Abstract
Maternal immune activation (MIA) and infection during pregnancy are known to reprogramme offspring phenotypes. However, the epigenetic effects of preconceptual paternal infection and paternal immune activation (PIA) are not currently well understood. Recent reports show that paternal infection and immune activation can affect offspring phenotypes, particularly brain function, behaviour, and immune system functioning, across multiple generations without re-exposure to infection. Evidence from other environmental exposures indicates that epigenetic inheritance also occurs in humans. Given the growing impact of the coronavirus disease 2019 (COVID-19) pandemic, it is imperative that we investigate all of the potential epigenetic mechanisms and multigenerational phenotypes that may arise from both maternal and paternal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as well as associated MIA, PIA, and inflammation. This will allow us to understand and, if necessary, mitigate any potential changes in disease susceptibility in the children, and grandchildren, of affected parents.
Keywords: COVID-19, infection, immune activation, inflammation, SARS-CoV-2, transgenerational epigenetic inheritance
Parental reprogramming of offspring health in the context of COVID-19
Most chronic diseases have complex aetiologies that cannot be explained by genetic factors alone [1]. Accumulating evidence suggests that both maternal and paternal environmental exposures can reprogramme offspring phenotypes via germline epigenetic modifications [2,3]. Both human and animal studies show that inherited epigenetic changes can promote the development of various chronic diseases, including schizophrenia, cardiovascular disease, and type 2 diabetes [4., 5., 6.]. Maternal stress, dietary changes, and infection during gestation can have intergenerational (see Glossary) and transgenerational impacts on offspring, including neurodevelopmental and metabolic dysfunctions [2,7,8]. However, due to the mature sperm’s tightly packaged chromatin and reduced transcriptional activity, the paternal epigenetic contributions to offspring (F1) and grand-offspring (F2) phenotypes have been previously disregarded [9]. Nevertheless, recent discoveries show that the sperm delivers intergenerational information, particularly in the form of noncoding RNAs, to the oocytes at fertilisation [10,11]. Furthermore, the small noncoding RNA ‘payload’ of the sperm can alter embryonic development and offspring phenotypes [12]. Paternal preconceptual exposure to various environmental factors, including stress, toxins, and dietary changes, can modulate the sperm epigenome, potentially increasing offspring disease susceptibility [13., 14., 15., 16., 17.]. Although there is substantial evidence that maternal infection and immune activation have detrimental intergenerational (and even transgenerational) outcomes [18], these exposures are largely unexplored in paternal epigenetic inheritance models.
Pathogenic infections, and associated immune activation, can impact an organism’s physiology and epigenetic profile [19,20]. Furthermore, maternal immune activation and infection in both humans and animals are known to increase offspring predisposition to neurodevelopmental disorders, including schizophrenia and autism spectrum disorder (ASD) [21,22]. Intriguingly, Tyebji and colleagues recently discovered that paternal preconceptual infection with Toxoplasma gondii can alter sperm epigenetics, leading to F1 and F2 cognitive and behavioural abnormalities without re-exposure to infection [19]. Nevertheless, the mechanisms underlying how infection and immune activation can reprogramme the sperm epigenome are not well understood [19].
Children of those affected by major disasters, including survivors of the Holocaust, famines, and the World Trade Centre attacks, often display altered stress responsivity and metabolism [23]. Furthermore, epigenetic mechanisms, including modified DNA methylation at imprinted genes, have been associated with these changes [24]. At the time of writing, over 500 million people worldwide have been infected with COVID-19, resulting in over 6 million reported deaths (World Health Organization, April 2022). Given the enormous physical and mental health burden associated with the COVID-19 pandemic, there is a significant possibility that long-term public health repercussions of the pandemic may arise due to epigenetic inheritance. Therefore, there is a clear imperative to investigate the potential intergenerational and transgenerational COVID-19-related health effects in preclinical studies, so that we can prevent these from arising in humans and, if they do occur, develop targeted therapeutic approaches. Henceforth, we discuss how the evidence from both MIA and paternal infection models of epigenetic inheritance can inform future research into the intergenerational and transgenerational impacts of COVID-19.
Brief overview of epigenetic inheritance and parental reprogramming
Epigenetics broadly refers to the mitotically stable molecular processes that regulate gene expression independently of the DNA sequence itself [25]. The main epigenetic processes include DNA methylation (and other modifications), histone modifications, as well as the actions of small and long noncoding RNAs [25]. Importantly, these epigenetic mechanisms work together to regulate gene expression. For example, DNA methylation modifications can regulate the expression of microRNAs (miRNAs) and in turn, miRNAs can modulate the expression of DNA methyltransferases and histone deacetylases [5]. These epigenetic processes are also dynamic and susceptible to changing environmental conditions during development [5]. The timing and severity of particular environmental insults, including diet and stress, can also affect whether epigenetic changes occur in germ cells [2].
Global erasure of epigenetic marks, including DNA methylation patterns and histone modifications, occurs at two stages in a mammal’s lifetime [26]. These are immediately after fertilisation in the zygote and when the primordial germ cells (PGCs) enter the developing gonad during gametogenesis [26]. These reset periods restore totipotency in the embryo and prevent many epigenetic aberrations from being inherited [2]. Furthermore, the developing sperm undergoes extensive chromatin remodelling during maturation as shown in Box 1 [27], which has previously led scientists to believe that paternal epigenetic inheritance is unlikely to occur. Contrastingly, these reprogramming and chromatin remodelling periods have been identified as critical periods where environmental insults can cause heritable epigenetic changes [2]. These epigenetic marks can escape reprogramming and potentially confer increased disease risk in offspring [2]. Moreover, the sperm and oocyte retain small and long noncoding RNAs that can guide embryonic development and subsequently affect offspring phenotypes [6,12,28]. Therefore, both paternal and maternal epigenetic marks can escape erasure and be inherited.
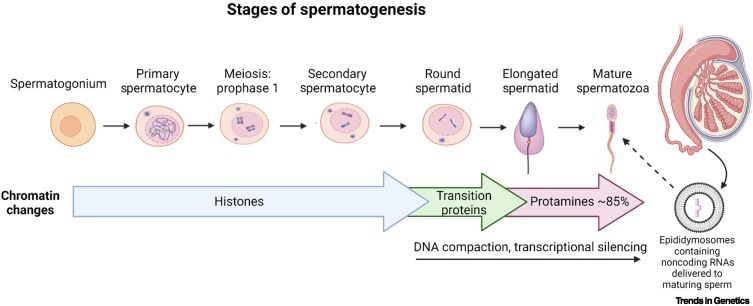
Figure 1.
The stages of spermatogenesis and sperm maturation.
Chromatin compaction occurs within the spermatid to spermatozoa stages where most histones (85% in humans) are replaced with transition proteins followed by protamines. Transcriptional activity is also reduced once the round spermatids are formed. Thus, the early stages of spermatogenesis represent a time window when epigenetic information can be altered. However, during maturation in the epididymis, the sperm receives noncoding RNAs, along with other factors required for development, from epididymal extracellular vesicles (epididymosomes). This is another mechanism whereby the sperm epigenome can be modified. Created with BioRender.com.
Box 1. Spermatogenesis and sperm epigenome remodelling.
In mammals, spermatogenesis involves the development and maturation of PGCs into mature spermatozoa (see Figure 1 in main text) [29]. Spermatogenesis is a continuous and efficient process that spans a mammal’s reproductive lifetime [29]. The process begins in the basement membrane and ends in the lumen of the seminiferous tubules in the testes. A full cycle of spermatogenesis typically takes approximately 35 days in mice and 74 days in humans [30]. It begins with the development of PGCs into diploid, self-renewing spermatogonia, which then mitotically divide to become spermatocytes [27,31]. Spermatocytes then undergo two meiotic divisions to become round haploid spermatids [27]. These spermatids morphologically mature into spermatozoa in a process known as spermiogenesis [32]. However, the spermatozoa exiting the testes after spermatogenesis still need to gain the motility and functional maturity to be able to fertilise the oocytes [33]. As they transition from the caput to the caudal region of the epididymis during their final stages of maturation, the spermatozoa receive growth factors and extracellular vesicles from the epithelial cells bordering the tubule [34,35].
During spermatogenesis, chromatin packaging occurs to attain nuclear compaction [27]. Histones that package the spermatozoal DNA are mostly replaced by intermediary transition proteins, which are then replaced by protamines [36]. Protamines are small basic proteins that can tightly pack the sperm DNA [37]. In humans, 15% of histones are retained in mature sperm, whereas only 1–2% of histones are retained in sperm from mice [38,39]. Sperm transcriptomic activity, including RNA synthesis, is also minimised during this process of nuclear compaction [39,40]. This entire compaction process has led many to disregard that the sperm carries any heritable information other than the paternal haploid genome. By contrast, researchers have found that the histones retained in human sperm map onto promotors for genes that are important in embryonic development [41]. Additionally, the mature sperm contain many noncoding RNA subtypes, most notably tRNAs, miRNAs, and PIWI-interacting RNAs (piRNAs) [38]. Rather than being a redundant remnant from spermatogenesis, these RNAs appear necessary for embryo implantation and development [12,42]. Furthermore, the extracellular vesicles delivered to the maturing sperm during epididymal transit contain noncoding RNAs that are also involved in fertility and implantation [12,42]. This highlights a mechanism by which crosstalk between somatic cells and the maturing spermatozoa can occur. Altogether, despite extensive chromatin compaction, there are multiple mechanisms whereby the sperm epigenome can reshape offspring embryonic and postnatal development.
Furthermore, the noncoding RNA profiles of sperm can be altered by environmental exposures experienced during a male’s reproductive lifetime [14,19,43]. Strikingly, microinjection of these differentially expressed noncoding RNAs into mouse zygotes can reproduce similar offspring phenotypes to those observed in epigenetic inheritance models involving paternal stress and infection [15,19]. Although the roles of sperm noncoding RNAs in intergenerational and transgenerational inheritance are yet to be fully characterised, this evidence highlights direct links between sperm epigenetic alterations and offspring phenotypes.
Alt-text: Box 1
Paternal environmental exposures and epigenetic inheritance
Epidemiological and rodent studies suggest that paternal preconceptual environmental conditions can also reprogramme offspring development via changes in the sperm epigenome. Early retrospective studies in human cohorts from Överkalix, Sweden, show that a surfeit of food in the paternal grandfather’s food supply during their slow growth period is associated with increased mortality and diabetes mortality risks in grandsons [44]. Since then, paternal epigenetic inheritance animal models have gained much attention amongst researchers in the epigenetics field [4]. An advantage of studying paternal epigenetic inheritance rodent models is that fathers only contribute the sperm and its contents at conception and thus, in well-designed and controlled experiments, any observed offspring phenotypic changes may be linked to the sperm DNA and associated epigenome [4]. Highly inbred (congenic) mouse strains with almost identically matched genomes allow researchers to probe the sperm epigenome more specifically. Rodent studies have shown that paternal preconceptual exposure to toxins, dietary changes, and stress can all lead to altered offspring behavioural and metabolic phenotypes [6,19,43,45., 46., 47.]. More recently, preliminary evidence indicates that paternal preconceptual exposure to infection and immune activation may also have both intergenerational and transgenerational health consequences [19,48,49].
The mammalian testes have a blood–testis barrier (BTB) that largely protects the developing sperm from potentially harmful immune factors and pathogens [50]. However, some pathogens, including mumps and Escherichia coli, can breach the BTB and disrupt spermatogenesis [51., 52., 53.]. For instance, E. coli can damage sperm by binding to mannose residues located on the sperm head and reduce sperm motility [54]. Otherwise, high levels of inflammatory mediators and immune factors associated with infections can disrupt the BTB and impair spermatogenesis [55]. An imbalance of both proinflammatory cytokines and reactive oxygen species (ROS) in the testicular and epididymal areas are often implicated [53]. For example, increased levels of the proinflammatory cytokine tumour necrosis factor (TNF)-α during mumps infection in mice can transiently reduce numbers of elongated spermatids [51]. Furthermore, elevated proinflammatory cytokines, including interleukin (IL)-6 and IL-1β, released by testicular macrophages and leukocytes during infection can promote oxidative stress, which can harm spermatogenesis by causing lipid peroxidation of the sperm membrane and sperm DNA fragmentation [52]. Damage to sperm DNA integrity and spermatogenesis are both associated with DNA methylation errors and changes in sperm miRNAs [52,56,57]. This suggests that the presence of certain pathogens and immune factors in the male reproductive tract may impact the sperm epigenome. However, the direct effects of infections and their associated immune responses on the sperm epigenome are not yet clearly defined.
Research into these impacts is critical, since it has been shown that preconceptual paternal infection with T. gondii in mice has detrimental intergenerational and transgenerational neurodevelopmental consequences [19]. In this study, F1 and F2 male offspring, in particular, showed abnormalities in learning, memory, sociability, anxiety and depressive behaviour [19]. Moreover, changes in sperm noncoding RNA profiles are involved, since injection of noncoding RNA fractions from T. gondii F0 sperm, which showed differentially expressed noncoding RNA profiles, into naïve zygotes recapitulated most of the offspring abnormalities observed in the natural mating experiments [19].
However, it is unclear whether T. gondii itself or the accompanying immune response alter the sperm epigenome, since cysts from T. gondii are present in the F0 testicular tissue at 4 weeks postinfection [19]. It is also unclear how T. gondii infection can modify the sperm small noncoding RNAs and how these modifications can produce transgenerational outcomes. Systemic inflammation may be mediating these epigenetic effects, since both preconceptual paternal sepsis and lipopolysaccharide (LPS) treatment can alter offspring metabolism and immune responsivity [48,49]. Indeed, systemic inflammation may modulate the sperm epigenome by changing the noncoding RNA payload delivered to maturing sperm during epididymal transit (Box 1). Alternatively, pathogens themselves may also directly cause sperm epigenome modifications, since some, including T. gondii, are able to manipulate somatic cell epigenetics to evade immune detection [58]. Therefore, a major challenge for future research will be to determine which particular pathogens and inflammatory mediators cause changes in the sperm epigenome.
Maternal environmental exposure and epigenetic inheritance
The effects of the maternal environment on reprogramming offspring phenotypes during gestation have been well studied [2,59,60]. The prenatal period is a critical window when maternal environmental exposures can alter foetal epigenetic programming [60]. Depending on the timing, nature, and severity of the exposure, this programming may have long-term consequences for offspring health [5]. For example, cohort studies show that individuals prenatally exposed to the Dutch Hunger Famine from 1944 to 1945 have elevated rates of chronic health conditions in adulthood, including schizophrenia [61], cardiovascular disease [62], and impaired glucose metabolism [63]. Animal studies have also shown that prenatal maternal stress, maternal undernutrition, and maternal overnutrition can all lead to adverse psychiatric and metabolic outcomes later in life [64., 65., 66., 67., 68.]. Although early gestational insults are often associated with foetal reprogramming, mid- and late gestational challenges can also have enduring offspring effects [69,70]. The details of the various effects of maternal nutrition and stress on offspring phenotypes are outside the scope of this article and have been reviewed extensively elsewhere [2,3,71].
Foetal neurodevelopment is a tightly regulated process which involves the participation of many immune factors at the maternal–foetal interface [72]. Maternal cytokines, Toll-like receptors, microglia, and the complement system are known to play roles in neurogenesis, synaptic pruning, and synaptogenesis [72]. Disturbances in the timing and levels of these immune factors due to maternal infection and inflammation can result in poorer foetal neurodevelopment [72].
Maternal infection and immune activation were identified as risk factors for offspring health when an association was found between high seasonal rates of infectious diseases and the birth dates of people diagnosed with schizophrenia [73]. Since then, human and animal studies have shown that maternal exposure to influenza and other infections during pregnancy can predispose offspring to neurodevelopmental disorders, such as schizophrenia and ASD [22,74., 75., 76., 77.]. Animal studies modelling maternal infection have been essential for uncovering the underlying mechanisms contributing to these offspring neurodevelopmental phenotypes. Rodent studies reveal many biochemical and structural changes in the brain associated with prenatal influenza exposure during mid-pregnancy [76,78,79]. Following this, behavioural and cognitive changes resembling the symptoms of schizophrenia and ASD, including decreased sociability, decreased exploration and prepulse inhibition (sensorimotor gating) deficits, have been observed in mice prenatally exposed to influenza [22]. Since many prenatal infections are associated with similar neurodevelopmental outcomes, researchers have investigated whether the immune responses are causing these abnormalities. With the use of immunostimulants, including the viral mimetic polyinosinic:polycytidylic acid (Poly I:C), maternal immune activation has been found to be the main driver of these offspring neurodevelopmental changes [80].
Poly I:C is a synthetic analogue of viral double-stranded RNA that binds to the Toll-like receptor 3 (TLR-3) [81]. Upon binding to TLR-3, Poly I:C triggers the release of proinflammatory cytokines, IL-6, IL-1β, interferon (IFN)-γ and TNF-α [82]. Prenatal Poly I:C exposure during mid- to late gestation in both male and female rodents recapitulate many of the effects seen in the maternal influenza model [18,83]. These offspring effects of MIA include increased amphetamine-induced locomotion [84], decreased prepulse inhibition [85], decreased social interaction [18], increased repetitive behaviour [86], impaired learning and memory [87], increased fear conditioning [18], increased depressive behaviour [84,88], and increased anxiety-like behaviour [89]. Accompanying these deficits are changes in limbic and cortical neurotransmitter systems, including changes in glutamatergic [90], GABAergic [91], dopaminergic [92], and serotonergic [93] signalling. Furthermore, some of the behavioural dysfunctions are ameliorated by antipsychotic drug administration, reinforcing the predictive validity of MIA as a model of schizophrenia [83,94]. The offspring effects of MIA, particularly anhedonia and amphetamine-induced locomotion, appear to be more serious with greater maternal weight loss induced by Poly I:C [84]. This reinforces the concept that the severity of the parental exposure influences the extent of offspring reprogramming.
Although the focus of MIA research has been on neurodevelopmental disorders, other intergenerational health concerns have been identified with preclinical models of MIA. For example, increased maternal TNF-α levels induced by LPS in an MIA model results in intrauterine foetal growth restriction and delayed skeletal development [95]. Furthermore, MIA alters the gut microbiome of offspring, potentially leading to changes in offspring health beyond the central nervous system (CNS) [87,96]. MIA has also been linked to other CNS disorders, including cerebral palsy [97] and Alzheimer’s disease [98]. Therefore, it is important for researchers to investigate the broad array of disease susceptibility phenotypes resulting from the different maternal and paternal environmental exposures.
The mechanisms underlying the effects of MIA are complex and not entirely understood. There is evidence that MIA disturbs foetal brain cytokine and oxidative stress levels, leading to epigenetic alterations in the developing brain that then lead to the long-term behavioural outcomes [89,99]. For example, DNA methylation and histone modification changes, including those affecting the schizophrenia risk factor gene DISC1, have been identified in the hippocampus and medial prefrontal cortex of MIA-exposed offspring [93,100., 101., 102.]. However, microglial changes in the foetal brain [88], maternal gut microbiome changes [103] and maternal care behaviour [104] are also linked to offspring abnormalities in the MIA model. Uncovering the relative contributions of each of these mechanisms and how they interrelate will be critical for our understanding of neurodevelopmental disorders.
Notably, some of the behavioural, molecular, and psychiatric MIA-related disturbances manifest in the second and third generations without re-exposure to an immune insult [18,92,104,105]. For example, increased fear conditioning, reduced sociability, and increased behavioural despair have been observed in the F2 and F3 generations of an MIA mouse model [18,92]. This implies that MIA can generate heritable germline epigenetic modifications. Indeed, Weber-Staudlbauer et al. have found increased DNA methylation at the promoter of nuclear receptor-related 1 protein (Nurr1), a transcription factor involved in dopaminergic signalling, in the sperm of MIA exposed offspring [92]. Intriguingly, the altered F2 and F3 phenotypes in this study are only observed in the paternal lineage, demonstrating that MIA-related inflammation may modify the sperm epigenome [92]. Further research is needed to fully characterise the transgenerational consequences and germline epigenetic mechanisms of MIA. Given that MIA can have such pervasive and prolonged outcomes, it is critical that we develop and utilise targeted public health approaches to identify exposed individuals, especially during and after the COVID-19 pandemic. This is important considering that early intervention may improve the mental health outcomes in individuals with genetic and/or environmental risk factors for schizophrenia [106].
Transgenerational COVID-19: MIA in the context of the global COVID-19 pandemic
Studies investigating the 1918–1919 Spanish influenza pandemic shed light on the potential long-term and multigenerational health complications that can arise due to prenatal viral exposure in a pandemic [107., 108., 109.]. The 1918 influenza pandemic had a rapid onset in October 1918 and resulted in 20–100 million deaths worldwide [107]. Women of child-bearing age were also disproportionately affected, with estimations that approximately one third of women in the USA aged 25–30 years were infected [107]. Using data registries such as the 1960–1980 US Decennial Census data, researchers have found that cohorts exposed to influenza in utero during the peak of the pandemic were more likely to suffer from increased physical disability, poorer social and economic outcomes, and decreased life expectancy [107., 108., 109.]. Furthermore, Richter and Olof Obling have shown that there are multigenerational impacts of the 1918 influenza pandemic, where daughters of women and sons of men prenatally exposed to influenza have lower educational attainments [109]. Interestingly, these effects are still present when nonbiological mechanisms, such as the socioeconomic status of the parent, are accounted for [109].
Although many of these studies are ecological and lack the controlled settings found in MIA animal studies, they provide an impetus to examine the potential for intergenerational outcomes resulting from prenatal exposure to COVID-19. Unlike the 1918 Spanish influenza pandemic, we now have more sophisticated diagnostic and data collection tools that allow us to study the neurodevelopment and overall health of large birth cohorts prenatally exposed to COVID-19.
SARS-CoV-2 is a single-stranded RNA betacoronavirus responsible for the COVID-19 pandemic [110]. SARS-CoV-2 infection in humans does not usually have fatal consequences, however, severe respiratory disease can occur in a minority of cases [111]. Symptoms can range from cough, fever, myalgia, and dyspnoea in mild presentations, to acute respiratory distress, cardiovascular complications, organ failure, and renal dysfunction in moderate to severe cases [111]. Severe illness and death from a SARS-CoV-2 infection are at least partially due to a cytokine storm originating from a dysregulated host immune response [112].
Although possible, emerging evidence suggests that vertical transmission of SARS-CoV-2 is unlikely to occur [113., 114., 115.]. This is supported by the lack of IgM antibodies targeting SARS-CoV-2 in the cord plasma and SARS-CoV-2 RNA in the placenta of pregnant women with a recent or ongoing infection [116,117]. However, there are some reports of adverse foetal outcomes following maternal SARS-CoV-2 infection, including preterm birth and intrauterine growth retardation [114,118]. Additionally, pregnant women with a SARS-CoV-2 infection appear to be at higher risk of severe illness, since hospitalisation rates and intensive care unit (ICU) admission rates are higher in this population group [119]. While the risk of a SARS-CoV-2 infection in neonates may be low, the associated maternal inflammatory response may threaten their neurodevelopment and long-term health (Figure 2 ).
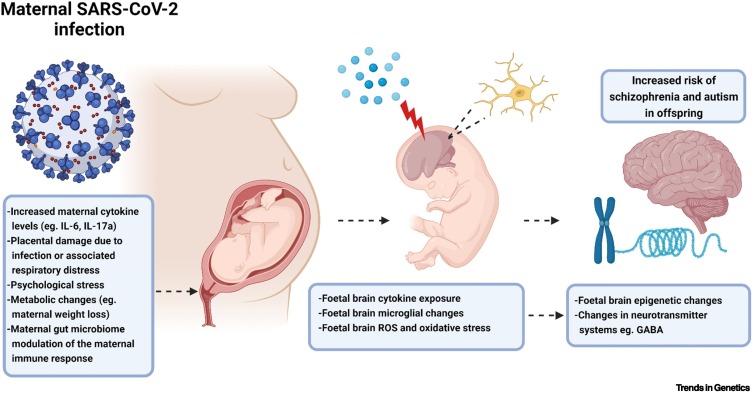
Figure 2.
Mechanisms contributing to offspring phenotypes from maternal immune activation (MIA) models.
Based on the MIA literature, increased maternal cytokine levels (particularly IL-6 and IL-17a) and placental damage from respiratory issues associated with a SARS-CoV-2 infection have the potential to alter foetal central nervous system development. In particular, MIA can lead to enhanced foetal brain cytokine levels, microglial changes, and an imbalance in reactive oxygen species. These can affect the epigenetic information contained within the various brain cells and the functioning of various neurotransmitter systems. This complex array of changes can increase the risk of neurodevelopmental disorders in the offspring, including schizophrenia and autism. Created with BioRender.com. Abbreviations: IL, interleukin; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Gee et al. (2021) have shown that a maternal SARS-CoV-2 infection can imprint on the neonatal immune system in the absence of vertical transmission of SARS-CoV-2, which includes changes in neonatal inflammatory cytokine levels and cytokine responsiveness of neonatal immune cells [117]. Furthermore, the inflammatory cytokines increased at the maternal-foetal interface during a SARS-CoV-2 infection include many of those that are upregulated by Poly I:C in MIA models, such as IL-1β, IL-10, IL-17A, and TNF [112,116,117,120]. Notably, IL-17a released by T-helper 17 cells has been linked to ASD-like behaviours in offspring exposed to MIA [121]. Patients with SARS-CoV-2 infection often have elevated plasma IL-6 levels, particularly in severe illness [112]. Importantly, elevated maternal IL-6 levels have been mechanistically linked to the behavioural and cognitive deficits seen in MIA [122,123]. Thus, the neonate may be exposed to several inflammatory mediators during a maternal SARS-CoV-2 infection that are known to increase the risk of neurodevelopmental disorders in adulthood.
Since the severity of neurodevelopmental phenotypes from MIA is increased with greater maternal immune induction [84], a SARS-CoV-2 cytokine storm during pregnancy may elevate both the likelihood and severity of offspring neurodevelopmental disorders. Based on recent MIA evidence [18,92,104], the maternal SARS-CoV-2 immune response may also have transgenerational neurodevelopmental repercussions, prolonging the consequences of the pandemic further still. Moreover, the increased maternal psychological stress from SARS-CoV-2 infection may also contribute to adverse foetal reprogramming [124]. There is some recent evidence to suggest that vaccination may reduce adverse outcomes and severity of illness in pregnant women with a SARS-CoV-2 infection [125]. However, it is currently unknown whether vaccinations can protect neonates against the inflammation associated with maternal SARS-CoV-2 infection.
Based on the current SARS-CoV-2 and previous Spanish influenza pandemic literature, we believe there is a strong imperative to urgently investigate COVID-19-specific MIA animal models to uncover any potential long-term mental health consequences. On this note, many SARS-CoV-2 mouse models have been developed to investigate the pathogenesis of SARS-CoV-2 and evaluate the efficacy of many antiviral therapeutics and vaccines [126]. Since murine angiotensin-converting enzyme (ACE)2, the receptor SARS-CoV-2 uses to enter cells, has a low affinity for the S protein of SARS-CoV-2, researchers have adapted the SARS-CoV-2 wild-type strains for murine ACE2 [126]. Alternatively, other researchers have developed transgenic mice expressing the human ACE2 receptor to allow for efficient viral infection and replication in mouse lungs [126]. A challenge for future MIA research will be to adapt these models so that they accurately mimic the pathophysiology and immunopathology found in pregnant humans with a SARS-CoV-2 infection. While adapting these SARS-CoV-2 models for future MIA studies may be difficult due to strict biosafety requirements, they will be valuable for us to understand the biological mechanisms contributing to any multigenerational effects of prenatal SARS-CoV-2 exposure. Researchers should also explore whether vaccinations can reduce any of the SARS-CoV-2 associated maternal inflammation that may be impacting on the foetus. These studies will allow us to develop therapeutic strategies aimed at preventing, or at least mitigating, disease development in individuals prenatally exposed to SARS-CoV-2.
Risk of long-term and multigenerational COVID-19 consequences from PIA
While the risks of maternal-related intergenerational and transgenerational COVID-19 health impacts can be appreciated from our knowledge of MIA, the possible risks from paternal infection and PIA are not obvious. Nonetheless, there is evidence accumulating to suggest that SARS-CoV-2 may breach the BTB and disrupt spermatogenesis [127]. Given that impaired spermatogenesis is linked to changes in the sperm epigenome, investigating the effects of paternal preconceptual SARS-CoV-2 infection on offspring phenotypes will be a valuable avenue of research.
ACE2 is present on Leydig and Sertoli cells of the testes, raising the possibility that SARS-CoV-2 can infect the male reproductive tract, and potentially impair spermatogenesis [128]. It has been recently shown that a severe SARS-CoV-2 infection can decrease protein expression of claudin-11 and connexin-43 and decrease Sertoli cells in the testes, which are all important for the integrity of the BTB [129]. There is also evidence that SARS-CoV-2 can lead to elevated levels of TNF-α in the testes [129]. High levels of TNF-α in the testes can inhibit testosterone release from Leydig cells and increase BTB permeability; both of which can adversely affect spermatogenesis [51]. Additionally, acute febrile illness, a common symptom reported in illness associated with a SARS-CoV-2 infection, can also have detrimental effects on sperm parameters for months after the episode [130]. Indeed, some studies show impaired spermatogenesis in COVID-19 patients with moderate to severe disease [131., 132., 133.]. Furthermore, a systematic review has found that semen quality of patients with a moderate SARS-CoV-2 infection is lower than that of patients with a mild infection [134]. Taken together, these suggest that severity of the SARS-CoV-2 infection may affect the degree of damage to male fertility outcomes.
However, there is currently very little published evidence showing changes in spermatogenesis and male fertility parameters in SARS-CoV-2-infected animal models [133]. It is also not clear whether the direct effects of SARS-CoV-2 or the associated immune response affect the level of impairment in spermatogenesis. Early data also suggest that COVID-19 mRNA vaccines themselves do not affect sperm parameters [135]. However, whether the current vaccines protect against detrimental SARS-CoV-2-related male reproductive parameters remains unclear. Answering these questions is essential for the mitigation of long-term male fertility issues that may arise due to the large number of young males affected by the virus.
Given the recent evidence that both paternal sepsis and infection with T. gondii can reprogramme offspring phenotypes [19,48,49], it will also be critical for researchers to investigate PIA and paternal SARS-CoV-2 epigenetic inheritance models. SARS-CoV-2 mouse models described earlier can also be adapted for this research so that all possible generational phenotypes and epigenetic mechanisms can be uncovered (Figure 3 ). Researchers should also pay careful attention to whether the timing and severity of COVD-19 exposure affects the sperm epigenome, since this can inform future public health measures aimed at reducing offspring disease burden. Other factors that affect spermatogenesis, such as cytokine levels and viral load, should also be considered when investigating the effects of SARS-CoV-2 on the sperm epigenome. Altogether, this research may prevent possible paternal-related intergenerational and transgenerational COVID-19 impacts from going unrecognised, and allow any negative consequence to be systematically addressed.
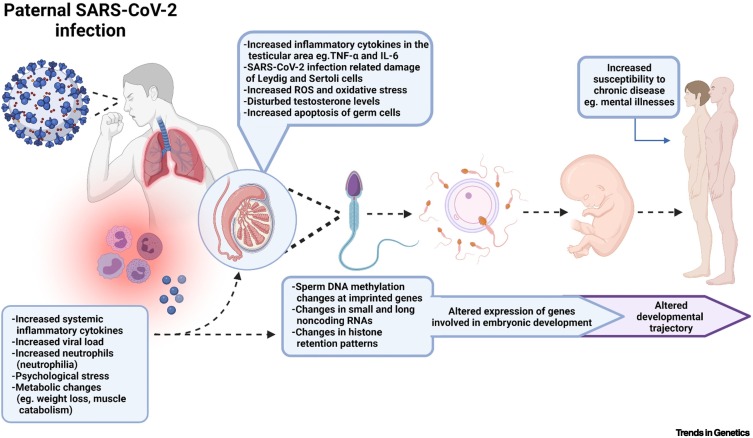
Figure 3.
Potential mechanistic pathways involved in the proposed reprogramming of offspring phenotypes due to paternal SARS-CoV-2 infection and immune activation.
High levels of systemic proinflammatory cytokines, such as TNF-α and IL-6, and neutrophils can potentially damage the testes and affect spermatogenesis. Since many epigenetic changes occur during spermatogenesis, these immune factors may be able to alter the sperm epigenome. Furthermore, stress and metabolic changes that may occur during SARS-CoV-2 infection could also affect specific contents of the sperm, such as noncoding RNAs. The sperm can transmit this epigenetic information to the oocyte at fertilisation, ultimately leading to changes in the expression of genes involved in embryonic development. This could modulate offspring development and function, and possibly alter disease susceptibility. Created with BioRender.com. Abbreviations: IL, interleukin; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumour necrosis factor-α.
Concluding remarks
There is mounting evidence that both maternal and paternal environmental exposures can modulate offspring and grand-offspring phenotypes via heritable epigenetic modifications [5]. This evidence fundamentally challenges the previously accepted dogma that only the parental genome contributes to biological inheritance. The main challenge for the future will be to delineate the biological mechanisms underpinning the various epigenetic inheritance models, particularly the less characterised paternal models (see Outstanding questions). Another challenge to consider in future human cohort studies is that offspring may be impacted by both parents being infected with SARS-CoV-2, since household spread of the more infectious COVID-19 variants has become increasingly common. By increasing our understanding of which exposures can induce heritable epigenetic changes and how they can reprogramme offspring phenotypes, we may be able to prevent, or at the very least alleviate, future generational health impacts.
Outstanding questions.
Will a high rate of prenatal SARS-CoV-2 exposures lead to an increase of neurodevelopmental disorders and other changes to disease susceptibility in affected offspring? If so, how can public health measures, including vaccines, and therapeutic approaches be developed to mitigate such potential increases to burdens of disease?
Can a SARS-CoV-2 specific MIA mouse model be adapted from the current SARS-CoV-2 animal models? Can these models provide information on the biological mechanisms underlying any potential intergenerational consequences of prenatal exposure to SAR-CoV-2?
Can infection, immune activation, and inflammation modify epigenetic information, including DNA modifications and noncoding RNAs, in sperm? If so, by which mechanisms?
Will paternal SARS-CoV-2 infection and PIA have detrimental intergenerational and transgenerational outcomes via changes to the sperm epigenome? If so, how severe does the SARS-CoV-2 infection and PIA need to be to reprogramme offspring phenotypes?
If paternal infection and PIA do affect the sperm epigenome, how long are these epigenetic modifications present in the sperm and capable of impacting offspring phenotypes?
If SARS-CoV-2 infection and COVID-19 affect sperm epigenetics and offspring phenotypes, do these changes occur with other viral (and nonviral) infections?
If SARS-CoV-2 infection and COVID-19 have intergenerational impacts, can these impacts transmit transgenerationally to one or more further generations?
Alt-text: Outstanding questions
While much is known about the offspring effects of MIA on neurodevelopment, the mechanisms by which MIA-related transgenerational outcomes can occur are yet to be fully characterised. Furthermore, little is known about the intergenerational and transgenerational effects of paternal infection and PIA. Due to the high infection rates of the global COVID-19 pandemic, we believe it is essential to focus available resources on determining whether (and, if so, how) SARS-CoV-2 infection, and the various associated immune responses, can reprogramme offspring and grand-offspring phenotypes. Research into any potential intergenerational and transgenerational effects of PIA also needs to be prioritised, given that there is emerging evidence suggesting inflammation and infection can alter the sperm epigenome. Well-controlled preclinical studies using SARS-CoV-2-infected animal models that accurately mimic the various presentations of the human disease will be vital for addressing these questions. Crucially, systematically investigating the reprogramming effects of MIA and PIA, in both humans and animal models, will provide insights into epigenetic mechanisms and other biological processes which may contribute to the complex aetiologies of many common disorders.
Acknowledgments
Acknowledgments
We would like to thank past and present members of the Hannan Laboratory whose research findings, together with those of collaborators and colleagues, informed our ideas on this topic. C.G. is supported by a Postdoctoral Fellowship (Mentor: A.J.H.) from the Hereditary Disease Foundation (HDF). A.J.H. is supported by a Principal Research Fellowship and Ideas Grant from the National Health and Medical Research Council (NHMRC), a Discovery Project Grant from the Australian Research Council (ARC), the DHB Foundation (Equity Trustees), and the Flicker of Hope Foundation.
Declaration of interests
No interests are declared.
Glossary
- DNA methylation
an epigenetic modification whereby methyl groups are added to the 5′ position of cytosine residues, converting them into 5-methylcytosines.
- DNA methyltransferases
a family of enzymes that catalyse the transfer of a methyl group to a DNA molecule.
- Histones
proteins found within nucleosomes that wrap around the DNA sequence in eukaryotic cells.
- Histone deacetylases
enzymes that catalyse the removal of acetyl groups from histone proteins.
- Intergenerational epigenetic inheritance
the transmission of epigenetic information from one generation to the next. In maternal intergenerational epigenetic inheritance (in utero), epigenetic changes appearing in both F1 and F2 generations are intergenerational since both generations are exposed to the environmental condition. In paternal intergenerational epigenetic inheritance, epigenetic changes appearing in the F1 generation are considered intergenerational.
- Lipopolysaccharide (LPS)
the main factor comprising the outer membrane in Gram-negative bacteria. It is a potent endotoxin that can stimulate the release of proinflammatory cytokines, including IL-1 and TNF-α.
- Long noncoding RNAs
RNA transcripts exceeding 200 nucleotides in length that do not translate into a protein.
- miRNA
a type of small noncoding RNA that directs post-transcriptional silencing of gene expression by partially binding to the 3′ untranslated region of target mRNAs.
- Oxidative stress
can result from an overproduction of ROS and can lead to cellular damage.
- Polyinosinic:polycytidylic acid (Poly I:C)
a synthetic analogue of viral double-stranded RNA that binds to TLR-3. It triggers the release of many proinflammatory cytokines, including IL-6, IL-1β, IFN-γ, and TNF-α.
- Primordial germ cells (PGCs)
primary undifferentiated stem cells that give rise to the gametes (sperm and oocytes).
- Reactive oxygen species (ROS)
reactive, unstable molecules derived from oxygen.
- Small noncoding RNAs
regulatory RNA molecules that are 18–200 nucleotides long and do not translate into a protein. They can include miRNAs, PIWI interacting RNAs (piRNAs), tRNAs, and endogenous small interfering RNAs (endo-siRNAs).
- T-helper 17 cells
proinflammatory T-helper lymphocytes that release IL-17a. They play an important role in regulating inflammation at mucosal surfaces in mammals.
- Toll-like receptor 3 (TLR-3)
a member of the TLR family that is involved in pathogen recognition and triggering the innate immune system. TLR-3 specifically recognises double-stranded RNA associated with viral infection and therefore plays a role in host viral defence.
- Toxoplasma gondii
an obligate intracellular protozoan parasite that causes the disease toxoplasmosis.
- Transgenerational epigenetic inheritance
the transmission of epigenetic information across generations without re-exposure to the environmental condition. In maternal transgenerational epigenetic inheritance, epigenetic changes appearing in F3 and beyond are considered transgenerational, since they have not been exposed to the environmental condition. In paternal transgenerational epigenetic inheritance, epigenetic changes appearing in F2 and beyond are transgenerational since they have not been exposed to the environmental condition.
References
- 1.Blanco-Gómez A., et al. Missing heritability of complex diseases: enlightenment by genetic variants from intermediate phenotypes. BioEssays. 2016;38:664–673. doi: 10.1002/bies.201600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale T.L. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015;16:332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sales V.M., et al. Epigenetic mechanisms of transmission of metabolic disease across generations. Cell Metab. 2017;25:559–571. doi: 10.1016/j.cmet.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins A.J., et al. Paternal programming of offspring health. Early Hum. Dev. 2020;150 doi: 10.1016/j.earlhumdev.2020.105185. [DOI] [PubMed] [Google Scholar]
- 5.Burton N.O., Greer E.L. Multigenerational epigenetic inheritance: Transmitting information across generations. Semin. Cell Dev. Biol. 2021;123:1–12. doi: 10.1016/j.semcdb.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q., et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 7.Weber-Stadlbauer U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl. Psychiatry. 2017;7:e1113. doi: 10.1038/tp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pembrey M., et al. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Blévec E., et al. Paternal epigenetics: mammalian sperm provide much more than DNA at fertilization. Mol. Cell. Endocrinol. 2020;518 doi: 10.1016/j.mce.2020.110964. [DOI] [PubMed] [Google Scholar]
- 10.Sharma U. Paternal contributions to offspring health: role of sperm small RNAs in intergenerational transmission of epigenetic information. Front. Cell Dev. Biol. 2019;7:215. doi: 10.3389/fcell.2019.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teperek M., et al. Sperm is epigenetically programmed to regulate gene transcription in embryos. Genome Res. 2016;26:1034–1046. doi: 10.1101/gr.201541.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conine C.C., et al. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell. 2018;46:470–480.e3. doi: 10.1016/j.devcel.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gapp K., et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gapp K., et al. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol. Psychiatry. 2020;25:2162–2174. doi: 10.1038/s41380-018-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgers A.B., et al. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. U. S. A. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodden C., et al. Diet-induced modification of the sperm epigenome programs metabolism and behavior. Trends Endocrinol. Metab. 2020;31:131–149. doi: 10.1016/j.tem.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Anway M.D., et al. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber-Stadlbauer U., et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol. Psychiatry. 2017;22:102–112. doi: 10.1038/mp.2016.41. [DOI] [PubMed] [Google Scholar]
- 19.Tyebji S., et al. Pathogenic infection in male mice changes sperm small RNA profiles and transgenerationally alters offspring behavior. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107573. [DOI] [PubMed] [Google Scholar]
- 20.Silmon de Monerri N.C., Kim K. Pathogens hijack the epigenome: a new twist on host-pathogen interactions. Am. J. Pathol. 2014;184:897–911. doi: 10.1016/j.ajpath.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knuesel I., et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 22.Shi L., et al. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yehuda R., et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J. Clin. Endocrinol. Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 24.Shen L., et al. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: the Genomic Research of the Chinese Famine (GRECF) study. Clin. Epigenetics. 2019;11:88. doi: 10.1186/s13148-019-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portela A., Esteller M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 26.Messerschmidt D.M., et al. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014;28:812–828. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larose H., et al. In: Current Topics in Developmental Biology. Wellik D.M., editor. Academic Press; 2019. Chapter eight - gametogenesis: a journey from inception to conception; pp. 257–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svoboda P. Long and small noncoding RNAs during oocyte-to-embryo transition in mammals. Biochem. Soc. Trans. 2017;45:1117–1124. doi: 10.1042/BST20170033. [DOI] [PubMed] [Google Scholar]
- 29.Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakberg E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 31.Hamatani T. Human spermatozoal RNAs. Fertil. Steril. 2012;97:275–281. doi: 10.1016/j.fertnstert.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Kimmins S., et al. Testis-specific transcription mechanisms promoting male germ-cell differentiation. Reproduction. 2004;128:5–12. doi: 10.1530/rep.1.00170. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan R., Mieusset R. The human epididymis: its function in sperm maturation. Hum. Reprod. Update. 2016;22:574–587. doi: 10.1093/humupd/dmw015. [DOI] [PubMed] [Google Scholar]
- 34.Sharma U., et al. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell. 2018;46:481–494.e6. doi: 10.1016/j.devcel.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma U., et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godmann M., et al. The dynamic epigenetic program in male germ cells: its role in spermatogenesis, testis cancer, and its response to the environment. Microsc. Res. Tech. 2009;72:603–619. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- 37.Wykes S.M., Krawetz S.A. The structural organization of sperm chromatin. J. Biol. Chem. 2003;278:29471–29477. doi: 10.1074/jbc.M304545200. [DOI] [PubMed] [Google Scholar]
- 38.Rando O.J. Intergenerational transfer of epigenetic information in sperm. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda R., et al. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J. Psychiatr. Res. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 40.Dadoune J.-P. Spermatozoal RNAs: what about their functions? Microsc. Res. Tech. 2009;72:536–551. doi: 10.1002/jemt.20697. [DOI] [PubMed] [Google Scholar]
- 41.Brykczynska U., et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 42.Yuan S., et al. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development (Cambridge, England) 2016;143:635–647. doi: 10.1242/dev.131755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Short A.K., et al. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry. 2016;6:e837. doi: 10.1038/tp.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaati G., et al. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 45.Azizi N., et al. Parental pre-conception stress status and risk for anxiety in rat offspring: specific and sex-dependent maternal and paternal effects. Stress. 2019;22:619–631. doi: 10.1080/10253890.2019.1619075. [DOI] [PubMed] [Google Scholar]
- 46.Claycombe-Larson K.G., et al. Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner. J. Nutr. Biochem. 2020;81 doi: 10.1016/j.jnutbio.2020.108373. [DOI] [PubMed] [Google Scholar]
- 47.Rodgers A.B., et al. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013;33:9003. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bomans K., et al. Paternal sepsis induces alterations of the sperm methylome and dampens offspring immune responses—an animal study. Clin. Epigenetics. 2018;10:89. doi: 10.1186/s13148-018-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z., et al. Paternal systemic inflammation induces offspring programming of growth and liver regeneration in association with Igf2 upregulation. Mol. Cell. Endocrinol. 2020;518 doi: 10.1016/j.mce.2020.111001. [DOI] [PubMed] [Google Scholar]
- 50.Mruk D.D., Cheng C.Y. The mammalian blood-testis barrier: its biology and regulation. Endocr. Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H., et al. Mumps virus infection disrupts blood-testis barrier through the induction of TNF-α in Sertoli cells. FASEB J. 2019;33:12528–12540. doi: 10.1096/fj.201901089R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henkel R., et al. The role of infections and leukocytes in male infertility. Andrologia. 2021;53 doi: 10.1111/and.13743. [DOI] [PubMed] [Google Scholar]
- 53.Azenabor A., et al. Impact of inflammation on male reproductive tract. J. Reprod. Infertil. 2015;16:123–129. [PMC free article] [PubMed] [Google Scholar]
- 54.Monga M., Roberts J.A. Spermagglutination by bacteria: receptor-specific interactions. J. Androl. 1994;15:151–156. [PubMed] [Google Scholar]
- 55.Loveland K.L., et al. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front. Endocrinol. 2017;8:307. doi: 10.3389/fendo.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curry E., et al. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology. 2011;76:1532–1539. doi: 10.1016/j.theriogenology.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura A., et al. Epigenetic alterations in sperm associated with male infertility. Congenit. Anomal. 2015;55:133–144. doi: 10.1111/cga.12113. [DOI] [PubMed] [Google Scholar]
- 58.Rosowski E.E., et al. Toxoplasma gondii inhibits gamma interferon (IFN-γ)- and IFN-β-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect. Immun. 2014;82:706–719. doi: 10.1128/IAI.01291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner M.K., et al. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marciniak A., et al. Fetal programming of the metabolic syndrome. Taiwan. J. Obstet. Gynecol. 2017;56:133–138. doi: 10.1016/j.tjog.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Susser E., et al. Schizophrenia after prenatal famine: further evidence. Arch. Gen. Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 62.Roseboom T.J., et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravelli A.C.J., et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 64.Mueller B.R., Bale T.L. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Mueller B.R., Bale T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28:9055. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lesage J., et al. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J. Endocrinol. 2004;181:291–296. doi: 10.1677/joe.0.1810291. [DOI] [PubMed] [Google Scholar]
- 67.Benoit J.D., et al. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav. Brain Res. 2015;281:1–8. doi: 10.1016/j.bbr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardikar Anandwardhan A., et al. Multigenerational undernutrition increases susceptibility to obesity and diabetes that is not reversed after dietary recuperation. Cell Metab. 2015;22:312–319. doi: 10.1016/j.cmet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Mueller B.R., Bale T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orso R., et al. How early life stress impact maternal care: a systematic review of rodent studies. Front. Behav. Neurosci. 2019;13:197. doi: 10.3389/fnbeh.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rando O.J., Simmons R.A. I’m eating for two: parental dietary effects on offspring metabolism. Cell. 2015;161:93–105. doi: 10.1016/j.cell.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu-Culligan A., Iwasaki A. The role of immune factors in shaping fetal neurodevelopment. Annu. Rev. Cell Dev. Biol. 2020;36:441–468. doi: 10.1146/annurev-cellbio-021120-033518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watson C.G., et al. Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch. Gen. Psychiatry. 1984;41:85–90. doi: 10.1001/archpsyc.1984.01790120089011. [DOI] [PubMed] [Google Scholar]
- 74.Buka S.L., et al. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 75.Brown A.S., et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 76.Fatemi S.H., et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell. Mol. Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown A.S., et al. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatr. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 78.Fatemi S.H., et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol. Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 79.Fatemi S.H., et al. Human influenza viral infection in utero increases nNOS expression in hippocampi of neonatal mice. Synapse. 1998;29:84–88. doi: 10.1002/(SICI)1098-2396(199805)29:1<84::AID-SYN8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 80.Meyer U., Feldon J. To poly(I:C) or not to poly(I:C): Advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62:1308–1321. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 81.Cunningham C., et al. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav. Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Fortier M.-E., et al. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 83.Zuckerman L., et al. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 84.Missault S., et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav. Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Meyer U., et al. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Malkova N.V., et al. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y., et al. Maternal immune activation alters adult behavior, gut microbiome and juvenile brain oscillations in ferrets. eNeuro. 2018;5 doi: 10.1523/ENEURO.0313-18.2018. ENEURO.0313-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chamera K., et al. Role of polyinosinic:polycytidylic acid-induced maternal immune activation and subsequent immune challenge in the behaviour and microglial cell trajectory in adult offspring: a study of the neurodevelopmental model of schizophrenia. Int. J. Mol. Sci. 2021;22:1558. doi: 10.3390/ijms22041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Talukdar P.M., et al. Maternal immune activation causes schizophrenia-like behaviors in the offspring through activation of immune-inflammatory, oxidative and apoptotic pathways, and lowered antioxidant defenses and neuroprotection. Mol. Neurobiol. 2020;57:4345–4361. doi: 10.1007/s12035-020-02028-8. [DOI] [PubMed] [Google Scholar]
- 90.Rahman T., et al. Effects of immune activation during early or late gestation on N-Methyl-d-aspartate receptor measures in adult rat offspring. Front. Psychiatry. 2017;8:77. doi: 10.3389/fpsyt.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richetto J., et al. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr. Bull. 2014;40:351–361. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weber-Stadlbauer U., et al. Transgenerational modification of dopaminergic dysfunctions induced by maternal immune activation. Neuropsychopharmacology. 2021;46:404–412. doi: 10.1038/s41386-020-00855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reisinger S.N., et al. Maternal immune activation epigenetically regulates hippocampal serotonin transporter levels. Neurobiol. Stress. 2016;4:34–43. doi: 10.1016/j.ynstr.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meyer U., et al. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology. 2010;208:531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- 95.Xu D.-X., et al. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol. Lett. 2006;163:20–29. doi: 10.1016/j.toxlet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 96.Juckel G., et al. Impact of Poly I:C induced maternal immune activation on offspring's gut microbiome diversity – implications for schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;110 doi: 10.1016/j.pnpbp.2021.110306. [DOI] [PubMed] [Google Scholar]
- 97.Saadani-Makki F., et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am. J. Obstet. Gynecol. 2008;199:651.e1–651.e7. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krstic D., et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conway F., Brown A.S. Maternal immune activation and related factors in the risk of offspring psychiatric disorders. Front. Psychiatry. 2019;10:430. doi: 10.3389/fpsyt.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richetto J., et al. Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol. Psychiatry. 2017;81:265–276. doi: 10.1016/j.biopsych.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Connor C.M., et al. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr. Res. 2012;140:175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang B., et al. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav. Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 103.Kim S., et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ronovsky M., et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav. Immun. 2017;63:127–136. doi: 10.1016/j.bbi.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 105.Pollak D.D., Weber-Stadlbauer U. Transgenerational consequences of maternal immune activation. Semin. Cell Dev. Biol. 2020;97:181–188. doi: 10.1016/j.semcdb.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Sommer I.E., et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2:16003. doi: 10.1038/npjschz.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almond D. Is the 1918 influenza pandemic over? Long term effects of in utero influenza exposure in the post-1940 U.S. Population. J. Pol. Econ. 2006;114:672–712. [Google Scholar]
- 108.Fletcher J.M. The effects of in utero exposure to the 1918 influenza pandemic on family formation. Econ. Hum. Biol. 2018;30:59–68. doi: 10.1016/j.ehb.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richter A., Robling P.O. Multigenerational effects of the 1918-19 influenza pandemic in Sweden. Swedish Inst. Soc. Res. (working paper series) 2013;5:1–45. [Google Scholar]
- 110.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delcuve G.P., et al. SARS-CoV-2 multifaceted interaction with human host. Part I: What we have learnt and done so far, and the still unknown realities. IUBMB Life. 2020;72:2313–2330. doi: 10.1002/iub.2380. [DOI] [PubMed] [Google Scholar]
- 112.Yang L., et al. The signal pathways and treatment of cytokine storm in COVID-19. Signal. Transduct. Target. Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen H., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salem D., et al. COVID-19 infection in pregnant women: review of maternal and fetal outcomes. Int. J. Gynecol. Obstet. 2021;152:291–298. doi: 10.1002/ijgo.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sinaci S., et al. Vertical transmission of SARS-CoV-2: a prospective cross-sectional study from a tertiary center. J. Med. Virol. 2021;93:5864–5872. doi: 10.1002/jmv.27128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Flores V., et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022;13:320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gee S., et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021;22:1490–1502. doi: 10.1038/s41590-021-01049-2. [DOI] [PubMed] [Google Scholar]
- 118.Chen R., et al. Interactions between specific immune status of pregnant women and SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2021;11:1–11. doi: 10.3389/fcimb.2021.721309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allotey J., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arrode-Brusés G., Brusés J.L. Maternal immune activation by poly I:C induces expression of cytokines IL-1β and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J. Neuroinflammation. 2012;9:83. doi: 10.1186/1742-2094-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi G.B., et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith S.E.P., et al. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007;27:10695. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rudolph M.D., et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018;21:765–772. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:320–327. doi: 10.1097/MCO.0b013e32835e8d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stock S.J., et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022;28:504–512. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shou S., et al. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.626553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo J., et al. An update on the relationship of SARS-CoV-2 and male reproduction. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.788321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Douglas G.C., et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 129.Peirouvi T., et al. COVID-19 disrupts the blood–testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm. Res. 2021;70:1165–1175. doi: 10.1007/s00011-021-01497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carlsen E., et al. History of febrile illness and variation in semen quality. Hum. Reprod. 2003;18:2089–2092. doi: 10.1093/humrep/deg412. [DOI] [PubMed] [Google Scholar]
- 131.Yang M., et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur. Urol. Focus. 2020;6:1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Achua J.K., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J. Mens. Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H., et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:1–8. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He Y., et al. Effect of COVID-19 on male reproductive system - a systematic review. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.677701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gonzalez D.C., et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]