Abstract
Background:
Medicare is a public insurer for whom many autistic adults are eligible in the United States, but little is known about autistic beneficiaries who are covered. A challenge in using claim data is identification of autism spectrum disorder (ASD) cases to ensure accurate characterization. Some work suggests that relying on one claim could identify probable ASD, although other works indicate that two claims are necessary for case identification. The purpose of the current study was to describe the sample of Medicare young adult beneficiaries, and determine whether using a 1+ versus 2+ claim case identification resulted in similar interpretation of sample demographic characteristics and primary care utilization patterns in Medicare professional service claims.
Methods:
We used Medicare Limited Data Sets (2008–2010) claims. After ASD case identification using ICD-9-CM (299.xx), 527 unique beneficiaries in the last claim year of 2010 professional service file were identified as having at least one claim of ASD. Of these, 69% (n = 364) had two or more claims. Proportions and zero-inflated negative binomial regression were used to examine differences in demographic characteristics and primary care utilization and costs for the 1+ and 2+ samples.
Results:
Medicare claims contain a sample of autistic adults with expected demographics identified in historic prevalence cohorts. No differences in age, gender, race/ethnicity, Hispanic status, or dual-eligibility months or Adjusted Clinical Groups (ACG)® concurrent risk scores were identified between the 1+ and 2+ samples. No difference was found in the overall estimation of primary care use or costs between the 1+ and 2+ samples based on Zellner's seemingly unrelated regression methods.
Conclusions:
This study is the first to describe a national sample of Medicare-insured autistic adults. We found that using a 1+ case identification results in a sample that is demographically similar to a 2+ claim sample, and produces similar estimates of utilization as a 2+ claim sample.
Keywords: autism spectrum disorder, Medicare, utilization, health services, claims
Lay Summary
Why was this study done?
In the United States health care system, Medicare provides health benefits primarily to individuals over the age of 65 years. However, it also includes individuals who have received a disability determination and meet eligibility criteria who are under 65 years. Approximately 25% of Medicare beneficiaries are under the age of 65 years, and these include individuals on the autism spectrum.
There are no published studies about the kinds of services used by autistic adults who receive Medicare health insurance benefits. Understanding the kinds of health services used by autistic adults who don't have private insurance will help us make sure that public health care benefits meet the needs of autistic adults in the future.
When conducting research using claims-based data sets, such as Medicare data sets, it can be challenging to identify which patients in the data set are autistic. Researchers disagree on whether to include anyone who has had at least one claim with an autism code, or if they should only include patients who have had at least two claims with an autism code.
What was the purpose of this study?
The purpose was to describe young autistic adults who are eligible for Medicare benefits, and to determine the best way that individuals can be identified in the claims records.
What did the researchers do?
We used claims files for Medicare Limited Data Sets in 2010 to identify fee-for-service beneficiaries with the diagnosis ‘autism spectrum disorder’. There were 527 people who had at least 1 claim with a code of ‘autism spectrum disorder’ (1+) and 364 people with at least 2 codes (2+) in the Medicare ‘Carrier’ file. We used statistical tests to look for differences in these two samples for age, gender, race/ethnicity, and use of primary care services and costs.
What did the researchers find?
• Medicare beneficiaries identified on the autism spectrum had similar characteristics as the young adult population we expect- most were men (more than 70%), most were white, non-Hispanic, and most (more than 80%) were also eligible for Medicaid (in addition to Medicare).
• We did not find differences in age, gender, race/ethnicity, dual enrollment in Medicaid, primary care services or costs between those with 1 compared with 2 or more claims with ‘autism spectrum disorder’.
What are the weaknesses of the study?
Weaknesses of our study include: lack of demographic information related to reasons for eligibility for Medicare benefits, income, or employment; and lack of any corresponding claims that may have been paid by Medicaid.
How will these findings help future researchers and autistic adults?
• The results help researchers understand that using a 1+ case identification is feasible, and results in a sample that is similar to a 2+ claim sample.
• This study will help autistic adults because very little research talks about how health care is used by those with public insurance such as Medicare. This study is the first to talk about this, and will pave the way for future studies.
As reported prevalence rates for children diagnosed on the autism spectrum continue to increase,1 an alarming number of studies of autistic adults† describe poor outcomes related to employment2 and health.3,4 Poor health outcomes include increased mortality,5,6 increased rates of co-occurring mental health conditions compared with same-aged peers,4 and increased rates of physical health conditions such as obesity, diabetes, and cardiovascular conditions.4,7 In the United States, coverage of health services to treat such health conditions in adulthood is provided by either private insurance companies, typically through employer-sponsored plans, or through public health insurance such as Medicare or Medicaid benefits. Published data on health care utilization and costs in the autistic adult population have relied largely on integrated medical system data in California,3,4 representing primarily privately insured individuals, and national all-payer hospital and emergency department discharge databases.8 It is imperative to understand utilization and services obtained for publicly insured individuals, given that ∼48,500 autistic teens9 age into adulthood each year, many without jobs.2
Autistic adults may disproportionately require publicly funded health insurance due to the high rates of unemployment among the autistic adult population.10 The literature indicates that ∼20%–80% of adults on the autism spectrum are insured by public insurers, such as Medicaid or Medicare.3,8,11,12 This broad range is likely due to the fact that while Medicare is a federal program with eligibility across the United States, Medicaid is a state-run program with eligibility criteria that vary by state for individuals on the autism spectrum. Among civilians (nonmilitary), two types of public health insurance are potentially available to autistic adults in the United States: an entitlement program (Medicare) best known for providing health insurance for individuals ages 65 years and older, and a means tested program (Medicaid) best known for providing health insurance to low-income children and pregnant women.
Although a significant body of pediatric autism health service research relies on Medicaid claims, the issue with relying on these data in examining adult services is that Medicaid eligibility is often based on income. Even when Medicaid waivers are available that cover young adults, the benefits differ by state, and therefore, analyses of Medicaid using state-level data are confounded by differences in eligibility and benefits available. In contrast, Medicare is a federal entitlement program, and health care coverage is available to persons in all 52 states and territories. Medicare applies consistent eligibility criteria for “disabled” adults across all states,13 and therefore, using data from Medicare allows inclusion of beneficiaries across the entire United States rather than from specific states, something that has not previously been described in the literature.
Persons younger than 65 years may become eligible for Medicare by virtue of a disability if they meet guidelines set by the U.S. Social Security Administration's (SSA) Old-Age, Survivors, and Disability Insurance program. Adults seeking coverage for Medicare due to a disability must undergo a disability determination process guided by federal regulations. Medicare applicants must meet work history requirements that are determined by the person's age at the time of the application, or meet criteria based on a parent's eligibility. Approximately, 25% of Medicare beneficiaries are younger than 65 years and have a disability determination.13
Policymakers, health care administrators, and others require information about populations that use and require publicly funded health care services. Entitlement programs such as Medicare are increasingly identified as a target for federal cost reductions, but to understand the impact of these policies on autistic adults, we first need to describe the population that would be impacted. Individuals on Medicare are likely to produce a different picture of costs and utilization of services, directly relevant to federal programs that fund them, and these individuals are likely to remain nonworking and observable for future longitudinal research in these claim data.
Medicare claims are used frequently for research and are valid sources of information.13 However, one challenge with using claim data for identification of beneficiary utilization and costs of services is relying on billing codes. Confirmation of coding with chart review is a time-consuming and costly approach. Identification of “autism spectrum disorder” (ASD) in claim records has primarily been validated in pediatric samples.14 Some research suggests that requiring a minimum of two claims with ASD diagnosis codes in a given claim year is the most sensitive and specific approach,15 and may prevent overidentification of children in a claim record, especially when a single billing code associated with ASD could be due to a diagnostic evaluation. Since diagnosis usually occurs in childhood, and adult ASD diagnostic evaluations are not billed medically, we question if relying on two claims in a given claim year when identifying adults may result in loss of eligible cases, and possibly result in a biased sample that relies on beneficiaries who utilize more care or who are more obviously autistic to providers. Two challenges with relying on a more restricted case definition (two claims) for identification are (1) not all care received may be associated with an ASD diagnosis; thus, a claim may not identify a beneficiary as autistic; and (2) physicians may not be aware of an ASD diagnosis for a patient and thus may not code for this diagnosis, unless a patient demonstrated greater autistic signs. Although autistic adults receive similar levels of primary care as nonautistic adults3,12 physicians underreport having autistic patients despite having these individuals on their caseload.4 This suggests that physicians may not always identify and use ICD codes for this diagnosis when billing in adult care. Given these differences in pediatric versus adult care, we need to consider the best way to identify adult ASD cases in claim data to minimize under- or overidentification.
Since no published literature describes the use of Medicare for identification of autistic adult cases, the purpose of this article was to describe the sample of autistic adults in Medicare fee-for-service (FFS) claims, and compare standard case identification (use of two or more claims of ASD) to a less stringent case identification criteria (one or more claims) before engaging in examination of utilization and costs broadly within this data source. The specific research questions we posed were as follows. (1) Does this 2010 young-adult Medicare sample reflect expected demographic characteristics of autistic adults from other published U.S. estimates, and (2) does relying on one, compared with two, instance of ICD-9-CM diagnosis-coded claims for ASD case identification criterion result in samples with similar demographic characteristics and similar predicted utilization and costs for primary care visits and costs?
Methods
This study received institutional review board approval at each author's respective institution before analysis.
Population
We examined health claims of young autistic adults between 18 and 27 years. This age range was selected so as to understand young adults from the first frequently characterized national cohort in the United States (those identified in early 2000s at the age of 8 years).16
Data source and variables
Our source data for case identification were national Centers for Medicare and Medicaid (CMS) claim LDS for 2008–2010, with the last claim year (2010) serving as the data used in our study.17 This was done to ensure that cases eligible in earlier years were retained in the 2010 analytic file. These LDS files were developed from provider claims for the CMS FFS program and are organized into six source files with different sources of data, and represent the most recent available data at the time of study initiation and funding. We chose to examine this sample in the professional or “carrier” file, which is a 5% sample of all Medicare beneficiaries and their professional service claims for that year. The CMS limits the size of professional service data files released for national studies due to the file size, but findings can be weighted to reflect a national population. The LDS data include a linkable denominator file that contains demographic and other enrollment information.
Only FFS claims were used. No encounter records from Medicare Advantage (Part C) or Part D (prescription) data were available. Over 90% of “disabled” (<65 years) Medicare beneficiaries select FFS coverage, so our data likely reflect the majority of autistic adults eligible for Medicare.13
Variables of interest included ICD-9-CM diagnosis, Healthcare Common Procedure Coding System/Current Procedure Terminology procedure codes, revenue center cost variables for service types, place of service, and provider specialty.
ASD and intellectual disability case identification
Our sampling frame included all Medicare beneficiaries whose current reason for eligibility was “blind or disabled.” We created a case finder file of unique identifiers for beneficiaries in three claim years (2008, 2009, and 2010) with at least one diagnosis of ASD in any of the annual six claim source files. ASD cases were identified if a beneficiary had one or more claim instances with ICD-9-CM diagnosis of 299.xx in any diagnosis field from any source file across the 3 years.
Beneficiaries were excluded if they had less than 12 months of FFS enrollment in the analytic year (2010); diagnosis of end-stage renal disease; or missing race/ethnicity data. Individuals with end-stage renal disease were excluded due to high costs of care and utilization that are not expected to resemble usual care. We flagged and retained beneficiaries between the ages of 18–25 years; if individuals turned 25 in 2008 or 2009, they were also retained in future claim years as “eligible.” Thus, our 2010 analytic file for this study includes individuals with ASD between 18 and 27 years.
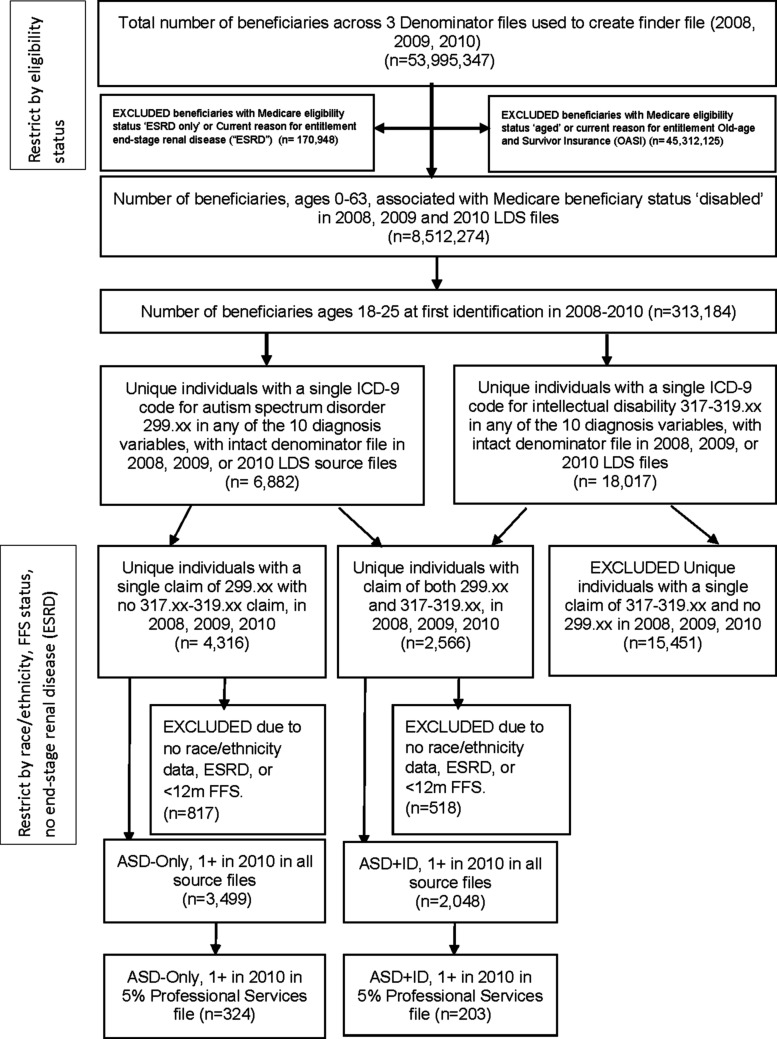
We implemented a similar process to identify beneficiaries with intellectual disability (ID) using ICD-9-CM codes 317.xx, 318.xx, or 319.xx across all three claim years. We merged the finder files for beneficiaries with ASD and those with ID to identify those beneficiaries with both ASD and ID. All beneficiaries were assigned to one of two categories: ASD-only (no claims of “intellectual disability” in any claim year) or ASD+ID (at least one claim with a diagnosis of an ASD and at least one claim code of ID in the same year). See Figure 1 for study sample identification.
FIG. 1.
Identification of young adults with ASD-only and ASD+ID in 2010 Medicare Limited Data Sets. ASD, autism spectrum disorder; FFS, fee-for-service; ID, intellectual disability; LDS, Limited Data Sets.
Analysis samples
We created additional analysis samples of those unique individuals who met a more restricted case definition of at least two claims of ASD for at least one claim year using similar methods described above. Therefore, there were four study samples for our analysis: ASD-only(1+), ASD+ID(1+), ASD-only(2+), and ASD+ID(2+).
Calculation of new derived variables
We merged denominator variables with source files. We created two age categories from the denominator file, 18–21 and 22–27 years, in the last enrolled month of 2010, and derived race/ethnicity (white vs. all other minorities) and Hispanic (yes vs. no) from the single available variable.
Individuals with Medicare may also have been eligible for state-funded Medicaid, which is referred to as “buy-in” or “dual-eligible” and can reflect additional income needs. Medicaid eligibility would not impact primary care analysis because Medicare is the primary payer, and Medicaid is considered the secondary payer. Beneficiary-enrolled months in the state Medicaid buy-in program were dichotomized into those with 12 months “buy-in” and those with 1–11 months of “buy-in” due to the skewed nature of this variable.
Utilization and cost variables
Utilization and cost variables for primary care visits were analyzed using the 5% sample of beneficiaries with professional service claims in 2010. These professional services represent health services received in nonhospital or nonemergent settings and include primary care settings where people are likely to be seen in their communities. We identified primary care visits as any professional service claim that met all three of the following criteria: (1) an evaluation and management code associated with primary care provider (PCP) visits (e.g., “Level 1 new patient office or other outpatient visit”); (2) a PCP specialist code (e.g., general/family practitioner, nurse practitioner, physician assistant); and (3) place of service codes typical of primary care (e.g., clinic/family practice, clinic/general classification, federally qualified health center, mass immunization center, public health clinic). We categorized utilization for each beneficiary as one or more PCP visits versus no PCP visits in 2010, and also created a count variable for PCP visits by summing the unique claims experienced by a given beneficiary in 2010. We calculated costs for PCP claims per beneficiary by summing payment made by the beneficiary to the provider, payment from Medicare to the provider, and any other payments from secondary payers to the provider.
Risk adjustment
Risk adjustment variables are used to understand the extent to which a particular patient, given prior health utilization and diagnoses in the claim data, is likely to use health care resources. Risk adjustment was achieved using Johns Hopkins Adjusted Clinical Groups (ACG)® Case-Mix System (Version 11)18 with unscaled concurrent weights to adjust for differences in overall disease burden between beneficiaries. The unscaled concurrent weight quantifies current resource use, compared to a national reference database expressed as a relative value.19 ACG scores were highly skewed, and thus, we report medians for bivariate comparisons and used log-transformed values for regression models.
Analytic approach
We conducted descriptive analysis using Stata Version 14.1.20 Characteristics of the sample were described using proportions and exact binomial 95% confidence intervals for each categorical variable using the Clopper/Pearson interval.21 We examined differences between the 1+ and 2+ sample demographic characteristics with a two-sample test of proportions. We tested the hypothesis that the samples were from the same population, and thus, a nonsignificant result supports our hypothesis. ACG unscaled concurrent risk score was treated as a continuous variable,18 and 1+ and 2+ sample differences were examined with median tests.
For our second research question about utilization and costs, we compared PCP counts adjusting for other demographic characteristics with zero-inflated negative binomial regression (ZINB) based on the presence of excessive zeros, examination of the distribution, and variance. We used Vuong22 test to determine model type, and tested model fit with Akaike's information criterion.23 Dual-eligibility status and risk score were used to predict zero PCP visits in the inflation model. Predictors for the ZINB count model included intellectual disability status, age, ethnicity, minority, dual eligibility, and risk score. Annual payments for PCP visits were modeled using a log transformation and alternately a gamma transformation of the dependent variable (total annual expenditures by beneficiary).
To evaluate the difference in 1+ and 2+ sample models for utilization and costs, we used Zellner's seemingly unrelated regression (SUR) method24 to identify if significant differences existed in the overall model estimates separately for the 1+ and 2+ count and cost models. The SUR approach combines the correlation matrices for both populations using model-based parameter estimates. This permits direct comparisons of the 1+ and 2+ parameter estimates, and in aggregate, using a Wald χ2 test of the postestimate combined results, accumulated across all predictors. We tested the hypothesis that the samples were from the same population. A nonsignificant result suggests support for our hypothesis. We used the unweighted 5% sample for the univariate and regression analysis and the weighted sample for our two-way descriptive tables. A post-hoc power analysis using G*Power (V.3.1.9.2)25 was conducted to assess the possibility of Type II error. Achieved power in unweighted samples for any PCP visit during the year was 0.79. Achieved power was 0.99 for the count model using a large sample z-test with variance correction for Poisson regression.
Results
In the 5% professional service file, we identified 527 total individuals with ASD between the ages of 18–27 years in FFS claims using the one-claim criteria: 324 adults (61%) classified as ASD-only, and 203 adults (39%) classified as ASD+ID. When the 5% sample is weighted to reflect the entire Medicare population from which this sample was taken, this reflects an approximate population of N = 6480 adults with ASD-only and N = 4060 adults with ASD+ID with the 1+ claim criterion.
Among the adults identified as ASD-only(1+), n = 233 (71.9%) had two or more claims of ASD. Among those identified as ASD+ID(1+), n = 131 (64.5%) had two or more claims in a given year. There were no significant differences in proportions between the 1+ or 2+ claim samples on the demographic variables for the ASD-only or ASD+ID groups: gender, age category, state buy-in, Hispanic status or minority status, or ACG risk (Table 1).
Table 1.
Comparison of the Proportional Values of Dichotomous Demographic Characteristics for the 1 + Versus 2+ Samples from 5% Limited Data Set Medicare File, 2010
| Variable | Study group | 1+ Diagnosis occurrence proportion % a | 95% CI for exact binomial proportions | 2+ Diagnosis occurrence proportion % b | 95% CI for exact binomial proportions | p-Value, difference in 1+ and 2+ |
|---|---|---|---|---|---|---|
| Gender: male | ||||||
| ASD-only | 79.63 | 74.83–83.88 | 81.12 | 75.49–85.93 | 0.66 | |
| ASD and ID | 77.83 | 71.49–83.35 | 79.39 | 71.45–85.96 | 0.74 | |
| Age group: 22–27 | ||||||
| ASD-only | 74.07 | 68.94–78.76 | 77.68 | 71.79–82.86 | 0.33 | |
| ASD and ID | 80.79 | 74.69–85.97 | 83.21 | 75.69–89.17 | 0.58 | |
| Hispanic status: Hispanic | ||||||
| ASD-only | 5.25 | 3.09–8.27 | 5.58 | 3.00–9.35 | 0.86 | |
| ASD and ID | 7.88 | 4.57–12.48 | 6.11 | 2.67–11.68 | 0.54 | |
| Minority status: nonwhite | ||||||
| ASD-only | 24.69 | 20.09–29.76 | 24.89 | 19.48–30.96 | 0.96 | |
| ASD and ID | 37.44 | 30.76–44.49 | 37.40 | 29.11–46.28 | 0.99 | |
| Medicaid: has 12 months state buy-in | ||||||
| ASD-only | 82.72 | 78.15–86.67 | 83.69 | 78.31–88.19 | 0.65 | |
| ASD and ID | 88.67 | 83.49–92.68 | 87.02 | 80.04–92.26 | 0.69 | |
| 1+ Diagnosis occurrence mean (SD) a | 1+ Diagnosis occurrence median | 2+ Diagnosis occurrence mean (SD) b | 2+ Diagnosis occurrence median | z-Test p-value | ||
| ACG® score | ||||||
| ASD-only | 2.25 (2.85) | 1.27 | 2.35 (2.95) | 1.27 | 0.69 | |
| ASD and ID | 3.60 (4.00) | 2.31 | 3.31 (3.41) | 2.31 | 0.48 | |
Data source: Centers for Medicare and Medicaid, Medicare Limited Data Set 2010 file for 5% carrier file (professional service file).
Comparison categories include gender: female; age: 18–21 years; Hispanic status: non-Hispanic; minority status: white; Medicaid: does not have Medicaid state buy-in.
1+ Sample included unweighted (5% file): ASD-only, n = 324, ASD+ID, n = 203; 1+ sample weighted ASD-only, N = 6480, ASD+ID, N = 4060.
2+ Sample included unweighted (5% file): ASD-only, n = 233, ASD+ID, n = 131; 2+ sample weighted ASD-only, N = 4660, ASD+ID, N = 2620.
ACG, Adjusted Clinical Groups; ASD, autism spectrum disorder; ASD+ID, autism spectrum disorder plus intellectual disability; CI, confidence interval; ID, intellectual disability.
No differences in unadjusted rates of any PCP visits or annual mean counts of PCP visits between the 1+ and 2+ samples existed for either the ASD-only or ASD+ID groups (Table 2).
Table 2.
Rates and Weighted Counts of Primary Care Utilization and Costs Among Young Adults Identified as ASD-Only or ASD+ID Using Different Definitions for Case Identification
| Weighted N | ASD-only, 1+ claim, n = 324 | ASD-only, 2+ claim, n = 233 | Difference in 1+ and 2+ a | ASD+ID, 1+ claim, n = 203 | ASD+ID, 2+ claim, n = 131 | Difference in 1+ and 2+ a |
|---|---|---|---|---|---|---|
| N = 6480 | N = 4660 | N = 4060 | N = 2620 | |||
| Any PCP visit in past yearb | ||||||
| Weighted N | 4220 | 3160 | z = −0.66, p = 0.51 | 2960 | 1980 | z = −0.54, p = 0.59 |
| % | 65.12 | 67.81 | 72.91 | 75.57 | ||
| 95% CI | 59.66–70.31 | 61.40–73.76 | 66.24–78.89 | 67.30–82.65 | ||
| Annual number of PCP visits per beneficiary | ||||||
| Mean (SD) | 2.18 (3.09) | 2.37 (2.91) | z = −0.74, p = 0.46 | 2.95 (3.38) | 3.01 (3.54) | z = −0.15, p = 0.88 |
| Median (IQR) | 1 (3) | 1 (3) | 2 (4) | 2 (4) | ||
| Annual PCP payment per beneficiaryc (in $) | ||||||
| Mean (SD) | 238 (279) | 243 (230) | z = −0.19, p = 0.85 | 289 (277) | 280 (289) | z = 0.24, p = 0.81 |
| Median (IQR) | 157 (234) | 164 (237) | 213 (260) | 188 (293) | ||
Data source: Centers for Medicare and Medicaid, Medicare Limited Data Set 2010 file for 5% carrier file (professional service file).
Beneficiaries with 12 months fee-for-service, no end-stage renal disease, no missing race/ethnicity.
For frequency data, the exact two-sample tests of proportions were used; for count data, the two-sample z-test was used.
The denominator for % is total unique beneficiaries in group.
Annual PCP payment per beneficiary is equal to the sum of the amount of money paid from Medicare to provider, amount paid from beneficiary to provider, and if there is one, payments made by a secondary payer across all PCP visits in a claim year.
PCP, primary care provider.
Following ZINB modeling of PCP visits (Table 3), the comparison of regressions run on the 1+ and 2+ samples using Zellner's SUR method resulted in no difference in the two models (χ2(7) = 2.69, p = 0.67), suggesting the models are reflecting similar estimates for PCP visits in each study sample.
Table 3.
Comparison of Two Zero-Inflated Negative Binomial Regression Models of Factors Associated with the Number of Primary Care Visits among Young Adults on the Autism Spectrum, 2010
| Regression 1+ sample a | Regression 2+ sample b | |||||||
|---|---|---|---|---|---|---|---|---|
| IRR | SE | Lower 95% CI | Upper 95% CI | IRR | SE | Lower 95% CI | Upper 95% CI | |
| Primary care visits | ||||||||
| ASD and ID | 0.986 | 0.089 | 0.826 | 1.178 | 0.926 | 0.100 | 0.750 | 1.144 |
| Age 22–27 | 1.046 | 0.110 | 0.851 | 1.284 | 1.003 | 0.127 | 0.782 | 1.286 |
| Minority | 1.027 | 0.100 | 0.849 | 1.242 | 0.993 | 0.114 | 0.792 | 1.244 |
| Female | 1.136 | 0.116 | 0.930 | 1.388 | 1.106 | 0.139 | 0.864 | 1.416 |
| Dual eligibility: Medicaid 1–12 months | 0.903 | 0.122 | 0.693 | 1.176 | 0.937 | 0.149 | 0.687 | 1.278 |
| Psychiatric primary claim | 0.914 | 0.102 | 0.735 | 1.136 | 0.814 | 0.106 | 0.630 | 1.051 |
| Log unscaled concurrent ACG score | 1.810*** | 0.086 | 1.649 | 1.988 | 1.883*** | 0.117 | 1.666 | 2.128 |
| Constant | 1.910*** | 0.326 | 1.370 | 2.670 | 1.993*** | 0.421 | 1.318 | 3.014 |
| Inflation model (0 PCP visits) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dual eligibility: Medicaid 1–12 months | 0.906** | 0.029 | 0.850 | 0.964 | 0.926 | 0.042 | 0.847 | 1.011 |
| Log unscaled concurrent ACG score | 1.052 | 0.178 | 0.755 | 1.467 | 0.968 | 0.242 | 0.593 | 1.579 |
| Constant (coefficient) | −0.710* | 0.341 | −1.370 | −0.038 | −1.066* | 0.529 | −2.102 | −0.300 |
| Log alpha (coefficient) | −1.150*** | 0.200 | −0.162 | −0.036 | −1.103*** | 0.238 | −1.57 | −0.637 |
| Alpha (coefficient) | 0.318 | 0.063 | 0.215 | 0.470 | 0.332 | 0.079 | 0.208 | 0.529 |
Data source: Centers for Medicare and Medicaid, Medicare Limited Data Set 2010 file for 5% carrier file (professional service file).
Reference group for indicator variables: ASD-only, 18–21 years, white, male, no dual Medicaid coverage, no primary psychiatric claim in past year.
p < 0.05; **p < 0.01; ***p < 0.001.
Number of observations = 527, number of zero observations = 168, Wald χ2 = 142.2, p < 0.001.
Number of observations = 364, number of zero observations = 107, Wald χ2 = 96.0, p < 0.001.
CI, confidence interval; IRR, incidence rate ratio; LR, likelihood ratio; SE, standard error.
Annual payments for PCP visits are displayed in Table 2. No difference in the annual mean or median payments per beneficiary for PCP visits was identified between the 1+ and 2+ samples (Table 2). Comparison of models using Zellner's SUR method (models not displayed) found no significant differences in either the log-transformed or gamma-transformed cost models (log-transformed model χ2(7) = 4.62, p = 0.71; gamma-transformed model χ2(7) = 5.2, p = 0.64). The findings suggest that the models reflect similar estimates of the annual payments for PCP visits in the 1+ and 2+ models.
Discussion
The purpose of this methodological study was to describe characteristics of young autistic adult Medicare beneficiaries relative to known historic cohort characteristics published from 2000 to 2002 prevalence studies, and to compare case identification with one-claim criteria to two claims of ASD. Individuals in our study reflect similar demographic characteristics as those described in the 2000–2002 surveillance year.26,27 The male to female ratio in our data is 3.5–3.9 (ASD+ID and ASD-only, respectively), which is similar to the 2000–2002 U.S. prevalence reports.16 Similarly, our rates of those with ID (39% of the analyzed Medicare sample) are similar to the expected U.S. 2000 estimates, which reported rates of ID of 33%–58%.26 However, our prevalence of Hispanic beneficiaries was much lower than expected; surveillance data from the 2000 to 2002 period suggest that ∼13% of 8-year-old autistic children were Hispanic,16,27 whereas we found ∼5%–8% of our Medicare 2010 sample were Hispanic. We wonder whether there are differences in how Hispanic and non-Hispanic families apply for and receive social security disability, which may eventually result in possible Medicare benefits.
We also demonstrated that comparing the 1+ and 2+ claim samples using bivariate and multivariate methods for utilization and costs of primary care visits resulted in similar estimates, suggesting that using the one-claim criterion results, in this study, comparable demographics, utilization, and cost findings as the two-claim criteria. Some nonautism studies using administrative claim data for case identification rely on one or more claims in a given period,28 and therefore, this procedure is not without precedent. In many adult claim studies, researchers report relying on two claims5 or one inpatient/two outpatient claims.4
However, the decision in claim data studies to rely on two claims versus one claim for case identification requires more investigation. The use of the 1+ claim approach increases sample size available for analysis. The limitation to relying on one claim is that possible misidentification or coding errors occurred, resulting in a sample that may include some non-ASD cases. Our study findings lessen this concern, because we found that the one-claim group was similar demographically to both the two-claim group and to national demographics of similar-aged autistics (from prevalence studies of when these adults were children in the early 2000s). The use of the 2+ approach may reduce misclassification, however, it decreases the available sample size and may inflate estimates of cost and utilization if the morbidity burden in the 2+ population is higher19,29 than in the 1+ population. While we did not find that ACG risk scores were statistically different in our one- versus two-claim samples in this primary care utilization study, there is evidence that suggests that autistic adults with greater health care needs utilize other types of non-PCP care at greater rates.3,8 Thus, relying on case identification for individuals with two or more claims may overinflate estimates of utilization and costs. We suggest that our initial findings lend support to future work that uses a 1+ claim criterion, but that other research could use chart review to determine the sensitivity and specificity of using a single claim for identification.
It is important to note that while claim records are useful for health service research, physician coding of care for individuals may not have always included an ICD-9 code of 299.xx, especially if that visit was not autism specific. We addressed this possible limitation by ensuring that once an individual was identified in our 3 years of claim data with at least one claim with ICD-9 code, that individual was flagged in our analytic year (2010) as having ASD. Thus, our analysis utilized all claims for identified cases, not just claims that included an ASD diagnosis code.
Each year, ∼50,000 young autistic adults age into adulthood,9 and given that our sample included 18–27-year olds, we would anticipate that nationally ∼450,000 young adults existed in the United States in 2010. Our sample was representative of ∼11,000 (weighted) beneficiaries who were eligible for publicly funded Medicare health insurance benefits in 2010 and who were identified as utilizing services in the professional service file (reflecting nonemergent professional services). This is a different population than those currently described in the health care costs and utilization literature, and is an important one to study because this population is likely eligible due to a disability determination and inability to maintain work. This sample is highly relevant to policymakers and others, as publicly funded Medicare should expect to see an increase in eligible autistic beneficiaries due to prevalence increases.16 As such, this study has important implications for our ability to understand future utilization and cost of services aimed at improving the mental and physical health of autistic adults who receive public medical benefits. Future work to examine services utilized in Medicare files beyond the professional services would be useful to determine how and why services are utilized by this population. Future research can also examine longitudinal cohort effects through use of Medicare data.
Limitations of our analyses include limited access to demographic characteristics that might be useful in characterizing this sample, such as education, employment, and income; and the inability to know for certain the reason for SSA determination that led to eligibility for Medicare. Also, given the high prevalence of dual eligibility for Medicare and Medicaid in the population, the addition of Medicaid claims would likely provide additional information, including more certain case identification through identification of beneficiaries enrolled in a state Medicaid autism waiver.30 Ideally, integrated data that include Medicare, Medicaid, and private insurance claims would be best for understanding utilization and costs in the adult population. In addition, our use of the 5% LDS restricted our sample size available for analysis, and future studies would benefit from using 100% samples to ensure adequate power to detect differences.
Despite these limitations, this study represents the first published work, to our knowledge, which documents demographic characteristics and preliminary utilization data from a national population of young adults with ASD covered by Medicare. Further work to characterize and understand service utilization of this population will contribute to improved understanding of access-to-care and service delivery questions.
Acknowledgments
We acknowledge Tingting Hu, Yuxia Wang, and Kelsey Houser at Florida State University for their assistance with programming and development of SAS algorithms necessary to structure the Medicare data set before analysis.
This study was supported by grant R40MC28854-01-02, MCH Research Program, from the Maternal and Child Health Bureau Health Resources and Services Administration, Department of Health and Human Services.
Footnotes
Due to the preferences expressed by the autistic population regarding identity-first language and the Journal guidelines supporting this recommendation, we have chosen to use identity-first language out of respect for the individuals represented in this article rather than person-first language.
Authors' Contributions
All authors have reviewed and approved the article before submission, and this article has not been submitted to any other journal for publication. The article is not published elsewhere. T.W.B. conceptualized the need for study and contributed to data analysis, data interpretation, and drafting the article for submission. H.J.C. also conceptualized the need for study, obtained the data from the Centers for Medicare and Medicaid, and oversaw the preparation of the data for analysis. H.J.C. contributed to data analysis and interpretation. He edited and contributed to significant revisions in the article as submitted. K.Y.G. contributed to data cleaning and preparation of the data files before analysis, contributed to data analysis, and edited the article as submitted.
Author Disclosure Statement
The authors declare that no competing financial interests exist, and that the funder did not play a role in the design, data analysis, or interpretation of this study.
Compliance with Ethical Standards
This research involved human subjects' health record data, but not human participants. All procedures performed (data analysis) involving human subjects' data were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review boards of each author's institution. No informed consent was required due to the nature of the health record data used in this study.
References
- 1. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(No. SS-6):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roux AM, Shattuck PT, Cooper BP, Anderson KA, Wagner M, Narendorf SC. Postsecondary employment experiences among young adults with an autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(9):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zerbo O, Qian Y, Ray T, et al. Health care service utilization and cost among adults with autism spectrum disorders in a U.S. integrated health care system. Autism Adulthood. 2019;1(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. [DOI] [PubMed] [Google Scholar]
- 5. Bilder D, Botts EL, Smith KR, et al. Excess mortality and causes of death in autism spectrum disorders: A follow up of the 1980s Utah/UCLA autism epidemiologic study. J Autism Dev Disord. 2013;43(5):1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan J, Li G. Injury mortality in individuals with autism. Am J Public Health. 2017;107(5):791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barber C. Meeting the healthcare needs of adults on the autism spectrum. Br J Nurs. 2017;26(7):420–425. [DOI] [PubMed] [Google Scholar]
- 8. Vohra R, Madhavan S, Sambamoorthi U. Emergency department use among adults with autism spectrum disorders (ASD). J Autism Dev Disord. 2016;46(4):1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shattuck PR, Roux AM, Hudson LE, Lounds Taylor J, Maenner MM, Trani J. Services for adults with an autism spectrum disorder. Can J Psychiatry. 2012;57(5):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roux A, Rast J, Rava J, Anderson K, Shattuck P. National Autism Indicators Report—Transition into Young Adulthood. Philadelphia, PA: Life Course Outcomes Research Program A.J. Drexel Autism Institute, Drexel University; 2015. http://drexel.edu/autisminstitute/research-projects/research/ResearchPrograminLifeCourseOutcomes/indicatorsreport/#sthash.31XId4lN.dpbs (accessed May 25, 2018) [Google Scholar]
- 11. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. [DOI] [PubMed] [Google Scholar]
- 12. Nicolaidis C, Raymaker D, McDonald K, et al. Comparison of healthcare experiences in autistic and non-autistic adults: A cross-sectional online survey facilitated by an academic-community partnership. J Gen Intern Med. 2013;28(6):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riley GF, Rupp K. Cumulative expenditures under the DI, SSI, Medicare, and Medicaid programs for a cohort of disabled working-age adults. Health Serv Res. 2015;50(2):514–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandell DS, Cao J, Ittenbach R, Pinto-Martin J. Medicaid expenditures for children with autistic spectrum disorders: 1994 to 1999. J Autism Dev Disord. 2006;36(4):475–485. [DOI] [PubMed] [Google Scholar]
- 15. Coleman KJ, Lutsky MA, Yau V, et al. Validation of autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. J Autism Dev Disord. 2015;45(7):1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Identified Prevalence of Autism Spectrum Disorder ADDM Network 2000–2012 Combining Data for All Sites. 2016. https://www.cdc.gov/ncbddd/autism/data.html (accessed June 28, 2018)
- 17. Centers for Medicare & Medicaid Services (CMS) Limited Data Sets (LDS) Files. Baltimore, MD: Buccaneer-A General Dynamics Company; 2008–2010. [Google Scholar]
- 18. Adjusted Clinical Groups (ACG)® Case-Mix System Version 11.0 [Computer Software] [computer program]. Version 11.0. Baltimore, MD: Johns Hopkins University; 2014. [Google Scholar]
- 19. The Johns Hopkins ACG System Version 11.0 Installation and Usage Guide [computer program]. Baltimore, MD: Johns Hopkins University; 2014. [Google Scholar]
- 20. Stata Statistics [Computer Program] Version 14.1 [computer program]. Version 14.1. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 21. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 22. Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1989;57(2):307–333. [Google Scholar]
- 23. Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. [Google Scholar]
- 24. Zellner A, Huang DS. Further properties of efficient estimators for seemingly unrelated regression equations. Int Econ Rev. 1962;3(3):300–313. [Google Scholar]
- 25. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 26. Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease Control & Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ. 2007;56(1):1–11. [PubMed] [Google Scholar]
- 27. Durkin MS, Maenner MJ, Baio J, et al. Autism spectrum disorder among US children (2002–2010): Socioeconomic, racial, and ethnic disparities. Am J Public Health. 2017;107(11):1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collard HR, Chen SY, Yeh WS, et al. Health care utilization and costs of idiopathic pulmonary fibrosis in U.S. Medicare beneficiaries aged 65 years and older. Ann Am Thorac Soc. 2015;12(7):981–987. [DOI] [PubMed] [Google Scholar]
- 29. Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Ann Fam Med. 2012;10(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Velott DL, Agbese E, Mandell D, et al. Medicaid 1915(c) Home- and community-based services waivers for children with autism spectrum disorder. Autism. 2016;20(4):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]