Abstract
Objectives:
To compare health care utilization patterns and cost among insured adults with autism spectrum disorder (ASD), adults with attention-deficit and hyperactivity disorder (ADHD), and adults with neither condition (general population [GP] controls).
Method:
We conducted a case–control study among adults (≥18 years) who were members of Kaiser Permanente Northern California (KPNC) for at least 9 months each year from 2008 to 2012. Cases (N = 1507) were adults with an ASD diagnosis (ICD-9-CM 299.0–299.8) recorded in the electronic medical record on at least two separate occasions by December 31, 2012. Two control groups, adults with ADHD (N = 9042) defined by ICD-9-CM code 314 and GP (N = 15,070), were randomly selected and frequency matched to cases on gender and age. Health care utilization and cost data were obtained from KPNC databases for the year 2012.
Results:
Compared with adults with ADHD, adults with ASD had significantly higher utilization of outpatient visits for primary care (74.2% vs. 66.6%), mental health (43.3% vs. 33.2%), and laboratory services (60.9% vs. 54.4%). Hospitalizations for ambulatory care sensitive diagnoses (5.4% vs. 2.3%) were less frequent overall but more common among adults with ASD than with ADHD. Group differences were larger comparing adults with ASD with GP controls. Gynecology visits and screening for cervical cancer were significantly less common among women with ASD than among women with ADHD (35% vs. 50%) or GP (35% vs. 49%). Total annual mean healthcare costs for adults with ASD were 20% higher than costs for adults with ADHD and double costs for GP.
Conclusion:
Adults with ASD had significantly higher rates of utilization across most health care service areas compared with adults with ADHD or GP; however, women with ASD were significantly less likely to have gynecology visits and have screening for cervical cancer.
Lay Summary
We conducted a study among adults (≥18 years) who were members of Kaiser Permanente Northern California (KPNC) from 2008 to 2012. We compared how often people attended different types of health care and costs of health care among adults with autism spectrum disorder (ASD), adults with attention-deficit and hyperactivity disorder (ADHD), and adults with neither condition (general population [GP] controls). The study included 1507 adults with ASD, 9042 with ADHD but not ASD, and 15,070 GP controls with no ASD or ADHD. Health care and cost data were obtained from KPNC databases for the year 2012. The study found that adults with ASD used more outpatient visits for primary care, mental health, and laboratory services than adults with ADHD. Gynecology visits and screening for cervical cancer were less common among women with ASD than among women with ADHD or GP. Health care costs for adults with ASD were higher than costs for adults with ADHD and costs for GP. In conclusion, adults with ASD had higher rates of use of most health care service areas than adults with ADHD or GP; however, women with ASD were less likely to have gynecology visits and have screening for cervical cancer.
Keywords: : autism spectrum disorders, health care costs, health care service utilization
Introduction
Autism spectrum disorders (ASDs) are a group of developmental disabilities typically diagnosed in childhood and persisting into adulthood. The prevalence of ASD in children has increased dramatically since the 1980s,1–4 suggesting that the prevalence in the adult population, estimated at 1%,5 will also increase.
Studies conducted in the United States and Europe found that children and adults with ASD have higher rates of co-occurring medical and mental health conditions than children and adults without,6–18 and a twofold or more increased mortality rates,19 and may, therefore, require more intensive use of health care services to address these health problems. In fact, previous studies in the United States have shown that among children, utilization of pediatric, psychiatric, and neurology services and prescription medications is higher among individuals with ASD than controls.20–25 Children with ASD are also more likely than controls to visit emergency departments (EDs) for psychiatric reasons22 and to be hospitalized for ambulatory care sensitive conditions (conditions, such as epilepsy, which, if well monitored, should not result in a hospitalization).26,27 In addition, when hospitalized, autistic children and youth have a longer length of stay, on average, than children without ASD.23 High health care service utilization, especially specialty care and hospitalization, leads to higher health care costs for children with ASD.21,23,28–32
Although previous research suggests that health care utilization patterns may change over the life course,33 very little is known about health care utilization patterns among adults with ASD. Recent studies reported higher rates of ED visits34–37 and lower rates of tetanus vaccination and cervical cancer screening among adults with ASD than among controls.34 The reasons for higher utilization of ED visits by ASD cases are not well understood and may be multifactorial.36 A recent study found that adults with ASD had higher mean annual outpatient office visits, prescription drug use claims, and higher health care costs.18 However, utilization of other types of health care services and preventive care among adults with ASD has not been reported. A number of studies have reported that children and adults with ASD do not have adequate access to health care and expressed unmet health care needs.34,38,39 There is a lack of comprehensive data on health care utilization of adults with ASD who are members of a comprehensive health care organization. The objective of this study was to determine and compare health care utilization and cost among adults with ASD, adults with attention-deficit and hyperactivity disorder (ADHD), and adults with neither condition receiving care in the same large integrated health care delivery system in the United States.
Methods
Study population
Adults ≥18 years of age as of January 2008 who were members of Kaiser Permanente Northern California (KPNC) for at least 9 months in each calendar year from January 2008 to December 2012 were eligible for inclusion (N = 1,578,657). KPNC is the largest and oldest group model prepaid, integrated health care delivery organization in the United States providing health care to ∼4 million members in the San Francisco and Sacramento metropolitan areas and surrounding counties. KPNC members are broadly socioeconomically representative of the northern California population in the geographic region covered by KPNC except for the extremes of income distribution.40,41 Cases (N = 1507) were defined as adults with ASD diagnoses (Autism: International Classification of Diseases-9-Clinical Modification [ICD-9-CM] 299.0; Asperger's Disorder [ICD-9-CM 299.8]; Pervasive Developmental Disorder, Not Otherwise Specified [ICD-9-CM 299.9]) recorded in KPNC electronic medical records on at least two separate occasions anytime through December 2012. We include all cases across the autism spectrum who met our case definition. Two comparison groups were chosen. The first comprised adults with a diagnosis of ADHD (ICD-9-CM 314), frequency matched at a 6:1 ratio to the ASD group on gender and 5-year age group (N = 9042). The second comprised adults with no ASD or ADHD diagnoses ([GP] controls) recorded in their medical records by December 2012, randomly sampled at a 10:1 ratio and frequency matched to cases on gender and 5-year age group (N = 15,070). We required that both the ADHD and GP controls had at least one encounter with health care services anytime through December 2012. We included the ADHD comparison group to determine whether health care utilization and costs for adults with ASD differed from that among adults with another common neurodevelopmental disorder.
Health care utilization
Health care utilization data were obtained from the KPNC inpatient, outpatient, and pharmacy databases for the period January–December 2012. Utilization was defined in terms of outpatient clinic visits, which included primary care (visits made to the patient's primary care physician or other internal/family medicine physician) and specialty care (visits made to a provider in one of the following departments: mental health/psychiatry, neurology, obstetrics/gynecology, speech therapy, physical/occupational therapy) that occurred at KPNC facilities and authorized providers outside of KPNC; ED visits; hospitalizations (inpatient hospitalization, hospitalization for ambulatory care sensitive diagnoses,42 and same-day hospitalizations); preventive services according to KPNC gender and age specific recommendations (annual influenza vaccination, diabetes screening, prostate cancer screening, breast cancer screening, cholesterol and colon cancer screening, and cervical cancer screening); prescribed medications (any medications, psychotherapeutics, anticonvulsants, analgesics/antirheumatics, anti-infectives, gastrointestinal agents, respiratory/allergy medications, diabetes medications, lipid/cholesterol medications, and hypertension medications). Preventive screening was based on tests for a health condition (identified by ICD-9 and CPT codes) among individuals who did not have the health condition and who were within the recommended age range for the preventive screening. For example, women who did not have a breast cancer diagnosis, who were at least 40 years of age, and who received a mammogram were considered to have preventive screening for breast cancer. Measures of health care utilization were defined as dichotomous (yes/no) and mean and median number of utilization for categories already listed.
Costs
Costs for services provided directly by the KPNC were obtained from the Cost Management Information System, an automated system that integrates hospital, laboratory, radiology, outpatient, and home health utilization databases with the program's financial ledger. Costs (including program and facility overhead) are generated for each service, as defined by the system, using standard accounting methods and program-specific relative value units for each service. Costs for KPNC-approved services provided by non-KPNC vendors (hospitals and individual providers) were also included. Because the cost of non-KPNC ED visits could not be distinguished from any immediately subsequent hospitalization, those costs were counted as hospital costs instead of ED costs. Pharmacy costs were obtained directly from the KPNC Pharmacy Information Management System, an automated, region-wide clinical database that records each prescription dispensed at an outpatient KPNC pharmacy. Costs not included in our analyses were dental costs, and patient out-of-pocket expenses including copayments. Costs were limited to those that occurred from January to December 2012.
Covariates
All covariates were obtained from the KPNC electronic medical record. Demographic characteristics included gender (male/female), age as of January 2008, continuous and categorical (18–24, 25–49, 50+), and race/ethnicity (white non-Hispanic, white Hispanic, black, Asian, and other). Measures of co-occurring medical health conditions included dichotomous (yes/no) indicators for immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions. Each condition was considered present if an individual had an ICD-9-CM diagnostic code for the condition documented in their KPNC electronic medical record on at least two separate occasions from January 2008 to December 2012.8
Statistical analysis
For dichotomous measures of health care utilization, we used multivariable logistic regression models to examine the association between having an ASD diagnosis (compared with ADHD or GP) and each health care service category while controlling for demographic factors (gender, age, and race/ethnicity), and major medical and psychiatric comorbidities. Multivariable negative binomial regression models were used when comparing the number of visits between adults with ASD and adults with ADHD or GP. Since this method yields inaccurate confidence intervals around the rate ratios (which are robust),43,44 we used a bootstrapping method to calculate accurate confidence intervals around the point estimates. In each analysis, we adjusted for the matching variables in addition to major medical and psychiatric comorbidities and race/ethnicity. We also conducted stratified analyses by gender and age.
Health care cost was measured by the mean and median annual cost per member. We calculated the total costs and service type-specific costs. We used generalized linear modeling with gamma distribution and log-link function to compare the mean cost of health care services for adults with ASD with those for adults with ADHD and adults with GP. Finally, we conducted two similar sets of analyses post hoc (1) restricting the ADHD and GP controls to individuals who had at least two encounters with the health care system during the study period, to match the eligibility criteria of the ASD cases, and (2) using a broader definition of ASD that included all adults with one or more ASD diagnoses recorded in their medical record.
All cost estimates were adjusted for the same variables included in the utilization analyses. All study procedures were approved by the KPNC Institutional Review Board.
Results
A total of 1507 adults with ASD, 9042 with ADHD, and 15,070 GP controls were included in this study. The demographic characteristics of the sample have been described previously.8 In brief, the mean age of the study population at the start of the study was 29 years (standard deviation 12 years). Approximately half (52%) were between 18 and 24 years, and 9.5% were 50 years or older. The overall male/female ratio was 2.7. Adults with ASD were less likely to be white non-Hispanic (66% vs. 73%) and more likely to be Asian (11% vs. 6%) than adults with ADHD. In contrast, adults with ASD were more likely to be white non-Hispanic (66% vs. 44%) and less likely to be Asian (11% vs. 17%) than GP controls. Adults with ASD were more likely to be covered by Medicaid or Medicare than adults with ADHD (47.9% vs. 9.5%) or GP controls (47.9% vs. 5.0%). The mean length of KPNC membership for each study group was 11.9 years (Supplementary Table S1).
Outpatient and inpatient health care service utilization
Compared with adults with ADHD, adults with ASD had higher utilization of outpatient visits for primary care (74.2% vs. 66.6%), mental health (43.3% vs. 33.2%), and laboratory services (60.9% vs. 54.4%) (Table 1). Visits for speech therapy were uncommon but also more frequent among adults with ASD than among adults with ADHD (0.8% vs. 0.1%) (Table 1). Adults with ASD were less likely to receive physical/occupational therapy (3.8% vs. 7.7%), radiology (25% vs. 30.6%), or have an ED visit (20.3% vs. 21%) than adults with ADHD (Table 1). Although there were no differences for inpatient and same-day hospitalization between the ASD and ADHD groups, the proportion of adults with ASD with a hospitalization for ambulatory care sensitive diagnoses was double that of adults with ADHD (5.4% vs. 2.3%). After adjustment for gender, age, race/ethnicity, total length of KPNC membership, and medical and psychiatric comorbidities, these differences remained statistically significant. Similar group differences were observed for ASD versus GP comparisons with the exception of neurology visits, which were significantly more common in the ASD group, and ED visits and hospitalizations for ambulatory care sensitive diagnoses, which did not differ significantly between the two groups (Table 1).
Table 1.
Health care Utilization Among Adults with Autism Spectrum Disorder, Attention-Deficit and Hyperactivity Disorder, and General Population Controls, Kaiser Permanente Northern California, 2012
| ASD (N = 1507), n (%) | ADHD (N = 9042), n (%) | GP (N = 15070), n (%) | ASD vs. ADHD adjusted OR a (95% CI) | ASD vs. GP adjusted OR a (95% CI) | |
|---|---|---|---|---|---|
| Any outpatient visit | 1299 (86.2) | 7387 (81.7) | 10648 (70.7) | 1.4 (1.2–1.7) | 2.1 (1.8–2.4) |
| Primary care | 1118 (74.2) | 6020 (66.6) | 8907 (59.1) | 1.4 (1.3–1.6) | 1.6 (1.4–1.8) |
| Mental health/psychiatry | 653 (43.3) | 3001 (33.2) | 816 (5.4) | 1.6 (1.4–1.8) | 11.5 (10.0–13.2) |
| Neurology | 85 (5.6) | 314 (3.5) | 223 (1.5) | 1.1 (0.8–1.5) | 1.7 (1.3–2.4) |
| Speech therapy | 12 (0.80) | 11 (0.1) | 10 (0.07) | 5.0 (2.0–12.2) | 16.0 (6.4–40.1) |
| Physical/occupational therapy | 57 (3.8) | 694 (7.7) | 702 (4.7) | 0.5 (0.4–0.6) | 0.62 (0.5–0.8) |
| Laboratory | 918 (60.9) | 4914 (54.4) | 7158 (47.5) | 1.3 (1.2–1.5) | 1.5 (1.3–1.7) |
| Radiology | 377 (25.0) | 2765 (30.6) | 3842 (25.5) | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) |
| Emergency department visit | 306 (20.3) | 1901 (21.0) | 2170 (14.4) | 0.8 (0.7–0.9) | 1.0 (0.9–1.2) |
| Inpatient hospitalization | 84 (5.6) | 377 (4.2) | 480 (3.2) | 1.1 (0.9–1.5) | 1.3 (1.0–1.7) |
| Same-day hospitalization | 52 (3.4) | 336 (3.7) | 500 (3.3) | 0.9 (0.7–1.3) | 0.7 (0.5–1.0) |
| Hospitalization for ambulatory care sensitive diagnoses | 81 (5.4) | 210 (2.3) | 475 (3.1) | 2.1 (1.6–2.9) | 1.2 (1.0–1.6) |
Adjusted for gender, age, race/ethnicity, total length of KPNC membership, and nine categories of medical and psychiatric comorbidities (immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions) identified with diagnosis codes for primary and secondary reasons for visit.
ASD, autism spectrum disorder; ADHD, attention-deficit/hyperactivity disorder; GP, general population; OR, odds ratio; CI, confidence interval; KPNC, Kaiser Permanente Northern California.
Adults with ASD had a significantly higher mean number of visits for primary care, mental health, and speech therapy, and a significantly lower mean number of visits for gynecology, physical/occupational therapy, and radiology than adults with ADHD and GP controls (Table 2). In addition, adults with ASD had a significantly higher mean number of neurology visits and laboratory visits than GP controls (Table 2).
Table 2.
Mean Annual Number of Outpatient and Inpatient Visits per Member Among Adults with Autism Spectrum Disorder, Attention-Deficit and Hyperactivity Disorder, and General Population Controls, Kaiser Permanente Northern California, 2012
| ASD | ADHD | GP | Adjusted mean ratios a (RR) (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ASD vs. ADHD | ASD vs. GP | |
| Any outpatient clinic visit | 8.0 (19.5) | 4 (1, 8) | 7.1 (14.4) | 3 (1, 8) | 3.9 (8.8) | 2 (0, 4) | 1.2 (1.1–1.2) | 1.7 (1.5–1.8) |
| Primary care visit | 2.5 (3.5) | 1 (0, 3) | 2.1 (3.5) | 1 (0, 3) | 1.5 (2.8) | 1 (0, 2) | 1.2 (1.1–1.3) | 1.3 (1.2–1.4) |
| Mental health/psychiatry | 2.8 (8.7) | 0 (0, 2) | 1.5 (5.0) | 0 (0, 1) | 0.3 (2.0) | 0 (0, 0) | 1.9 (1.7–2.2) | 11.7 (9.3–14.8) |
| Neurology | 0.1 (0.6) | 0 (0, 0) | 0.1 (0.4) | 0 (0, 0) | 0.0 (0.2) | 0 (0, 0) | 1.3 (0.9–1.7) | 2.2 (1.6–3.2) |
| Gynecology | 0.7 (1.6) | 0 (0, 0) | 0.4 (2.1) | 0 (0, 0) | 1.6 (3.5) | 0 (0, 0) | 0.6 (0.5–0.8) | 0.4 (0.3–0.5) |
| Speech therapy | 0.0 (0.2) | 0 (0, 0) | 0.0 (0.1) | 0 (0, 0) | 0.0 (0.1) | 0 (0, 0) | 7.5 (2.4–23.8) | 26.7 (6.6–109.0) |
| Physical/occupational therapy | 0.1 (0.6) | 0 (0, 0) | 0.2 (1.3) | 0 (0, 0) | 0.1 (1.1) | 0 (0, 0) | 0.4 (0.3–0.5) | 0.4 (0.3–0.6) |
| Laboratory | 2.1 (4.9) | 1 (0, 2) | 1.9 (4.1) | 1 (0, 2) | 1.4 (2.9) | 0 (0, 2) | 1.1 (1.0–1.2) | 1.3 (1.2–1.4) |
| Radiology | 0.6 (1.7) | 0 (0, 1) | 0.7 (1.6) | 0 (0, 1) | 0.5 (1.4) | 0 (0, 1) | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) |
| Emergency department visit | 0.4 (1.3) | 0 (0, 0) | 0.4 (1.2) | 0 (0, 0) | 0.2 (1.0) | 0 (0, 0) | 0.9 (0.8–1.0) | 1.2 (1.0–1.4) |
| Inpatient hospitalization | 0.1 (0.4) | 0 (0, 0) | 0.1 (0.4) | 0 (0, 0) | 0.0 (0.3) | 0 (0, 0) | 1.1 (0.8–1.4) | 1.3 (1.0–1.7) |
| Same-day hospitalization | 0.1 (0.5) | 0 (0, 0) | 0.1 (0.5) | 0 (0, 0) | 0.1 (0.5) | 0 (0, 0) | 0.8 (0.5–1.1) | 0.8 (0.5–1.1) |
| Hospitalization for ambulatory case sensitive diagnoses | 0.1 (0.9) | 0 (0, 0) | 0.1 (0.6) | 0 (0, 0) | 0.0 (0.3) | 0 (0, 0) | 1.1 (0.9–1.4) | 1.26 (1.0–1.7) |
Adjusted for gender, age, race/ethnicity, total length of KPNC membership, and nine categories of medical and psychiatric comorbidities (immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions) identified with diagnosis codes for the primary and secondary reasons for visit.
RR, rate ratio; SD, standard deviation; IQR, interquartile range.
Among both men and women, adults with ASD had significantly higher rates of outpatient visits for primary care and mental health, and significantly lower rates of visits for physical/occupational therapy and radiology than adults with ADHD (Supplementary Table S2). Among men only, utilization of laboratory services and hospitalizations for ambulatory care sensitive diagnoses were significantly more common among adults with ASD than among adults with ADHD. Among women only, gynecology visits and same-day hospitalizations were significantly less common among adults with ASD than among adults with ADHD. Results were similar for ASD versus GP comparisons (Supplementary Table S2).
Mental health visits, ED visits, inpatient hospitalizations, and laboratory visits all increased with age for adults with ASD (Supplementary Fig. S1). In each of these four service categories, adults with ASD had significantly higher service utilization than gender-matched ADHD and GP controls for most age groups. For ED visits, case–control (ADHD or GP) differences were only statistically significant for the ≥50-year age group (Supplementary Fig. S1).
Preventive health care service utilization
A significantly higher proportion of adults with ASD received a seasonal influenza vaccination, cholesterol screening, and diabetes screening than adults with ADHD or GP controls after adjustment for demographic factors and comorbidities. Among women, breast cancer screening rates did not differ between the three study groups; however, cervical cancer screening was significantly less common among adults with ASD than among adults with ADHD or GP controls (Table 3).
Table 3.
Preventive Health care Utilization Among Adults with Autism Spectrum Disorder, Attention-Deficit and Hyperactivity Disorder, and General Population Controls, Kaiser Permanente Northern California, 2012
| Preventive service | ASD (N = 1507), n (%) | ADHD (N = 9042), n (%) | GP (N = 15070), n (%) | ASD vs. ADHD adjusted OR a (95% CI) | ASD vs. GP adjusted OR a (95% CI) |
|---|---|---|---|---|---|
| Seasonal influenza vaccination | 659 (43.7) | 2380 (26.3) | 3487 (23.1) | 2.3 (2.1–2.7) | 2.6 (2.3–2.9) |
| Cholesterol screening (age ≥40)b | 48 (24.1) | 206 (15.9) | 345 (14.8) | 1.7 (1.2–2.5) | 1.7 (1.2–2.5) |
| Diabetes screening (age ≥45)c | 80 (29.4) | 308 (18.3) | 512 (18.5) | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) |
| Prostate cancer screening (male age ≥40)d | 37 (14.0) | 179 (11.3) | 314 (11.9) | 1.3 (0.9–2.0) | 1.2 (0.8–1.8) |
| Cervical cancer screening (female age ≥21)e | 82 (20.3) | 674 (28.2) | 1166 (29.2) | 0.7 (0.5–0.9) | 0.6 (0.5–0.8) |
| Breast cancer screening (female age ≥40)f | 56 (43.1) | 331 (42.4) | 573 (43.7) | 1.0 (0.7–1.5) | 1.1 (0.7–1.6) |
Adjusted for gender, age, race/ethnicity, total length of KPNC membership, and nine categories of medical and psychiatric comorbidities (immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions) identified with diagnosis codes for the primary and secondary reasons for visit.
Cholesterol screening defined by ICD-9 (V77.91, V70.0) and CPT4 (80061, 82465, 83718, 83719, 83721, 84478, 36415, 36416).
Diabetes screening defined by ICD-9 (V77.1, V70.0) and CPT4 (82947, 82948, 82950, 82951, 82952, 84520, 83036, 36415, 36416).
Prostate cancer screening defined by ICD-9 (V76.44, V70.0) and CPT4 (84152, 84153, 84154).
Cervical cancer screening defined by ICD-9 (V76.2, V72.31, V70.0) and laboratory report data.
Breast cancer screening defined by ICD-9 (V76.12, V76.11, V72.31, V70.0) and CPT4 (77057, 76092, 76083).
Medication utilization
After adjusting for demographic factors and medical and psychiatric comorbidities, a significantly higher proportion of adults with ASD received a prescription for psychotherapeutic, anticonvulsant, diabetes, cardiovascular, and lipid/cholesterol medications, and a significantly lower proportion received a prescription for analgesics/antirheumatics and anti-infectives than adults with ADHD or GP (Table 4). Receipt of a prescription for hypertensive medications was significantly more common among adults with ASD only in comparison with GP controls (Table 4).
Table 4.
Prescribed Medication Utilization Among Adults with Autism Spectrum Disorder, Attention-Deficit and Hyperactivity Disorder, and General Population Controls, Kaiser Permanente Northern California, 2012
| Medication group a | ASD N = 1507, n (%) | ADHD N = 9.042, n (%) | GP N = 15,070, n (%) | ASD vs. ADHD adjusted OR b (95% CI) | ASD vs. GP adjusted OR b (95% CI) |
|---|---|---|---|---|---|
| Any medications (overall) | 1199 (79.6) | 7139 (79.0) | 9313 (61.8) | 1.0 (0.8–1.1) | 1.9 (1.7–2.2) |
| Psychotherapeutic medications | 927 (61.5) | 4478 (49.5) | 2273 (15.1) | 1.7 (1.5–1.9) | 8.0 (7.1–9.1) |
| Antidepressants | 592 (39.3) | 2681 (29.7) | 1245 (8.3) | 1.7 (1.5–1.9) | 6.1 (5.4–7.0) |
| Antipsychotics | 457 (30.3) | 591 (6.54) | 158 (1.1) | 6.2 (5.4–7.2) | 32.7 (26.7–40.0) |
| ADHD medications | 129 (8.6) | 2126 (23.5) | 148 (1.0) | 0.3 (0.3–0.4) | 7.4 (5.7–9.5) |
| Other | 543 (36.0) | 2047 (22.6) | 1436 (9.5) | 1.9 (1.7–2.2) | 4.1 (3.6–4.7) |
| Anticonvulsants | 97 (6.4) | 103 (1.1) | 82 (0.5) | 3.4 (2.5–4.7) | 4.4 (3.1–6.3) |
| Analgesics/antirheumatics | 293 (19.4) | 2954 (32.7) | 3616 (24.0) | 0.4 (0.4–0.5) | 0.6 (0.5–0.6) |
| Anti-infectives | 425 (28.2) | 3314 (36.7) | 4323 (28.7) | 0.6 (0.6–0.7) | 0.8 (0.7–0.9) |
| Gastrointestinal medications | 273 (18.1) | 1571 (17.4) | 1801 (12.0) | 1.0 (0.8–1.2) | 1.2 (1.0–1.4) |
| Respiratory/allergy medications | 320 (21.2) | 2122 (23.5) | 2748 (18.2) | 0.9 (0.8–1.0) | 1.0 (0.8–1.1) |
| Diabetes medications | 87 (5.8) | 301 (3.3) | 515 (3.4) | 1.6 (1.2–2.1) | 1.7 (1.3–2.2) |
| Cardiovascular medications | 312 (20.7) | 1437 (15.9) | 2005 (13.3) | 1.4 (1.2–1.7) | 1.7 (1.5–2.1) |
| Lipid/cholesterol medications | 165 (11.0) | 684 (7.6) | 979 (6.5) | 1.7 (1.4–2.1) | 2.0 (1.6–2.5) |
| Hypertension medications | 205 (13.6) | 1034 (11.4) | 1406 (9.3) | 1.2 (1.0–1.5) | 1.4 (1.2–1.7) |
KPNC groups medications into therapeutic classes based on the National Drug Code (NDC) system.
Adjusted for gender, age, race/ethnicity, total length of KPNC membership and nine categories of medical and psychiatric comorbidities (immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions) identified with diagnosis codes for the primary and secondary reasons for visit.
In a post hoc sensitivity analysis, we found that only 1 (0.01%) ADHD case and 330 (2.2%) GP controls did not have at least two visits for health care services. After restricting the sample to those (ASD, ADHD, and GP) who had at least two visits for health care services, our results were virtually unchanged (data not shown). We identified an additional 157 adults with ASD using the broader definition of ASD, which required only one recorded ASD diagnosis. Results were virtually the same using this definition.
Cost of health care utilization
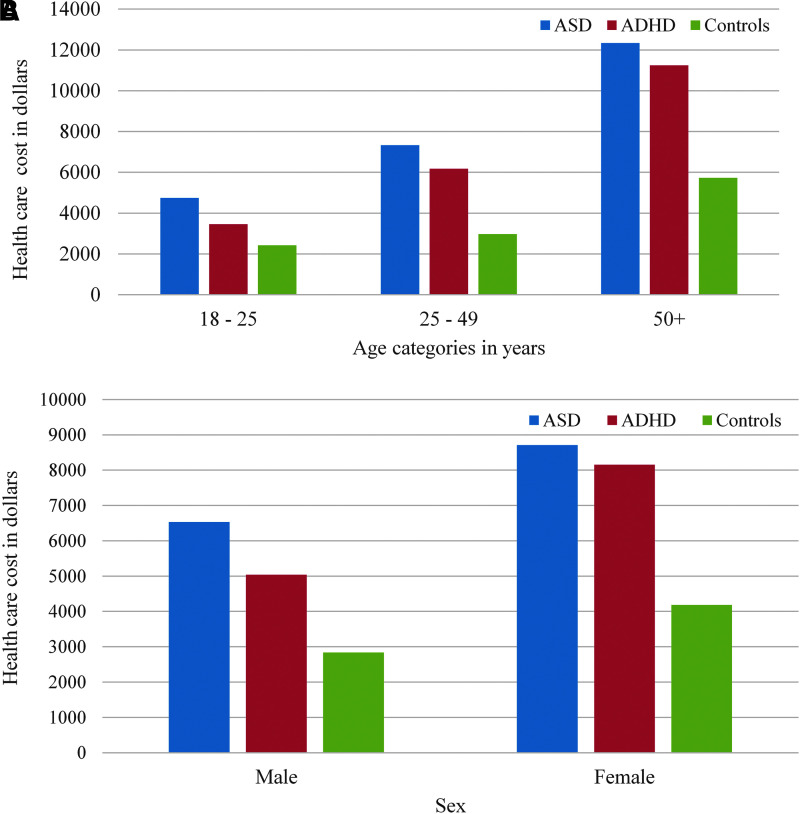
The crude mean cost of health care services for the 1-year study period was $7118.7 for adults with ASD, $5876.7 for adults with ADHD, and $3197.3 for GP controls. After adjusting for demographic covariates and major medical and psychiatric conditions, the overall cost of health care services for adults with ASD was 20% higher than the cost for adults with ADHD and 70% higher than the cost for GP controls (Table 5). The specific health services with the highest cost differences between cases and controls included mental health and neurology services and prescribed medications, specifically psychotherapeutic agents and anticonvulsants (Table 5). Annual total mean costs of utilization increased as age increased and was significantly higher for adults with ASD than for adults with ADHD or GP in each age group (Fig. 1A). The total mean cost was lower for men than for women, and costs were significantly higher in the ASD group than in the ADHD and GP groups for both men and women (Fig. 1B).
Table 5.
Health care Costs for Adults with Autism Spectrum Disorder, Attention-Deficit and Hyperactivity Disorder, and General Population Controls, Kaiser Permanente Northern California, 2012
| ASD (N = 1507) | ADHD (N = 9042) | GP (N = 15070) | Adjusted mean ratios a (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean cost (SD) | Median cost (IQR) | Mean cost (SD) | Median cost (IQR) | Mean cost (SD) | Median cost (IQR) | ASD vs. ADHD | ASD vs. GP | |
| Overall costb | 7118.7 (15647.8) | 2520 (716, 6949) | 5876.7 (17916.2) | 1984 (652, 5043) | 3197.3 (17480.9) | 806.0 (120, 2140) | 1.2 (1.1–1.3) | 1.7 (1.6–1.9) |
| Any outpatient clinic | 2955.9 (5773.2) | 1455 (499, 3310) | 2546.7 (5405.11) | 1224 (342, 2943) | 1407.2 (4616.1) | 655.0 (0, 1545) | 1.1 (1.0–1.2) | 1.6 (1.5–1.7) |
| Primary care | 946.8 (1616.6) | 491 (149, 1130) | 829.6 (1416.2) | 342 (0, 1026) | 579.7 (1009.2) | 342.0 (0, 707) | 1.1 (1.0–1.2) | 1.2 (1.1–1.4) |
| Mental health/psychiatry | 1078.9 (2719.0) | 0 (0, 998) | 601.0 (1693.3) | 0 (0, 499) | 94.6 (663.2) | 0 (0, 0) | 1.8 (1.6–2.1) | 11.6 (10.2–13.1) |
| Neurology | 68.4 (373.4) | 0 (0, 0) | 42.0 (322.8) | 0 (0, 0) | 12.6 (124.8) | 0 (0, 0) | 1.8 (1.6–2.0) | 2.3 (2.1–2.6) |
| Ob/gyn | 248.0 (531.3) | 0 (0, 426) | 534.5 (1043.8) | 381 (0, 426) | 601.9 (1246.3) | 0 (0, 426) | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) |
| Other specialties | 525.5 (2010.3) | 0 (0, 401) | 648.6 (1683.9) | 0 (0, 563) | 301.3 (1539.2) | 0 (0, 0) | 0.8 (0.7–0.9) | 1.0 (0.9–1.1) |
| Emergency department | 385.0 (1145.0) | 0 (0, 0) | 320.3 (1111.0) | 0 (0, 0) | 186.4 (766.6) | 0 (0, 0) | 0.9 (0.8–1.0) | 1.1 (1.0–1.3) |
| Hospitalizations | 1762.6 (9621.0) | 0 (0, 0) | 1735.2 (12914.3) | 0 (0, 0) | 1160.8 (14646.1) | 0 (0, 0) | 0.9 (0.8–1.0) | 1.0 (0.8–1.1) |
| Home health services | 138.6 (1556.2) | 0 (0, 0) | 61.1 (1657.6) | 0 (0, 0) | 33.3 (1636.4) | 0 (0, 0) | 16.2 (14.5–18.1) | 9.1 (8.1–10.2) |
| Skilled nursing | 18.7 (407.6) | 0 (0, 0) | 12.9 (525.4) | 0 (0, 0) | 4.7 (267.9) | 0 (0, 0) | 1.7 (1.6–1.9) | 2.5 (2.3–2.6) |
| Any medication | 1857.9 (7466.6) | 254 (26, 1387) | 1200.5 (3913.2) | 181 (17, 885) | 404.9 (2441.8) | 29 (0, 157) | 1.9 (1.7–2.1) | 4.5 (4.0–5.0) |
| Psychotherapeutic | 1013.6 (2857.9) | 43 (0, 310) | 324.8 (1204.3) | 0 (0, 90) | 37.0 (448.0) | 0 (0, 0) | 4.0 (3.5–4.5) | 26.3 (23.5–29.3) |
| Anticonvulsive | 162.9 (995.3) | 0 (0, 17) | 25.5 (328.2) | 0 (0, 0) | 8.2 (217.8) | 0 (0, 0) | 8.4 (7.6–9.4) | 23.5 (21.7–25.4) |
| Other medication | 681.4 (6796.7) | 56 (0, 261) | 850.2 (3633.7) | 97 (0, 476) | 359.7 (2374.2) | 27 (0, 137) | 0.8 (0.7–0.8) | 1.7 (1.5–1.9) |
All costs are in U.S. dollars and are adjusted to reflect 2016 costs.
Adjusted for gender, age, race/ethnicity, total length of KPNC membership, and nine categories of medical and psychiatric comorbidities (immune conditions, cardiovascular diseases, metabolic disorders, neurological disorders, gastrointestinal disorders, sleep disorders, psychiatric conditions, nutritional conditions, and endocrine conditions) identified with diagnosis codes for the primary and secondary reasons for visit.
Overall costs include all hospitalizations, emergency room visits, outpatient visits (including primary and specialty care), home health care, skilled nursing, and medications. Costs for durable medical equipment were not included.
FIG. 1.
(A) Annual mean health care costs for adults with ASD, ADHD, and typical controls, Kaiser Permanente 2012. (B) Annual mean health care cost for adults with ASD, ADHD, and typical controls by gender, Kaiser Permanente 2012. ASD, autism spectrum disorder; ADHD, attention-deficit/hyperactivity disorder.
Discussion
Within the same integrated health care delivery system, we found that utilization of health care services was significantly different between adults with ASD and adults with ADHD or adults with neither condition. Utilization of outpatient services for primary care, mental health, and speech therapy occurred significantly more often, and utilization of services for radiology and physical/occupational therapy occurred significantly less often among adults with ASD than either comparison group. Utilization of laboratory services and hospitalizations for ambulatory care sensitive diagnoses were significantly more common among men with ASD, and utilization of gynecology services and same-day hospitalizations were significantly less common among women with ASD than either comparison group. Utilization of ED services was significantly higher among adults with ASD over the age of 50 years than either comparison group. Most preventive care services were utilized at a higher rate among adults with ASD than either comparison group, with the exception of cervical cancer screening, which occurred at nearly half the rate among women with ASD, and breast cancer screening, which occurred at the same rate among women with ASD as women with ADHD or women with neither condition. The proportion of adults receiving a prescription for psychotherapeutic medications and medications for chronic medical conditions was higher for adults with ASD as well. These differences persisted after controlling for demographic factors and medical and psychiatric comorbidities. Higher utilization of health care services translated into higher costs of health care among adults with ASD than among both adults with ADHD and adults with neither condition.
The present results are similar to previously reported results on health care utilization patterns for children and adolescents with ASD.21,45–48 Our results are also similar to those reported in a recent study of Medicaid recipients that found that, on average, adults with ASD have more outpatient visits and more prescription drug claims per year than adults without ASD.18 Like a number of previous studies,34–36 we found that adults with ASD were more likely than adults without ASD to have emergency room visits. However, after controlling for covariates and stratifying by age category, we found that this difference was only significant for adults who were 50 years or older. Cidav et al.32 similarly reported higher utilization of the ED among older adults with ASD than among younger adults with ASD, but their study was limited by the lack of a non-ASD comparison group.
Like Nicolaidis et al.,34 we found that adult women with ASD were less likely to be screened for cervical cancer compared with their age-matched controls. Hypersensitivity to touch among adult women with ASD may cause them to refuse the procedure. It is also possible that health care providers may assume that women with ASD are not sexually active and, therefore, may forego the procedure. Low rates of Pap smear test could lead to missed opportunity for early cervical cancer diagnosis.
Our results indicate that adults with ASD have a higher rate of utilization of many health service areas than adults with ADHD and adults with neither ASD or ADHD, even after controlling for the excess burden of medical and psychiatric comorbidities in the ASD population.8 It is possible that the higher utilization may be a consequence of adult physicians' lack of knowledge and experience with the ASD population, resulting in increased referrals or patient's “shopping” for the right doctor. In support of this hypothesis, a recent survey of more than 900 adult health care providers found that the vast majority reported that they lacked the adequate training to care for the ASD population.49 Further investigation into the reasons for higher utilization among the ASD group, including the nature of the utilization of specialty visits (i.e., related to diagnosis or treatment), is warranted.
Our results should be considered in light of some study limitations. For outpatient visits including specialty visits, we based our metrics on the department code assigned to that encounter. It is possible that this method may have led to some misclassification of visits categorized as primary care. Although utilization was limited to a 1-year period, most preventive health care services are not performed on an annual basis. However, given that adults with ASD had higher utilization of nonpreventive health care services, it is possible that they had a greater opportunity to receive preventive care than controls. We were not able to control for whether people with ASD lived independently or in residential care, which may have affected their health care utilization. In this study, we included individuals with ASD, ADHD, and GP controls without ASD and ADHD, regardless of their other disability status. It is possible that the GP control group included a small fraction of individuals with other disabilities, which may have affected our results in a direction that is difficult to predict. We were not able to examine utilization patterns for people with intellectual disability and those without intellectual disability because the data on intellectual disability are incomplete. It is possible that the utilization patterns in these two groups are different and should be investigated in future studies.
Although we adjusted our results for major health conditions found to occur at higher rates in the ASD group compared with GP controls,8 our utilization results could still be affected by residual confounding from other conditions or condition severity. Future analyses using a more comprehensive risk adjustment approach, for example, 3 M50 or Johns Hopkins Case Mix System,51 are warranted. Our ASD cases were selected without regard to their level of functioning and included people who were covered by private and government insurance. Thus, our findings are likely to be most representative of adults who receive their care in a health care setting, and may not be generalizable to all adults with ASD. We focused on health services and costs incurred within the health plan, thus our results are likely to be most representative of adults who receive their care in a health care setting similar to KPNC and may not be generalizable to other settings or to the population of uninsured adults. We did not estimate costs associated with lost productivity of care takers, out-of-pocket expenses, behavioral interventions, or other nonmedical treatments and services.
Our study was strengthened by its large size and inclusion of two internal comparison groups, each matched to the ASD group on gender and age. For each study participant, health care service utilization was determined by documentation in their electronic medical record and thus the results are not subject to recall bias. Unlike previous studies, all results were adjusted for important covariates and medical and psychiatric comorbidities.
Conclusion
With the exception of gynecologic services among women, utilization and costs of health care services are significantly higher among adults with ASD than among adults with ADHD or adults with neither condition, even after controlling for medical and psychiatric comorbidities. More research is needed to determine reasons for these utilization differences and to develop strategies to improve delivery of health care to adults with ASD. Our study suggests that health care systems will need to plan ahead to adequately accommodate the needs of this growing and aging population.
Supplementary Material
Acknowledgment
This study was supported by grants from The Special Hope Foundation.
Authorship Confirmation Statement
Drs. Zerbo, Croen, Sidney, Rich, and Massolo conceptualized and designed the study. Dr. Zerbo drafted the initial and final article with substantial contribution from Dr. Croen. Dr. Qian and Mr. Ray conducted all statistical analyses with input from Drs. Zerbo and Croen. All authors critically reviewed the article and approved the final article as submitted.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Baio J. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2. Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168(9):904–912. [DOI] [PubMed] [Google Scholar]
- 3. Durkin MS, Maenner MJ, Baio J, et al. Autism spectrum disorder among US Children (2002–2010): Socioeconomic, racial, and ethnic disparities. Am J Public Health. 2017;107(11):1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459–465. [DOI] [PubMed] [Google Scholar]
- 6. Bauman ML. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics. 2010;7(3):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley E, Bolton P. Episodic psychiatric disorders in teenagers with learning disabilities with and without autism. Br J Psychiatry. 2006;189:361–366. [DOI] [PubMed] [Google Scholar]
- 8. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. [DOI] [PubMed] [Google Scholar]
- 9. de Bruin EI, Ferdinand RF, Meester S, de Nijs PF, Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord. 2007;37(5):877–886. [DOI] [PubMed] [Google Scholar]
- 10. Ghaziuddin M, Weidmer-Mikhail E, Ghaziuddin N. Comorbidity of Asperger syndrome: A preliminary report. J Intellect Disabil Res. 1998;42(Pt 4):279–283. [DOI] [PubMed] [Google Scholar]
- 11. Hofvander B, Delorme R, Chaste P, et al. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joshi G, Petty C, Wozniak J, et al. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. J Autism Dev Disord. 2010;40(11):1361–1370. [DOI] [PubMed] [Google Scholar]
- 13. Joshi G, Wozniak J, Petty C, et al. Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: A comparative study. J Autism Dev Disord. 2013;43(6):1314–1325. [DOI] [PubMed] [Google Scholar]
- 14. Kohane IS, McMurry A, Weber G, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7(4):e33224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schieve LA, Gonzalez V, Boulet SL, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Res Dev Disabil. 2012;33(2):467–476. [DOI] [PubMed] [Google Scholar]
- 16. Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. [DOI] [PubMed] [Google Scholar]
- 17. Tyler CV, Schramm SC, Karafa M, Tang AS, Jain AK. Chronic disease risks in young adults with autism spectrum disorder: Forewarned is forearmed. Am J Intellect Dev Disabil. 2011;116(5):371–380. [DOI] [PubMed] [Google Scholar]
- 18. Vohra R, Madhavan S, Sambamoorthi U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism. 2017;21(8):995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schendel DE, Overgaard M, Christensen J, et al. Association of psychiatric and neurologic comorbidity with mortality among persons with autism spectrum disorder in a Danish population. JAMA Pediatr. 2016;170(3):243–250. [DOI] [PubMed] [Google Scholar]
- 20. Aman MG, Lam KS, Collier-Crespin A. Prevalence and patterns of use of psychoactive medicines among individuals with autism in the Autism Society of Ohio. J Autism Dev Disord. 2003;33(5):527–534. [DOI] [PubMed] [Google Scholar]
- 21. Croen LA, Najjar DV, Ray GT, Lotspeich L, Bernal P. A comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics. 2006;118(4):e1203–e1211. [DOI] [PubMed] [Google Scholar]
- 22. Kalb LG, Stuart EA, Freedman B, Zablotsky B, Vasa R. Psychiatric-related emergency department visits among children with an autism spectrum disorder. Pediatr Emerg Care. 2012;28(12):1269–1276. [DOI] [PubMed] [Google Scholar]
- 23. Lokhandwala T, Khanna R, West-Strum D. Hospitalization burden among individuals with autism. J Autism Dev Disord. 2012;42(1):95–104. [DOI] [PubMed] [Google Scholar]
- 24. Cummings JR, Lynch FL, Rust KC, et al. Health services utilization among children with and without autism spectrum disorders. J Autism Dev Disord. 2016;46(3):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deavenport-Saman A, Lu Y, Smith K, Yin L. Do children with autism overutilize the emergency department? Examining visit urgency and subsequent hospital admissions. Matern Child Health J. 2016;20(2):306–314. [DOI] [PubMed] [Google Scholar]
- 26. McDermott S, Hardin JW, Royer JA, et al. Emergency department and inpatient hospitalizations for young people with fragile x syndrome. Am J Intellect Dev Disabil. 2015;120(3):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carbone PS, Young PC, Stoddard GJ, Wilkes J, Trasande L. A comparison of ambulatory care sensitive hospitalizations among children with and without autism spectrum disorder. Acad Pediatr. 2015;15(6):626–635. [DOI] [PubMed] [Google Scholar]
- 28. Leslie DL, Martin A. Health care expenditures associated with autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):350–355. [DOI] [PubMed] [Google Scholar]
- 29. Liptak GS, Stuart T, Auinger P. Health care utilization and expenditures for children with autism: Data from U.S. national samples. J Autism Dev Disord. 2006;36(7):871–879. [DOI] [PubMed] [Google Scholar]
- 30. Peacock G, Amendah D, Ouyang L, Grosse SD. Autism spectrum disorders and health care expenditures: The effects of co-occurring conditions. J Dev Behav Pediatr. 2012;33(1):2–8. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Leslie DL. Health care expenditures for children with autism spectrum disorders in Medicaid. J Am Acad Child Adolesc Psychiatry. 2010;49(11):1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cidav Z, Lawer L, Marcus SC, Mandell DS. Age-related variation in health service use and associated expenditures among children with autism. J Autism Dev Disord. 2013;43(4):924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimabukuro TT, Grosse SD, Rice C. Medical expenditures for children with an autism spectrum disorder in a privately insured population. J Autism Dev Disord. 2008;38(3):546–552. [DOI] [PubMed] [Google Scholar]
- 34. Nicolaidis C, Raymaker D, McDonald K, et al. Comparison of healthcare experiences in autistic and non-autistic adults: A cross-sectional online survey facilitated by an academic-community partnership. J Gen Intern Med. 2013;28(6):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vohra R, Madhavan S, Sambamoorthi U. Emergency department use among adults with autism spectrum disorders (ASD). J Autism Dev Disord. 2016;46(4):1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu G, Pearl AM, Kong L, Leslie DL, Murray MJ. A profile on emergency department utilization in adolescents and young adults with autism spectrum disorders. J Autism Dev Disord. 2017;47(2):347–358. [DOI] [PubMed] [Google Scholar]
- 37. Lunsky Y, Paquette-Smith M, Weiss JA, Lee J. Predictors of emergency service use in adolescents and adults with autism spectrum disorder living with family. Emerg Med J. 2015;32(10):787–792. [DOI] [PubMed] [Google Scholar]
- 38. Saqr Y, Braun E, Porter K, Barnette D, Hanks C. Addressing medical needs of adolescents and adults with autism spectrum disorders in a primary care setting. Autism. 2018;22(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liptak GS, Orlando M, Yingling JT, et al. Satisfaction with primary health care received by families of children with developmental disabilities. J Pediatr Health Care. 2006;20(4):245–252. [DOI] [PubMed] [Google Scholar]
- 40. Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011-2012 California Health Interview Survey. Kaiser Permanente Division of Research, Oakland, CA; June 2015. Available at: www.dor.kaiser.org/external/chis_non_kp_2011/ [Google Scholar]
- 41. Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Millman M, ed. Access to Health Care in America. Washington, DC: National Academies Press; 1993. [PubMed] [Google Scholar]
- 43. Onukwugha E, Bergtold J, Jain R. A primer on marginal effects—Part II: Health services research applications. Pharmacoeconomics. 2015;33(2):97–103. [DOI] [PubMed] [Google Scholar]
- 44. Onukwugha E, Bergtold J, Jain R. A primer on marginal effects—Part I: Theory and formulae. Pharmacoeconomics. 2015;33(1):25–30. [DOI] [PubMed] [Google Scholar]
- 45. Logan SL, Nicholas JS, Carpenter LA, King LB, Garrett-Mayer E, Charles JM. High prescription drug use and associated costs among Medicaid-eligible children with autism spectrum disorders identified by a population-based surveillance network. Ann Epidemiol. 2012;22(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121(3):e441–e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17(3):348–355. [DOI] [PubMed] [Google Scholar]
- 48. Rosenberg RE, Mandell DS, Farmer JE, Law JK, Marvin AR, Law PA. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. J Autism Dev Disord. 2010;40(3):342–351. [DOI] [PubMed] [Google Scholar]
- 49. Zerbo O, Massolo ML, Qian Y, Croen LA. A study of physician knowledge and experience with autism in adults in a large integrated healthcare system. J Autism Dev Disord. 2015;45(12):4002–4014. [DOI] [PubMed] [Google Scholar]
- 50. Goldfield N, Kelly WP, Patel K. Potentially preventable events: An actionable set of measures for linking quality improvement and cost savings. Qual Manag Health Care. 2012;21(4):213–219. [DOI] [PubMed] [Google Scholar]
- 51. Smith NS, Weiner JP. Applying population-based case mix adjustment in managed care: The Johns Hopkins Ambulatory Care Group system. Manag Care Q. 1994;2(3):21–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.