 ± 0.6 | 15.8 ± 5.7 | 10.4 ± 2.7 |
| 3a (n = 1) | 32 | 32 | 64 | 1.92 ± NA | 1.6 ± NA | 19.8 ± NA | 16.0 ± NA |
| 4a (n = 4) | 40 | 40 | 80 | 2.87 ± 1.63 | 2.0 ± 1.2 | 24.0 ± 9.7 | 16.7 ± 7.2 |
| 5a (n = 2) | 50 | 50 | 100 | 4.63, 3.17 | 3.0, 3.6 | 16.7, 14.0 | 6.3, 15.4 |
| 6a (n = 1) | 62.5 | 62.5 | 125 | 18.98 ± NA | 5.5 ± NA | 11.4 ± NA | 3.5 ± NA |
Area under the concentration–time curve (0 to infinity);
systemic clearance;
volume of distribution;
dose levels 1 and 1a were combined for PK purposes as the preinfusion PK for dose level 1a were lower than lower limit of quantitation.
Fig. 1.
Melphalan plasma concentration versus time curves on day –3 from six patients representing each dose level. The dose of melphalan is the total dose given over 2 days. X-axis: minutes from end of melphalan (L-PAM) infusion. Open circle: observed concentrations; solid line: fitted for two-compartment model. Dashed line: 10 μM concentration of melphalan.
DISCUSSION
We demonstrated that this BSO-melphalan regimen is tolerable with hematopoietic stem cell support and achieved responses in patients with recurrent/refractory high-risk neuroblastoma after extensive prior therapy including myeloablative doses of melphalan. In contrast to the prior pediatric neuroblastoma pilot of BSO-melphalan,[33] there were no serious renal or neurological toxicities, which may reflect restriction of concomitant cephalosporins, acetaminophen, and sulfonamides and/or requiring adequate renal function at enrollment. While the etiology of the two of 32 deaths due to neurologic and renal toxicity on the prior pilot study [33] cannot be definitively confirmed, we demonstrated that this regimen can be given safely using our eligibility and monitoring criteria. Based on our toxicity data, future trials could be safely amended to allow less complex eligibility criteria to facilitate patient accrual.
Systemic exposure to melphalan (AUC) in our study patients ranged from 0.7 to 5.5 μg/ml‧hr, with higher exposure seen with higher dose levels. The systemic clearance ranged from 7.8 to 33.4l/hr/m2, but did not appear to be correlated with dose level. Our pharmacokinetic results (Table V) of melphalan given with concurrent BSO are similar to those reported in children for melphalan alone or given with other agents excluding carboplatin, which is known to decrease melphalan clearance and volume of distribution.[38,39] This suggests that BSO does not affect melphalan PK. Other studies of high-dose melphalan (140–180 mg/m2) showed similar systemic clearance results ranging from 15.4 to 29.9 l/hr/m2.[40–42]
The melphalan Cmax achieved in this study remained below the 10 μM levels needed for significant preclinical activity with BSO against multidrug resistant neuroblastoma until a melphalan dose of 125 mg/m2 (Table V). However, only one of three patients treated at the 125 mg/m2 dose level had PK. Despite suboptimal melphalan levels, two PRs occurred at melphalan doses of 40 and 80 mg/m2. One patient with a PR and two patients with MRs had received previous myeloablative melphalan doses. Overall response rates were not significantly different for patients who had or had not received prior melphalan, which supports our hypothesis that BSO may reverse resistance to melphalan. It is also possible that these responses indicate that high-dose melphalan remains active against neuroblastoma that has progressed after prior therapy with myeloablative melphalan doses. Based on the acceptable systemic toxicities and the lack of DLT for 125 mg/m2 of melphalan given with BSO, further dose escalation of melphalan in this regimen is feasible. This may enhance tumor responses if melphalan plasma levels ≥ 10 μM are tolerable, based on the dose–response data from our preclinical studies.[19,20] This study was stopped prior to determination of the melphalan MTD due to slow accrual, which was due to several factors. Accrual was suspended for a total of 30 months for the IND transfer and an amendment to add complex eligibility and monitoring criteria due to the toxic deaths on the predecessor pilot. These complex criteria limited the number of eligible patients.
The Phase 2 Children’s Oncology Group (COG) study of irinotecan/temozolomide for neuroblastoma patients in first relapse found a CR/PR rate of 15%.[43] Our study was not limited to first relapse, and 39% of patients had received a prior transplant. One of the PRs occurred in a patient who had received a prior carboplatin, etoposide, melphalan (CEM) transplant. Our 8% CR/PR rate and 28% CR/PR/MR rate in this BSO-melphalan study occurred in a patient population with extensive prior therapy and a very poor prognosis.
Melphalan is currently a standard component of frontline myeloablative regimens for high-risk neuroblastoma. The CEM regimen that utilized carboplatin, etoposide, and melphalan (180–210 mg/m2) was employed in multiple COG trials for high-risk neuroblastoma.[4,44] Current COG trials utilize busulfan-melphalan (Bu-Mel; 140 mg/m2) based on a randomized International Society of Pediatric Oncology Europe Neuroblastoma (SIOPEN) trial demonstrating superior EFS and OS with Bu-Mel versus CEM.[45] While CEM [4] was associated with an 8% incidence of grades 3–5 sinusoidal obstruction syndrome (SOS), and Bu-Mel [45] had an 18% incidence of grade 2 or greater SOS in the SIOPEN trial, SOS was not seen with BSO-melphalan. BSO-melphalan could potentially be randomized against these other regimens to determine if it achieves better EFS and/or OS.
We conclude that BSO 75 g/m2 with melphalan 125 mg/m2 and autologous stem cell support is feasible and tolerable in recurrent/refractory neuroblastoma patients. Future BSO-melphalan studies in neuroblastoma could include exploring its use in patients with refractory disease at the end of induction therapy, or as an alternative myeloablative consolidation regimen for high-risk patients. Our study also provides safety data to support further clinical trials of myeloablative BSO-melphalan, which are in development for multiple myeloma (which has demonstrated activity with this regimen in preclinical models [46]), and pediatric solid tumors.
Acknowledgments
The authors would like to acknowledge the contribution of the NANT Operations Center Staff, the physicians at NANT sites who enrolled patients on this trial, and the National Cancer Institute Developmental Therapeutics Program, which provided clinical-grade BSO for this study.
Grant sponsors: National Institutes of Health; (Grant numbers: PO1 CA81403, RO1 CA82830); Dougherty Foundation; Milken Family Foundation; Evan Dunbar Foundation; Alex Lemonade Stand; Children’s Neuroblastoma Cancer Foundation; Neuroblastoma Children’s Cancer Society; Douglas Michael Fuller Foundation; Because of Ezra Foundation.
Abbreviations:
- ANC
absolute neutrophil count
- AUC
area under the curve
- BM
bone marrow
- BSO
buthionine sulfoxime
- Bu-Mel
busulfan-melphalan
- CEM
carboplatin, etoposide, melphalan
- COG
Children’s Oncology Group
- CR
complete response
- DHM
dihydroxymelphalan
- DLT
dose-limiting toxicity
- EFS
event-free survival
- GCL
glutamate-cysteine ligase
- GSH
glutathione
- IND
investigational new drug
- IV
intravenous
- MIBG
metaiodobenzylguanidine
- MHM
monohydroxymelphalan
- MR
mixed response
- MTD
maximum tolerated dose
- NANT
New Approaches to Neuroblastoma Therapy
- OS
overall survival
- PBSC
peripheral blood stem cell
- PD
progressive disease
- PPB
propylparaben
- PR
partial response
- psi
pounds per square inch
- SD
stable disease
- SIOPEN
International Society of Pediatric Oncology Europe Neuroblastoma
- SOS
sinusoidal obstruction syndrome
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children’s Oncology Group study. J Clin Oncol 2009;27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dini G, Lanino E, Garaventa A, Rogers D, Dallorso S, Viscoli C, Castagnola E, Manno G, Brisigotti M, Rosanda C. Myeloablative therapy and unpurged autologous bone marrow transplantation for poor-prognosis neuroblastoma: Report of 34 cases. J Clin Oncol 1991;9:962–969. [DOI] [PubMed] [Google Scholar]
- 4.Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, Haas-Kogan DA, Laquaglia MP, Yu AL, Diller L, Buxton A, Park JR, Cohn SL, Maris JM, Reynolds CP, Villablanca JG. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol 2013;14:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer 2005;44:348–357. [DOI] [PubMed] [Google Scholar]
- 6.Suzukake K, Vistica BP, Vistica DT. Dechlorination of L-phenylalanine mustard by sensitive and resistant tumor cells and its relationship to intracellular glutathione content. Biochem Pharmacol 1983;32:165–167. [DOI] [PubMed] [Google Scholar]
- 7.Suzukake K, Petro BJ, Vistica DT. Reduction in glutathione content of L-PAM resistant L1210 cells confers drug sensitivity. Biochem Pharmacol 1982;31:121–124. [DOI] [PubMed] [Google Scholar]
- 8.Fahey RC, Sundquist AR. Evolution of glutathione metabolism. Adv Enzymol Relat Areas Mol Biol 1991;64:1–53. [DOI] [PubMed] [Google Scholar]
- 9.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 1979;254:7558–7560. [PubMed] [Google Scholar]
- 10.Griffith OW, Anderson ME, Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem 1979;254:1205–1210. [PubMed] [Google Scholar]
- 11.Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 1982;257:13704–13712. [PubMed] [Google Scholar]
- 12.Somfai Relle S, Suzukake K, Vistica BP, Vistica DT. Reduction in cellular glutathione by buthionine sulfoximine and sensitization of murine tumor cells resistant to L-phenylalanine mustard. Biochem Pharmacol 1984;33:485–490. [DOI] [PubMed] [Google Scholar]
- 13.Green JA, Vistica DT, Young RC, Hamilton TC, Rogan AM, Ozols RF. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res 1984;44:5427–5431. [PubMed] [Google Scholar]
- 14.Hamilton TC, Winker MA, Louie KG, Batist G, Behrens BC, Tsuruo T, Grotzinger KR, McKoy WM, Young RC, Ozols RF. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol 1985;34:2583–2586. [DOI] [PubMed] [Google Scholar]
- 15.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Res 1985;45:2110–2115. [PubMed] [Google Scholar]
- 16.Ozols RF, Louie KG, Plowman J, Behrens BC, Fine RL, Dykes D, Hamilton TC. Enhanced melphalan cytotoxicity in human ovarian cancer in vitro and in tumor-bearing nude mice by buthionine sulfoximine depletion of glutathione. Biochem Pharmacol 1987;36:147–153. [DOI] [PubMed] [Google Scholar]
- 17.Ongaro A, Pellati A, De Mattei M, De Terlizzi F, Rossi CR, Campana LG. Enhancement of melphalan activity by buthionine sulfoximine and electroporation in melanoma cells. Anticancer Drugs 2015;26:284–292. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CP, Seeger RC, Statake N, Monforte-Munoz HL, Keshelava N, Bailey HH, Reynolds CP. Buthionine sulfoximine and myeloablative concentrations of melphalan overcome resistance in a melphalan-resistant neuroblastoma cell line. J Pediatr Hematol Oncol 2001;23:500–505. [DOI] [PubMed] [Google Scholar]
- 19.Anderson CP, Reynolds CP. Synergistic cytotoxicity of buthionine sulfoximine (BSO) and intensive melphalan (L-PAM) for neuroblastoma cell lines established at relapse after myeloablative therapy. Bone Marrow Transplant 2002;30:135–140. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CP, Tsai JM, Meek WE, Liu RM, Tang Y, Forman HJ, Reynolds CP. Depletion of glutathione by buthionine sulfoxine is cytotoxic for human neuroblastoma cell lines via apoptosis. Exp Cell Res 1999;246:183–192. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CP, Tsai J, Chan W, Park CK, Tian L, Lui RM, Forman HJ, Reynolds CP. Buthionine sulphoximine alone and in combination with melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur J Cancer 1997;33:2016–2019. [DOI] [PubMed] [Google Scholar]
- 22.Jordan J, d’Arcy Doherty M, Cohen GM. Effects of glutathione depletion on the cytotoxicity of agents toward a human colonic tumour cell line. Br J Cancer 1987;55:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemann DW, Beyers KL. In vivo therapeutic potential of combination thiol depletion and alkylating chemotherapy. Br J Cancer 1993;68:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prezioso JA, Fitzgerald GB, Wick MM. Melanoma cytotoxicity of buthionine sulfoximine (BSO) alone and in combination with 3,4-dihydroxybenzylamine and melphalan. J Invest Dermatol 1992;99:289–293. [DOI] [PubMed] [Google Scholar]
- 25.Prezioso JA, Fitzgerald GB, Wick MM. Effects of tyrosinase activity on the cytotoxicity of 3,4-dihydroxybenzylamine and buthionine sulfoximine in human melanoma cells. Pigment Cell Res 1990;3:49–54. [DOI] [PubMed] [Google Scholar]
- 26.Yang B, Keshelava N, Anderson CP, Reynolds CP. Antagonism of buthionine sulfoximine cytotoxicity for human neuroblastoma cell lines by hypoxia is reversed by the bioreductive agent tirapazamine. Cancer Res 2003;63:1520–1526. [PubMed] [Google Scholar]
- 27.Sheard MA, Ghent MV, Cabral DJ, Lee JC, Khankaldyyan V, Ji L, Wu SQ, Kang MH, Sposto R, Asgharzadeh S, Reynolds CP. Preservation of high glycolytic phenotype by establishing new acute lymphoblastic leukemia cell lines at physiologic oxygen concentration. Exp Cell Res 2015;334:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman HS, Colvin OM, Griffith OW, Lippitz B, Elion GB, Schold SCJ, Hilton J, Bigner DD. Increased melphalan activity in intracranial human medulloblastoma and glioma xenografts following buthionine sulfoximine-mediated glutathione depletion. J Natl Cancer Inst 1989;81:524–527. [DOI] [PubMed] [Google Scholar]
- 29.Skapek SX, VanDellen AF, McMahon DP, Postels DG, Griffith OW, Bigner DD, Friedman HS. Melphalan-induced toxicity in nude mice following pretreatment with buthionine sulfoximine. Cancer Chemother Pharmacol 1991;28:15–21. [DOI] [PubMed] [Google Scholar]
- 30.Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: An attempt at modulation of glutathione. J Clin Oncol 1994;12:194–205. [DOI] [PubMed] [Google Scholar]
- 31.O’Dwyer PJ, Hamilton TC, LaCreta FP, Gallo JM, Kilpatrick D, Halbherr T, Brennan J, Bookman MA, Hoffman J, Young RC, Comis RL, Ozols RF. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol 1996;14:249–256. [DOI] [PubMed] [Google Scholar]
- 32.Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT, Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst 1997;89:1789–1796. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CP, Matthay K, Perentesis J, Neglia JP, Bailey HH, Villablanca JG, Groshen S, Hasenauer B, Maris JM, Seeger RC, Reynolds CP. Pilot study of intravenous melphalan combined with continuous infusion L-S,R-buthionine sulfoximine for children with recurrent neuroblastoma. Pediatr Blood Cancer 2015;62:1739–1746. [DOI] [PubMed] [Google Scholar]
- 34.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009;101:708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A, Park JR, Tsao-Wei D, Hawkins R, Shulkin BL, Jackson H, Goodarzian F, Shimada H, Courtier J, Hutchinson R, Haas-Kogan D, Hasenauer CB, Czarnecki S, Katzenstein HM, Matthay KK. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A New Approaches to Neuroblastoma Therapy (NANT) phase II study. Biol Blood Marrow Transplant 2015;21:673–681. [DOI] [PubMed] [Google Scholar]
- 36.Messina JA, Cheng S-C, Franc BL, Charron M, Shulkin B, To B, Maris JM, Yanik G, Hawkins RA, Matthay KK. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer 2006;47:865–874. [DOI] [PubMed] [Google Scholar]
- 37.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 38.Nath CE, Shaw PJ, Montgomery K, Earl JW. Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 2007;64:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath CE, Shaw PJ, Montgomery K, Earl JW. Melphalan pharmacokinetics in children with malignant disease: Influence of body weight, renal function, carboplatin therapy and total body irradiation. Br J Clin Pharmacol 2005;59:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tranchand B, Ploin YD, Minuit MP, Sapet C, Biron P, Philip T, Ardiet C. High-dose melphalan dosage adjustment: Possibility of using a test-dose. Cancer Chemother Pharmacol 1989;23:95–100. [DOI] [PubMed] [Google Scholar]
- 41.Ardiet C, Tranchand B, Biron P, Rebattu P, Philip T. Pharmacokinetics of high-dose intravenous melphalan in children and adults with forced diuresis. Report in 26 cases. Cancer Chemother Pharmacol 1986;16:300–305. [DOI] [PubMed] [Google Scholar]
- 42.Gouyette A, Hartmann O, Pico JL. Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 1986;16:184–189. [DOI] [PubMed] [Google Scholar]
- 43.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, Kretschmar C, Cohn SL. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J Clin Oncol 2011;29:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 1999;341:1165–1173. [DOI] [PubMed] [Google Scholar]
- 45.Ladenstein R, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, Papadakis V, Laureys G, Malis J, Balwierz W, Ruud E, Kogner P, Schroeder H, Forjaz De Lacerda A, Beck Popovic M, Bician P, Garami M, Trahair T, Pearson AD, Valteau Couanet D. Busulphan-melphalan as a myeloablative therapy (MAT) for high-risk neuroblastoma: Results from the HR-NBL1/SIOPEN trial. J Clin Oncol 2011;29(Suppl 2):abstract 2. [Google Scholar]
- 46.Tagde A, Singh H, Kang MH, Reynolds CP. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J 2014;4:e229. [DOI] [PMC free article] [PubMed] [Google Scholar]