Abstract
The delineation of optimal regimens for combinations of agents is a difficult problem, in part because, to address it, one needs to (i) have effect relationships between the pathogen in question and the drugs in the combination, (ii) have knowledge of how the drugs interact (synergy, antagonism, and additivity), and (iii) address the issue of true between-patient variability in pharmacokinetics for the drugs in the population. We have developed an approach which employs a fully parametric assessment of drug interaction using the equation of W. R. Greco, G. Bravo, and J. C. Parsons (Pharmacol. Rev. 47:331–385, 1995) to generate an estimate of effects for the two drugs and have linked this approach to a population simulator, using Monte Carlo methods, which produce concentration-time profiles for the drugs in combination. This software automatically integrates the effect over a steady-state dosing interval and produces an estimate of the mean effect over a steady-state interval for each simulated subject. In this way, doses and schedules can be easily evaluated. This software allows for a rational choice of dose and schedule for evaluation in clinical trials. We evaluated different schedules of administration for the combination of the nucleoside analogue abacavir plus the human immunodeficiency virus type 1 protease inhibitor amprenavir. Amprenavir was simulated as either 800 mg every 8 h (q8h) or 1,200 mg q12h, each along with 300 mg q12h of abacavir. Both regimens produced excellent effects over the simulated population of 500 subjects, with average percentages of maximal effect (as determined from the in vitro assays) of 90.9% ± 11.4% and 80.9% ± 18.6%, respectively. This difference is statistically significant (P ≪ 0.001). In addition, 68.8 and 46.0% of the population had an average percentage of maximal effect which was greater than or equal to 90% for the two regimens. We can conclude that the combination of abacavir plus amprenavir is a potent combination when it is given on either schedule. However, the more fractionated schedule for the protease inhibitor produced significantly better effects in combination. Clinicians need to explicitly balance the improvement in antiviral effect seen with the more fractionated regimen against the loss of compliance attendant to the use of such a regimen. This approach may be helpful in the preclinical evaluation of multidrug anti-infective regimens.
Recent data have proven that combination chemotherapy is a necessity for the long-term suppression of human immunodeficiency virus (HIV) (1, 5). Once one posits that combination chemotherapy is a necessity for successful HIV chemotherapy, the problem of determining optimal chemotherapeutic regimens is posed. Combination chemotherapy is an inherently difficult problem, as there is true between-patient variability in the pharmacokinetics of each agent in the combination. In addition, the pharmacokinetics of one agent may not provide clues to the handling of the second agent in the combination, particularly when the drugs are primarily cleared by different organs (e.g., clearance by the kidneys versus that by the liver). Finally, the drugs may interact in a synergistic, additive, or antagonistic fashion. Other variables which influence the observed outcome include the drug dose and schedule of administration.
Determination of the drug interaction in a quantitative fashion is central to the delineation of optimal dosing regimens. Clearly, this is an important problem. The multiple regimens which need to be evaluated in a phase I-II trial of combination therapy means that many patients will be exposed to suboptimal regimens. Further, the cost in time and money of evaluating multiple regimens for dose and schedule is staggering. The ability to examine the impact of both dose and schedule in a quantitative fashion for combinations of agents can winnow the search for an optimal regimen to a manageable number, which can be appropriately studied in the clinical trial arena in the shortest time with the fewest number of patients.
Recently, our group has published a paper detailing the interaction of abacavir plus amprenavir with regard to antiviral effect in a fully parametric fashion (2) by employing the equation of Greco et al (4). In this analysis, we demonstrated that the combination of abacavir and amprenavir was fully synergistic.
In this study, our objective was to develop an approach which allows such data to be used to evaluate doses and schedules for combinations of agents. We have previously shown for an HIV protease inhibitor that time being greater than the 95% effective concentration (EC95) is the dynamically linked variable (G. L. Drusano, S. L. Preston, J. A. Bilello, B. Sadler, J. McDowell, W. Symonds, M. Rogers, S. LaFon, D. S. Stein, K. Muir, and A. Bye, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A5, 1998), and we wished to explicitly assess the effect of schedule of administration of the protease inhibitor amprenavir on the antiviral effect of its combination with the potent nucleoside analogue abacavir. We examined the simulated effects of 300 mg of abacavir given orally every 12 h (q12h) in combination with either 1,200 mg q12h or 800 mg q8h of amprenavir.
MATERIALS AND METHODS
Drug interaction parameters.
Drug interaction parameters were determined as previously described by fitting the Greco et al. drug interaction model to drug interaction data developed in triplicate in an HIV inhibition assay. These data have been previously published (2).
Pharmacokinetic parameter values.
Plasma drug concentration-time data were obtained from the sponsor and were derived from clinical patients receiving either abacavir or amprenavir as monotherapy for HIV disease in phase I or II trials. Abacavir was modeled by employing the NPEM III population modeling program of Schumitzky (7). One- and two-compartment open models with first-order elimination and input were examined. Model discrimination was by Akaikie's information criterion (9). The weighting scheme was determined by the high-level search algorithm of NPEM III. Seventy-eight patients contributed to this analysis. The mean parameter vector and major diagonal covariance matrix were employed in the Monte Carlo simulation described below.
For amprenavir, examination of the data demonstrated a clear-cut lag time to absorption. NPEM III does not currently have a lag time evaluation built in. Consequently, for this drug, these robust data sets (n = 40) were modeled individually by employing the ADAPT II program of D'Argenio and Schumitzky (ADAPT II User's Guide: Pharmacokinetics/Pharmacodynamics System Analysis Software; Biomedical Simulations Resource, Los Angeles, Calif.) and a two-compartment, open model with first-order input and elimination with a lag time was chosen. The mean parameter vector was formed as the mean of the individual patient estimates (n = 40). For the covariance matrix, the major diagonal matrix was employed.
Monte Carlo simulation.
The ADAPT II software for pharmacokinetic and pharmacodynamic systems analysis developed by D'Argenio and Schumitzky (ADAPT II User's Guide) was used for population simulations of 500 subjects for both abacavir and amprenavir. For abacavir, a dose of 300 mg orally q12h was simulated. For amprenavir, two simulations were performed. In one, a dose of 1,200 mg orally q12h was simulated. In the next, the 2,400-mg daily dose was divided into three 800-mg oral doses given q8h. In both simulations, the same initial seed integer value was used. The mean parameter vectors and covariance matrices estimated from the analyses described above were used to define the population distribution. The choice of a normal or lognormal prior population distribution was made by examining the marginal distributions of the parameters (part of the output of NPEM III) for abacavir and by generating frequency histograms of parameter values for amprenavir. In addition, both normal and lognormal choices were employed and the ability of the 500-subject simulation to recreate the parameter means and variances was examined.
Effect simulation.
The identified parameters from the drug interaction analysis previously published (2) were inserted into the Greco et al. equation. Each of the 500 simulated subjects had their parameter values inserted into a simulation module of the ADAPT II program in which the model of Greco et al. was coded and linked to the appropriate drug structural model. The effect profile for a 24-h period at steady state was then simulated and integrated from values from 0 to τ in the output module of ADAPT II and divided by 24 to provide the mean effect for that period. After the main simulation, the alpha (interaction parameter) was varied over a wide range to determine if the results were dependent upon a specific value of alpha.
Statistical analysis.
The fraction of subjects in each group whose average 24-h effect was ≥70 and ≥90% of the maximal effect was determined. Differences in proportions between groups were tested for significance by the Fisher exact test. The mean 24-hour effect ± standard deviation for each group (abacavir q12h plus amprenavir q12h versus abacavir q12h plus amprenavir q8h) was determined. Differences between means of the groups were tested for significance by the paired t test. All statistical testing was performed using SYSTAT for Windows version 8.0 (SPSS, Inc., Chicago, Ill.).
RESULTS
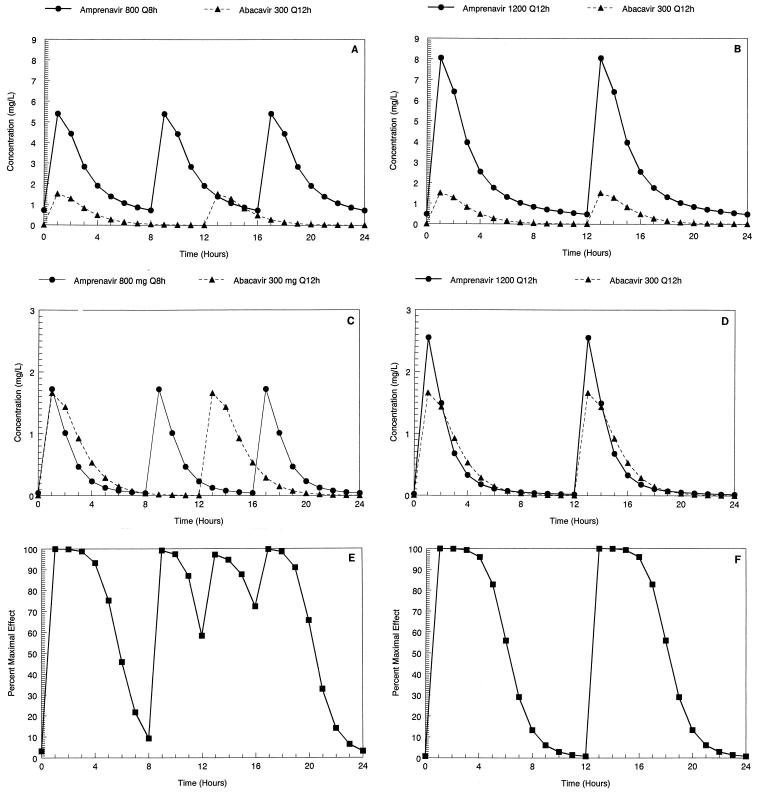
The parameter values and their dispersions for both abacavir and amprenavir are displayed in Table 1. The mean concentration-time curves for abacavir and amprenavir derived by simulation from the mean values of the 500-subject simulations are displayed in Fig. 1A and B. In Fig. 1C and D, we display the concentration-time profiles for a specific, but random, subject from among the 500 simulated subjects for each of the regimens. In Fig. 1E and F, the effect-time curves derived from the concentration-time curves of Fig. 1C and D are displayed. Clearly, the q8h regimen for amprenavir gives a very different shape to the effect-time curve because the doses of the two drugs are administered out of synchrony.
TABLE 1.
Parameter values and their dispersions for abacavir and amprenavir employed in the Monte Carlo simulations
| Agent and parametera | Value (mean ± SD) |
|---|---|
| Abacavir | |
| SCL (liters/h) | 60.0 ± 3.24 |
| Vc (liters) | 67.6 ± 5.0 |
| Ka (h−1) | 0.813 ± 0.233 |
| Amprenavir | |
| Vc (liters) | 79.0 ± 53.3 |
| Ke (h−1) | 0.958 ± 0.456 |
| Ka (h−1) | 2.359 ± 2.335 |
| K12 (h−1) | 0.738 ± 1.428 |
| K21 (h−1) | 1.251 ± 4.338 |
| Tlag (h) | 0.517 ± 0.295 |
SCL, clearance from serum; Vc, volume of the central compartment; Ke, first-order rate constant for drug removal from the central compartment; Ka, first-order absorption rate constant from the gut; K12 and K21, first-order transfer rate constants between the central and peripheral compartments; Tlag, lag time to absorption.
FIG. 1.
(A) Mean concentration-time curve for a steady-state dosing interval derived from a 500-subject Monte Carlo simulation with abacavir (300 mg q12h) and amprenavir (800 mg q8h). (B) Mean concentration-time curve for a steady-state dosing interval derived from a 500-subject Monte Carlo simulation with abacavir (300 mg q12h) and amprenavir (1,200 mg q12h). (C) Concentration-time curve for a steady-state dosing interval simulated for one of 500 simulated subjects using abacavir (300 mg q12h) and amprenavir (800 mg q8h). (D) Concentration-time curve for a steady-state dosing interval simulated for one of 500 simulated subjects using abacavir (300 mg q12h) and amprenavir (1,200 mg q12h). (E) Steady-state effect-time curve as calculated from the drug interaction parameters (3) for abacavir plus amprenavir at the concentrations displayed in panel C. (F) Steady-state effect-time curve as calculated from the drug interaction parameters (3) for abacavir plus amprenavir at the concentrations displayed in panel D.
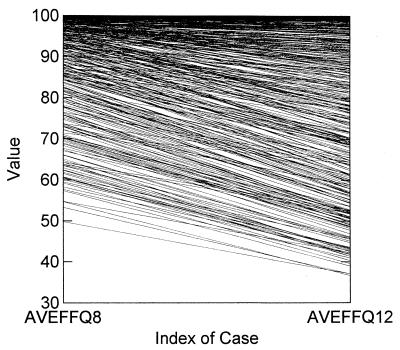
In Fig. 2, we display the graph for all 500 simulated subjects, with amprenavir being administered on an 800-mg-q8h and on a 1,200-mg-q12h schedule. It can be seen that, for the majority of patients, there is a negative slope for the line connecting each subject by regimen. This result indicates that, in general, the q8h regimen has a greater 24-h mean effect at steady state.
FIG. 2.
Average effect for each patient for a steady-state dosing interval displayed for the two simulated administration schedules. AVEFFQ8 and AVEFFQ12, amprenavir administered q8h and q12h, respectively. On average, the schedule on which amprenavir was administered q8h had a higher mean effect.
In Table 2, we display the mean 24-h effect for each group along with the dispersion. These differences are highly significant (P < 0.001) and favor the more fractionated schedule. We also display in Table 2 the fraction of the 500 simulated subjects whose mean 24-h steady-state effect was ≥70 and ≥90% of maximal. In each instance, the more fractionated (q8h) administration schedule was superior (for each contrast, P ≪ 0.001).
TABLE 2.
Mean percentages of the antiviral suppressive effect and the fractions of the simulated population (n = 500) exceeding the 70 and 90% maximal suppressive effects over a 24-hour steady-state dosing interval for two regimens of abacavir plus amprenavir differing only in the schedule of amprenavir administration
| Parameter | Value for regimen:
|
|
|---|---|---|
| Abacavir at 300 mg orally q12h plus amprenavir at 800 mg orally q8h | Abacavir at 300 mg orally q12h plus amprenavir at 1,200 mg orally q12ha | |
| Mean effect ± SD (%) | 90.9 ± 11.4 | 80.9* ± 18.6 |
| Fraction ≥70% of maximal effect | 459/500 | 354/500** |
| Fraction ≥90% of maximal effect | 344/500 | 230/500** |
*, P < 0.001 (paired t test); **, P < 0.001 (Fisher exact test).
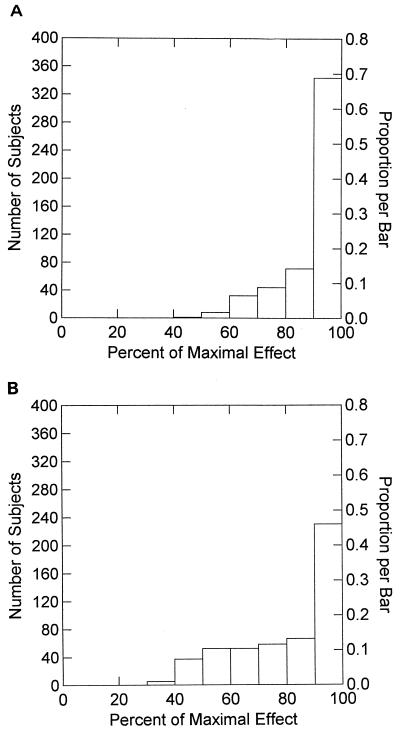
Finally, we display the complete frequency histogram of the mean 24-h effect for each of the regimens in Fig. 3. By examination, it is clear that the more fractionated schedule for amprenavir produced a greater fraction of patients at higher mean effect levels.
FIG. 3.
Frequency histogram for the average steady-state effect seen from the simulation for abacavir at 500 mg q12h plus amprenavir at 800 mg q8h. (B) Frequency histogram for the average steady-state effect seen from the simulation for abacavir at 500 mg q12h plus amprenavir at 1,200 mg q12h.
Further simulation with different values of the interaction parameter did not change the outcome meaningfully (data not shown).
DISCUSSION
We examined combination chemotherapy with two potent agents of differing classes, abacavir, a nucleoside analogue, and amprenavir, an HIV type 1 protease inhibitor. Both drugs have been tested as single agents and have been shown to be effective and well tolerated (M. Saag, D. Lancaster, A. Sonnerborg, J. Mulder, R. Torres, R. Schooley, R. Harrigan, D. Kelleher, and W. Symonds, Abstr. 3rd Conf. Retroviruses Opportunistic Infect., abstr. 195, 1996; R. T. Schooley and the 141W94 Int. Study Group, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LB7a, 1996). We demonstrated here that the dosing interval of the protease inhibitor portion of the combination has a major influence on the average in vitro anti-HIV effect of the regimen at steady state.
Prior in vitro evaluation (2) demonstrated that abacavir and amprenavir were significantly synergistic, whether they were evaluated relative to a Loewe additivity or Bliss independence model of drug interaction.
While it is helpful to know that two drugs interact synergistically, it begs the question of how this knowledge may be put to practical use in an evaluation of combination chemotherapy regimens. It was our intention to develop here, for the first time, a system which would allow the evaluation of different regimens in combination.
Variability in the pharmacokinetics of the agents exerts a major influence on the antiviral effect seen. All of the variability seen in this evauation is attributable to between-patient variability in the pharmacokinetic profiles of the two drugs and the differences in administration schedules. This is so because the evaluation was performed against a single strain of virus, so there was no variability in sensitivities to the drugs being evaluated.
The value of the Monte Carlo (stochastic) approach, when compared to a simpler method using only mean parameter values (deterministic), can be illustrated as follows. Using, for instance, the median value for clearance misses the fact that 50% of the population will have larger clearances, lower concentrations, and therefore lesser effects. Depending on the spread of clearance values in the population, a portion of the population may have suboptimal effects, even while evaluation of the mean values of parameters indicated that the typical results might be acceptable. Consequently, we felt that it was imperative to perform a stochastic simulation.
Our evaluation had clear-cut results. The more fractionated schedule was superior. A regimen of 300 mg of abacavir orally q12h plus 800 mg of amprenavir orally q8h was significantly better than the regimen in which the amprenavir was administered q12h, whether one examined the mean effect for the population or proportions of the population whose mean effect was greater than two arbitrary values we chose (≥70 and ≥90% of the maximal effect). It is important to note that the effect results are generated from in vitro data.
Such results need to be placed into proper perspective. First, it should be pointed out that even when administered on a 12-h basis, the combination of abacavir and amprenavir is very potent, with the overall mean effect for a steady-state dosing interval exceeding 80% of maximum for this regimen. Further, almost half (46%) of the simulated subjects had a mean effect which was ≥90% of the maximal effect. Also, the addition of a third drug into the regimen (e.g., lamivudine or efavirenz), as is the current standard, might well further reduce the differences between the two modes of administration.
Another issue which needs to be addressed is the believability of the results. There are other data which support our findings. The conclusion which can be drawn from our investigation is that amprenavir, the protease inhibitor, is a drug for which time is greater than the EC95 is the pharmacodynamically linked variable. More fractionated schedules of the same total daily dose tend in most instances (but not always) to extend the times that concentrations exceed the EC95. This result was also found in a single-agent evaluation of amprenavir by our hollow-fiber system, consistent with the findings here (Drusano et al., 38th ICAAC). In addition, while performed with different drugs, a trial in which nucleoside analogues (zidovudine and lamivudine) were administered on a 12-h schedule and another inhibitor of the HIV protease (indinavir) was administered at either 800 mg q8h or 1,200 mg q12h was recently reported (6). The outcome in that study was as clear-cut as our results. When this combination was administered q8h, 91% of patients had their viral loads decline to below 400 copies/ml, while 64% of patients in the group receiving 1,200 mg q12h had their viral loads decline to below this level. Consequently, it should not be surprising that our simulation demonstrated that the combination of abacavir and amprenavir was also more effective when the protease inhibitor was administered on a more fractionated schedule. Given its different pharmacokinetic profile, it is also not surprising that amprenavir works somewhat better on a 12-h schedule than does indinavir. The point of this evaluation is that the already potent activity of the abacavir-amprenavir combination might be positively affected by a change in the schedule of administration for the protease inhibitor.
Such findings pose a dilemma for the clinician. The advent of effective antiretroviral chemotherapy also clarified issues regarding the emergence of viral resistance. There are two major pathways to emergence of resistance: one is suboptimal chemotherapy which does not suppress the viral copy number to below the detectability of the assay (and allows ongoing viral replication) and the other is poor compliance with the therapeutic regimen. Clearly, maximal antiviral effect would be desirable, would result in the largest proportion of patients with undetectable viral loads, and would lead to the recommendation of q8h dosing. However, frequent dosing results in poorer compliance from patients with their therapeutic regimen and poor compliance leads to resistance (3, 8). It seems that clinicians must choose between maximal antiviral effect, possibly leading to emergence of resistance from poor compliance, and suboptimal therapeutic effect, possibly leading to emergence of resistance from inadequate suppression of viral replication.
While all solutions to such conundrums are, in essence, patient specific (i.e., clinicians will suspect that some patients are more likely than others to be compliant, even with an 8-h regimen), it may be that the 12-h regimen may be preferred, even if it is less virologically active.
In summary, the ability to understand the way in which drugs interact for antiviral effect is key to designing appropriate regimens for testing in clinical trials. Issues of dose and schedule can be robustly addressed, and the full variability of pharmacokinetics for each of the agents can be examined for effect upon virological activity. It should be realized that the investigation described above was for a single HIV isolate. Use of such an approach for clinical trial design purposes would benefit from having multiple HIV isolates examined for virologic interaction and from having the simulation performed for each of these isolates. In such a way, the effect of full or partial resistance of a viral isolate to one or more of the agents in a combination regimen can be examined. Hopefully, the result will be the choice of the best doses and schedules of agents for examination in the clinical trial arena.
ACKNOWLEDGMENT
This study was supported by Glaxo Wellcome, Inc.
REFERENCES
- 1.Drusano G L, Bilello J A, Stein D S, Nessly M, Meibohm A, Emini E A, Deutsch P, Condra J, Chodakewitz J, Holder D J. Factors influencing the emergence of resistance to indinavir: role of virologic, immunologic, and pharmacologic variables. J Infect Dis. 1998;178:360–367. doi: 10.1086/515631. [DOI] [PubMed] [Google Scholar]
- 2.Drusano G L, D'Argenio D Z, Symonds W, Bilello P A, McDowell J, Sadler B, Bye A, Bilello J A. Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro. Antimicrob Agents Chemother. 1998;42:2153–2159. doi: 10.1128/aac.42.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eraker S A, Kirscht J P, Becker M H. Understanding and improving patient compliance. Ann Intern Med. 1984;100:258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- 4.Greco W R, Bravo G, Parsons J C. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 5.Kempf D J, Rode R A, Xu Y, Sun E, Heath-Chiozzi M E, Valdes J, Japour A J, Danner S, Boucher C, Molla A, Leonard J M. The duration of viral suppression during protease inhibitortherapy for HIV-1 infection is predicted by plasma HIV-1 RNA at the nadir. AIDS. 1998;12:F9–F14. doi: 10.1097/00002030-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 6.McGee K. Ask the pharmacist: simpler is not always better. Am Druggist. 1999;2(Suppl.):25–26. [Google Scholar]
- 7.Schumitzky A. Nonparametric EM algorithms for estimating prior distributions. Appl Math Comput. 1991;45:141–157. [Google Scholar]
- 8.Wainberg M A, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 9.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]