Abstract
AMD-3100, a bicyclam, is a novel agent that uniquely inhibits the entry of human immunodeficiency virus type 1 (HIV-1) into CD4+ T cells via selective blockade of the chemokine CXCR-4 receptor. Twelve healthy volunteers were given AMD-3100 as a single 15-min intravenous infusion at 10, 20, 40, or 80 μg/kg. Five subjects also received a single subcutaneous injection of AMD-3100 (40 or 80 μg/kg). Three subjects received two escalating oral doses each (80 and 160 μg/kg). All subjects tolerated their dose(s) well without any grade 2 toxicity or dose adjustment. Six subjects experienced mild, transient symptoms, primarily gastrointestinal in nature and not dose related. All subjects experienced a dose-related elevation of the white blood cell count, from 1.5 to 3.1 times the baseline, which returned to the baseline 24 h after dosing. AMD-3100 demonstrated dose proportionality for the maximum drug concentration in serum (Cmax) and the area under the concentration-time curve from 0 h to ∞ (AUC0–∞) over the entire dose range. At the highest intravenous dose (80 μg/kg), the median Cmax was 515 (range, 470 to 521) ng/ml and the AUC0–∞ was 1,044 (range, 980 to 1,403) ng-h/ml. The median systemic absorption after subcutaneous dosing was 87% (range, 67 to 106%). No drug was detectable in the blood following oral dosing. Using a two-compartment model, the median pharmacokinetic parameter estimates (ranges) were as follows: volume of distribution, 0.34 (0.27 to 0.36) liter/kg; clearance, 1.30 (0.97 to 1.34) liters/h; elimination half-life, 3.6 (3.5 to 4.9) h. After a single, well-tolerated intravenous dose of AMD-3100, concentrations were sustained for 12 h above the in vitro antiretroviral 90% inhibitory concentrations and for 8 h above antiviral concentrations identified in the SCID-hu Thy/Liv mouse model of HIV infection.
Current anti-human immunodeficiency virus (HIV) therapy employs drugs that inhibit HIV replication via reverse transcriptase or protease inhibition (9). Although these two classes of antiretroviral drugs decrease the HIV load and prolong life, eradication of infection has not been achieved due, in part, to the viral reservoirs remaining in blood and infected tissue (5, 30, 33). This problem is exacerbated by viral resistance to the triple combination of drugs currently used for first-line treatment of HIV infection and cross-resistance to drugs within the same class. The identification of new classes of antiretroviral drugs with unique mechanisms of action therefore remains an important therapeutic objective.
The bicyclam AMD-3100 (Fig. 1) exhibits potent and selective inhibition of HIV type 1 (HIV-1) and HIV-2 replication by binding to the chemokine receptor CXCR4, the receptor used by T-tropic (X4) HIV in addition to CD4 for membrane fusion and entry into the cell (12, 14, 20, 25, 26). AMD-3100 does not bind to the other physiologically relevant chemokine receptor, CCR5, which has been found to mediate the entry of macrophage-tropic (R5) HIV (2, 6, 11, 13, 25). The coreceptor used by the virus for entry (or viral tropism) appears to correlate with disease progression. Whereas the most commonly transmitted strains are those with the macrophage-tropic, non-syncytium-inducing phenotype which utilize CCR5 for entry, the T-tropic, syncytium-inducing strains that use CXCR4 are rarely transmitted (15, 29, 34). The X4 strains are considerably more pathogenic, and their appearance during an extended time course of infection correlates with a decline in the CD4+ T-cell count and more rapid disease progression (7, 27, 28, 29). In vitro, AMD-3100 inhibits replication of T-tropic (syncytium-inducing) strains of HIV-1 and HIV-2 with a 90% effective concentration of below 1.0 to 10 ng/ml and provides complete (>99.9%) protection of lymphocytes and monocytes against HIV-1 at concentrations of 10 to 30 ng/ml, 4 orders of magnitude below its 50% cytotoxic concentration (10). AMD-3100 also inhibits syncytium formation between cocultures of persistently HIV-1 (IIIB)-infected HUT-78 cells and uninfected MOLT-4 cells, albeit at higher concentrations (1 to 5 μg/ml) than those required to inhibit viral replication (10). Resistance to AMD-3100 develops relatively slowly in serial passage studies in vitro (200-fold increased 50% inhibitory concentration [IC50] after 50 to 60 passages) when evaluated in parallel with TIBO (4,5,6,7-tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2[1H]-one) compounds (200-fold increased IC50 after 10 passages; AnorMED, data on file).
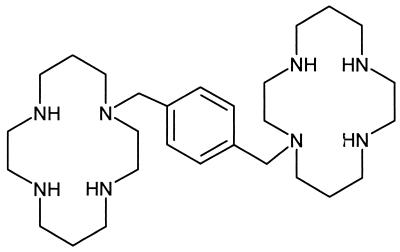
FIG. 1.
Structure of AMD-3100, 1,1′-[1,4-phenylenebis(methylene)]-bis-1,4,8,11-azatetradecane.
Preclinical animal studies have also suggested potential efficacy for AMD-3100. Using the SCID-hu Thy/Liv mouse model of HIV infection, multiple dosages of AMD-3100 were tested for the ability to decrease HIV loads (8). AMD-3100 was administered by daily injection for 2 weeks. The viral load in the transplanted thymocytes was then determined to have been decreased to a statistically significant degree at dosages at and above 1 mg/kg/day. In continuous-infusion studies with the SCID-hu mouse model, a similar total daily dose was found to be efficacious, resulting in statistically significant viral load reductions as measured by p24 antigen production (1.1 mg/kg/day, P = 0.004; 3.8 mg/kg/day, P = 0.003). Continuous infusion resulted in mean drug concentrations in plasma of <40 ng/ml (1.1 mg/kg/day) and 117 ng/ml (3.8 mg/kg/day).
In preclinical studies with rats, single oral doses (20 μg/kg) of AMD-3100 were poorly and variably absorbed (oral bioavailability, 3.9% [range, 0.5 to 9.6%]; AnorMED, data on file). The half-life of the total radioactivity of the parent drug was 0.9 h after a single intravenous dose and 0.7 to 0.8 h after repeated dosing. Renal clearance (CL) was the primary route of excretion; 72 and 63% of an intravenous dose was recovered in the urine of the rat and dog, respectively. In a 4-week subcutaneous dosing study with rats and dogs over a range of doses (0.25, 1, and 4 mg/kg/day) maximum drug concentrations in plasma occurred within 1 to 2 h with dose proportionality. In safety studies with rats and dogs, the maximum levels without observable adverse effects were 600 and 250 μg/kg, respectively. Adjusted for human surface area, this was estimated to be 120 and 125 μg/kg, respectively, in rats and dogs. In rats, the limiting toxicities included sedation, spasms, and dyspnea. In the dog, diarrhea and tachycardia were dose limiting.
In light of the need for new anti-HIV drugs, the preclinical efficacy of AMD-3100, and its reasonable toxicity profile, we undertook this phase I clinical investigation of AMD-3100. We report the first use of AMD-3100, the first HIV chemokine coreceptor blocker, in humans.
MATERIALS AND METHODS
Study design and population.
This was a phase I, single-center, single-dose, open-label dose escalation study of AMD-3100 with healthy volunteers using intravenous, subcutaneous, and oral doses. The intravenous dose was escalated after three subjects in the preceding cohort had received their dose without grade 3 or 4 toxicity (World Health Organization [WHO] scale). A total of 12 subjects intravenously received doses of 10, 20, 40, and 80 μg/kg. Some of these subjects were readmitted for subcutaneous or oral dosing as described below. The protocol was approved by the local Institutional Review Board, and all subjects gave written informed consent prior to their participation in this study.
Subjects were healthy, HIV-seronegative men and women 18 years of age or older with no active medical illness by history, physical, or laboratory evaluation. No concomitant medications were allowed 2 weeks prior to and throughout the study period. Exceptions were considered on a case-by-case basis only for drugs with a short half-life, and their use was discontinued 3 days prior to the study.
Procedures.
Prior to study enrollment, prospective volunteers were screened to assess their health by way of history and physical examination and laboratory evaluations, including HIV testing. Eligible subjects were admitted to the General Clinical Research Center for 2 nights. The dose of AMD-3100 was prepared from a standard solution and diluted with 0.9% saline in 50 ml to achieve the scheduled dose amount. The amount of AMD-3100 to be administered was determined by the volume of the 1-mg/ml solution added to the 50-ml bag. After an overnight fast, subjects were administered a single dose of AMD-3100 by 15-min intravenous infusion. Breakfast was withheld for 1 h after the dose.
Blood was collected for AMD-3100 analysis predosing, at the end of the infusion (approximately 15 min), and 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h postdosing. Subjects were monitored for electrocardiographic changes continuously via telemetry and observed frequently for adverse events during the inpatient period. Collections of blood and urine were made for safety evaluations on both inpatient days. Subjects were discharged from the hospital on the second morning and returned 1 week later for follow-up evaluation, which included an interim history, a directed physical examination, and laboratory evaluations (blood and urine). Dose escalations in the intravenous dosing cohorts proceeded to the next scheduled level if no more than one subject experienced dose-limiting toxicity (WHO grade 3 or 4).
Subjects from the higher-dose (40- and 80-μg/kg) cohorts were readmitted after they gave informed consent for a second, identical dose, this time by periumbilical subcutaneous injection. Sampling and safety monitoring were performed as for the intravenous dose observations, with the exception of the postinfusion blood sample.
Three of the 12 original subjects were readmitted on two occasions for a single oral dose of 80 μg/kg and then a single 160-μg/kg dose. The intravenous formulation used for these doses was prepared as follows. Distilled water was added to the stock intravenous AMD-3100 solution to make a 20-ml volume, which was squirted onto the back of the tongue, swallowed, and followed by 180-ml of water. Safety monitoring and blood sampling were performed as for the subcutaneous dosing cohort.
AMD-3100 assay.
Concentrations of AMD-3100 in plasma were determined by high-pressure liquid chromatography with electrochemical detection using a validated assay (Phoenix International Life Sciences, Inc., Saint Laurent, Quebec, Canada). Following addition of the internal standard (AMD-2763), plasma samples were extracted with methyl-t-butyl ether, followed by acidification with trifluoroacetic acid and back extraction. Chromatography was performed with an Isochrom pump and a PLRP-S column (5 μm; 250 by 4 mm) using an acetonitrile-water (33.7-65.5%) mobile phase containing 0.5% t-butyl ammonium hydroxide, 0.3% sodium lauryl sulfate, and EDTA. Electrochemical detection was carried out using a Coulochem II electrochemical detector. AMD-3100 standards were prepared in the range of 5 to 250 ng/ml using pooled human plasma (lower limit of quantification, 5 ng/ml). Standards and samples were extracted and assayed in the same manner using an internal standard (AMD-2763). Quality control samples (15, 120, and 200 ng/ml) were used to determine interday precision and accuracy. The quality control sample interday precision (percent coefficient of variation [%CV]) ranged from 4.4 to 8.6%. The interday accuracy (percent nominal concentration) ranged from 97.9 to 100.4%. Intraday precision and accuracy (from assay validation) ranged from 1.1 to 5.7% and from 96.5 and 98.4%, respectively. Correlation coefficients for standard curves from sample analysis runs were equal to or greater than 0.9914 for AMD-3100.
Pharmacokinetic analysis.
All compartmental and noncompartmental analyses were performed using WinNonlin Professional (version 2.0) software (Pharsight, Cary, N.C.). Individual pharmacokinetic parameters were calculated and then summarized by dose cohort for dose proportionality evaluations. The maximum drug concentration in serum (Cmax) and the time to Cmax were determined directly from the concentration-time curves. The area under the concentration-time curve extrapolated to infinity (AUC0–∞) was calculated using the log-linear trapezoidal rule. Terminal elimination half-life was determined by linear regression of the log-transformed final concentration-time points.
Compartmental parameter estimates for AMD-3100 were determined for each individual after fitting of concentration data to one-, two-, and three-compartment models. Final model selection was based on goodness-of-fit comparisons among the models using several criteria, including, among others, visual inspection of the data, the CV of parameter estimates, condition numbers, the Aikake information criterion, and the Schwartz criterion (16). Various weighting schemes were used (1/C, 1/C2, actual and estimated), but there was no improvement in the final model, so the simplest unweighted results are shown.
Subcutaneous absorption and oral bioavailability were calculated as the AUC0–∞ following subcutaneous or oral dosing divided by the AUC0–∞ following intravenous dosing. Results were expressed as percentages.
RESULTS
Enrollment.
Thirteen healthy volunteers were enrolled, and 23 total doses were administered as follows: 12 by intravenous infusion (three subjects each at 10, 20, 40, and 80 μg/kg), 5 by subcutaneous injection (two subjects at 40 μg/kg and three at 80 μg/kg), and 6 by oral dosing (three subjects each received 80- and 160-μg/kg doses). A subject enrolled in the 40-μg/kg subcutaneous cohort was not able to return for personal reasons. A thirteenth subject was enrolled in the 80-μg/kg intravenous cohort, but during the infusion, the intravenous tubing leaked an uncertain volume. He completed the protocol but was not included in any pharmacokinetic analyses, given the uncertainty of the actual dose received, although his Cmax and AUC0–∞ suggest a dose between 40 and 80 μg/kg. He experienced no adverse events.
Clinical effects.
The study drug was well tolerated by all subjects. No clinically significant changes in physical examination results, vital signs, or cardiac telemetry were identified. There were no serious adverse events, and no dose-limiting toxicity was identified.
Symptomatic changes from the baseline occurred in 6 (50%) of 12 subjects receiving parenteral doses; all were transient and mild (WHO grade 1) and required no intervention (Table 1). The most common complaints of subjects were gastrointestinal and included bloating, increased stool frequency, loose stools, and a feeling of excess lower abdominal gas though not increased flatulence. Several subjects had a headache or dry mouth. The timing and nature of the gastrointestinal symptoms were similar after both intravenous and subcutaneous dosing in two of the five subjects who received doses by both routes. No dose-response relationship could be identified for symptoms. Among the three subjects receiving oral doses, two (67%) developed new symptoms which included lightheadedness and loose bowel movements in one subject and headache in the other.
TABLE 1.
Clinical and laboratory changes from baseline
| Dose (μg/kg) and subject no. | Change(s)a after drug administered:
|
|
|---|---|---|
| Intravenously | Subcutaneously | |
| 10 | ||
| 1 | Headache | |
| Dry mouth | ||
| Upper respiratory infection | ||
| 2 | None | |
| 3 | None | |
| 20 | ||
| 4 | Headache | |
| 5 | None | |
| 6 | None | |
| 40 | ||
| 7 | Cramps, frequent stools | Cramps, gas |
| 13% lactate dehydrogenase rise | ||
| 8 | Loose stool | |
| 9 | Dry mouth | Dry mouth |
| Bloating, gas, diarrhea | Abdominal distention | |
| 13% Mg2+ rise | ||
| 80 | ||
| 10 | None | None |
| 11 | None | None |
| 12 | Diaphoresis | None |
All changes were grade 1.
White blood cell (WBC) counts were elevated relative to the baseline in all subjects receiving intravenous or subcutaneous dosing. This increase ranged from 1.5 to 3.1 times the baseline WBC count; the WBC counts largely returned to the baseline at 24 h (Fig. 2). The peak WBC count elevation (6 h in all subjects) lagged behind the peak drug concentration in blood (0.25 to 1.20 h). Elevations in polymorphonuclear leukocytes, lymphocytes, and monocytes were seen based on electronic differential analysis. There was no increase in immature band forms based on a manual differential analysis performed in all cases of leukocytosis. The magnitude of the WBC count elevation increased with increasing doses across the lower-dose cohorts, but the peak effects observed at 6 h after dosing were similar with 40 μg/kg (ratio, 2.72 ± 0.41) and 80 μg/kg (ratio, 2.67 ± 0.20), the two highest doses.
FIG. 2.
WBC ratio versus time compared to AMD-3100 concentration versus time. Each line represents a different AMD-3100 dose cohort given 10 to 80 μg/kg by a short intravenous infusion. The WBC ratio is calculated as the ratio of WBC count at a given time to the predose WBC count. All values are displayed as the mean ± the standard deviation.
Urine calcium was slightly elevated in several subjects based on normal ranges established by the reference laboratory. These were not dose related. One 40-μg/kg subject experienced a 13% rise in lactate dehydrogenase (Table 1). Another subject in the same dose cohort had a magnesium elevation of 13%. Both of these elevated levels returned to the baseline by the 1-week follow-up evaluation. All other laboratory abnormalities were either less than a 10% change from the normal limits, an abnormal baseline, or limited to the 1-week follow-up evaluation without previous changes.
Noncompartmental pharmacokinetics.
AMD-3100 concentration-versus-time plots of the 12 evaluable intravenous infusion subjects demonstrated parallel decays after the single dose (Fig. 3). The rate of decay changed over time, evidencing at least biexponential logarithmic decay. The interindividual variability in concentrations was small, and the dose cohorts were discretely separate from each other. The highest-dose (80-μg/kg) cohort reached a median peak concentration of 515 (range, 470 to 521) ng/ml (Table 2). The Cmax and AUC0–∞ demonstrated dose proportionality across the four dose levels (Fig. 4).
FIG. 3.
AMD-3100 concentration versus time (log10) following a 15-min intravenous infusion at four dose levels. The inset shows the linear concentration-versus-time curve. All values are the mean ± the standard deviation.
TABLE 2.
Pharmacokinetic parameters by dose cohort after intravenous infusion
| Dose (μg/kg) and subject no. | Wt (kg) | Noncompartmental model
|
Compartmental modela
|
|||||
|---|---|---|---|---|---|---|---|---|
| Maximum concn (ng/ml) | AUC0–∞ (ng-h/ml) | AUC/dose (103) | CL (ml/min/kg), % CV | Vol of distributionb (liters/kg), %CV | Distribution half-life (h), %CV | Elimination half-life (h), %CV | ||
| 10 | ||||||||
| 1 | 107 | 48 | 96 | 0.09 | 1.73, 3 | 0.30, 4 | 0.19, 14 | 2.3, 6 |
| 2 | 73 | 56 | 86 | 0.12 | 1.97, 4 | 0.27, 6 | 0.11, 28 | 1.8, 8 |
| 3 | 60 | 38 | 73 | 0.12 | 2.23, 5 | 0.50, 7 | 0.30, 13 | 3.2, 13 |
| 20 | ||||||||
| 4 | 95 | 96 | 232 | 0.12 | 1.43, 1 | 0.34, 2 | 0.59, 4 | 3.6, 3 |
| 5 | 75 | 119 | 213 | 0.14 | 1.63, 5 | 0.36, 9 | 0.24, 31 | 3.1, 12 |
| 6 | 92 | 118 | 274 | 0.15 | 1.23, 3 | 0.27, 4 | 0.25, 24 | 2.8, 6 |
| 40 | ||||||||
| 7 | 62 | 241 | 526 | 0.21 | 1.30, 3 | 0.32, 6 | 0.46, 20 | 3.6, 8 |
| 8 | 84 | 369 | 617 | 0.18 | 1.03, 7 | 0.27, 11 | 0.17, 37 | 3.5, 11 |
| 9 | 82 | 268 | 528 | 0.16 | 1.32, 4 | 0.33, 7 | 0.35, 26 | 3.6, 9 |
| 80 | ||||||||
| 10 | 73 | 515 | 1,044 | 0.18 | 1.30, 4 | 0.31, 7 | 0.44, 19 | 3.6, 9 |
| 11 | 104 | 470 | 1,403 | 0.17 | 0.97, 4 | 0.34, 7 | 0.74, 30 | 4.9, 9 |
| 12 | 91 | 521 | 980 | 0.14 | 1.34, 4 | 0.36, 7 | 0.17, 25 | 3.6, 7 |
| Median values | ||||||||
| 10 | 73 | 48 | 86 | 0.12 | 1.97, 4 | 0.30, 6 | 0.19, 14 | 2.3, 8 |
| 20 | 92 | 118 | 232 | 0.14 | 1.43, 3 | 0.34, 4 | 0.25, 24 | 3.1, 6 |
| 40 | 82 | 268 | 528 | 0.18 | 1.30, 4 | 0.32, 7 | 0.35, 26 | 3.6, 9 |
| 80 | 91 | 515 | 1,044 | 0.17 | 1.30, 4 | 0.34, 7 | 0.44, 25 | 3.6, 9 |
Two compartment model results are partially shown. %CV is for a given parameter fit to individual data (values of <20% are typically statistically significant). The one compartment model fit data much less well. The three-compartment model fit marginally better than the two-compartment model.
Volume of distribution = mean residence time · CL.
FIG. 4.
Dose proportionality of AMD-3100 pharmacokinetics (AUC0–∞ [AUCinf] and Cmax) across the four intravenous dose levels studied. The solid and dotted lines represent the mean and 95% confidence intervals, respectively, for the ordinary least-squares linear regression.
Terminal half-life increased and CL declined over the two lower-dose cohorts, stabilizing with the two higher-dose (40- and 80-μg/kg) cohorts. Inspection of the concentration-time plots (Fig. 3) showed that the concentrations in the lower-dose cohorts dropped below the limit of assay quantitation (5 ng/ml) at the later sampling times, obscuring final elimination rate changes seen in higher-dose cohorts. Based on compartmental analyses (see below), the final measurable levels in the lower-dose cohorts were still within the distribution phase.
Compartmental pharmacokinetics.
One-, two-, and three-compartment models were fitted to the individual-subject data and compared. Different weighting schemes were evaluated, but they did not reduce the variance in the parameter estimates and so were not used in the final analyses. The lack of later time points for the lower-dose cohorts noted above prevented fitting of the data from these subjects to a three-compartment model, so comparisons between models were made using the 40- and 80-μg/kg dose cohorts. The data fit a three-compartment model better than a two-compartment model, judging by several criteria (including the Aikake information criterion and the Schwartz criterion), but the difference was very small. When doing continuous-infusion simulations based on the pharmacokinetic parameter estimates of the 40- and 80-μg/kg cohort subjects, two- compared to three-compartment models yielded steady-state concentrations within ±5% of each other (data not shown). The one-compartment model was a poor fit with large residuals and a large standard error for all subjects. The simpler two-compartment model parameter estimates are summarized in Table 2.
Absorption and bioavailability.
Subcutaneous concentration-time profiles, when compared to intravenous profiles, showed much lower initial peaks during the period of absorption and distribution, followed by nearly overlapping values for the elimination phase. Subcutaneous dosing of AMD-3100 demonstrated a median absorption of 87% and median peak concentrations equivalent to 48% of the peak concentrations achieved after a 15-min intravenous infusion, with identical elimination rates (Table 3). Modeling of the subcutaneous data yielded inconsistent models across subjects, even within a dose cohort, and parameter estimates were highly variable (data not shown).
TABLE 3.
Pharmacokinetic parameters after subcutaneous dosing
| Subject no. | Dose (μg/kg) | Wt (kg) | Cmax (ng/ml), %IVa | Tmaxb (h) | AUC0–∞ (ng-h/ml), %IV |
|---|---|---|---|---|---|
| 7 | 40 | 63 | 103, 43 | 0.3 | 365, 67 |
| 9 | 40 | 83 | 145, 54 | 0.5 | 504, 96 |
| 10 | 80 | 73 | 258, 50 | 0.6 | 906, 87 |
| 11 | 80 | 102 | 226, 48 | 1.2 | 1,147, 82 |
| 12 | 80 | 89 | 229, 44 | 1.2 | 1,029, 106 |
| Median | 40 | 73 | 124, 48 | 0.4 | 435, 81 |
| 80 | 89 | 229, 48 | 1.2 | 1,029, 87 |
%IV, percentage of value obtained by intravenous administration.
Tmax, time to Cmax.
No AMD-3100 was detected after oral dosing in the six subjects who received both intravenous and oral doses (limit of assay detection, 5 ng/ml).
DISCUSSION
AMD-3100 was well tolerated by the healthy volunteers in this single-dose study; all adverse events were mild and reversible. Lack of a contemporaneous placebo group somewhat limits our ability to attribute specific symptoms to the drug. The reported effects are not dissimilar in frequency or type to the effects reported in orally dosed subjects (who had no detectable AMD-3100 in their blood). The only laboratory abnormalities noted were WBC count elevations (discussed below) and findings of urine calcium just beyond the reported normal range. However, because the diets were not controlled and urine sodium was not consistently assessed, interpretation of these urine calcium results is problematic.
The leukocytosis noted in all subjects receiving parenteral AMD-3100 was reversible and dose related, with evidence of saturation noted at higher doses just below a threefold elevation from the baseline. This is consistent with a demargination effect in which WBCs are released from attachment to the endothelial cell surface into the central circulation and therefore counted in assays of whole blood. There are several reports which suggest an increase in WBCs may be CXCR4 mediated. First, chemokines such as stromal cell-derived factor 1α (SDF-1α) are produced locally in tissue and their primary purpose is the trafficking and chemoattraction of lymphocytes (3, 22, 24). Second, whereas CCR5 is expressed on the surface of leukocytes, particularly monocytes, and T lymphocytes upon activation, CXCR4 is widely expressed (3). For example, in addition to leukocytes, T lymphocytes, and neutrophils, CXCR4 is expressed in human endothelial cells (17, 32) and microglia and neuronal cells in the brain (18, 19, 21). Third, in mechanistic experiments, AMD-3100 has been shown to completely inhibit binding of 125I-labeled SDF-1α to CXCR4 in the MT-2 cell line, to dose dependently inhibit signal transduction (indicated by an increase in calcium flux) in response to SDF-1α in SUP-T1 and THP-1 cells, and to completely block the response of THP-1 cells to the chemotactic effect of SDF-1α (4, 25). These combined observations suggest that binding of AMD-3100 to CXCR4 may inhibit the chemotactic effects of SDF-1α, causing release of WBCs from the endothelium and/or stem cells from bone marrow (1, 23, 31). In clinical use, this leukocytosis may complicate the interpretation of CD4 and CD8 concentrations.
Much of the time in the elimination phase, the drug was below the limit of detection of the assay in the two lower-dose cohorts. Accordingly, noncompartmental curve stripping to determine the terminal half-life yielded a falsely elevated estimate of the terminal half-life in the two lower-dose cohorts. This also resulted in inaccurate noncompartmental estimates of the volume of distribution and CL for these cohorts, since their calculations are dependent upon half-life estimates from curve stripping. Compartmental estimates of pharmacokinetic parameters, therefore, are most accurate, especially for the two highest-dose cohorts.
The in vitro IC90s for clinical HIV isolates were all less than 10 ng/ml, except for two of four non-syncytium-inducing isolates (10). Statistically significant p24 reductions (∼0.4 log) were seen with continuous infusions that achieved steady-state concentrations below 40 ng/ml (8). These in vitro and SCID-hu mouse antiviral concentrations were safely achieved or exceeded by all subjects in all parenteral-dose cohorts in this study, for periods ranging up to several hours, with minimal side effects. The half-life of AMD-3100 was 3.6 h in the higher-dose cohorts, much longer than expected based on the animal studies (0.7 to 1.7 h in dogs; AnorMED, data on file). Again, with reference to the preclinical anti-HIV responses noted above, in our subjects, concentrations remained above 30 ng/ml for 8 h and above 10 ng/ml for 12 h with a single 80-μg/kg intravenous dose. While we are encouraged by the tolerability of this drug when preclinical antiviral concentrations are sustained for several hours, the tolerability of sustained concentrations of AMD-3100 remains to be determined. Further, the potential antiviral impact of different protein binding and free-AMD-3100 concentrations in humans relative to SCID-hu mice is unknown. The volume of distribution of AMD-3100 is greater than typical for the blood volume and suggests that this drug is distributed widely throughout the body beyond the central (blood) compartment, probably indicating redistribution from plasma proteins to body fat, given the drug's high lipophilicity. Alternatively, AMD-3100 may bind to epithelial cells or other sites within the central (blood) compartment, therefore lowering the apparent AMD-3100 concentration detected from flowing blood sampled by venipuncture.
Because no AMD-3100 assay is validated for urine samples, we cannot draw any inferences about modes of metabolism or excretion. In preclinical multiple-dose studies of radiolabeled AMD-3100 with rats, 72% of the radioactivity appeared in the urine. Of these compounds in the urine, 70% of the radioactivity was recovered in the urine as the parent compound, representing 41% of the administered AMD-3100. There was also evidence of multiple metabolites (AnorMED, data on file).
Subcutaneous dosing yielded concentration profiles nearly identical to those obtained by intravenous dosing, with the exception of peak concentrations only half as high. Oral absorption, however, was not detectable at doses of up to 160 μg/kg, twice the size of the highest intravenous dose given. If the oral absorption profile were similar to that seen with subcutaneous dosing (Cmax 48% of intravenous Cmax) with a peak concentration at the limit of detection (5 ng/ml), the maximum oral bioavailability would be estimated to be <2%. In preclinical studies, oral bioavailability in rats was very low (3.9%) but not zero (AnorMED, data on file). Experiments with CACO-2 cells in vitro have demonstrated no transepithelial transport of AMD-3100 (29a).
We have completed the first human studies of AMD-3100, a chemokine receptor blocker with an antiviral mechanism of action completely different from that of drugs currently licensed for HIV infection. We achieved total drug concentrations, associated with an antiviral effect in preclinical SCID-hu mice studies that were sustained for 8 to 12 h after a single intravenous dose in our healthy volunteers. These single-dose-related concentrations were associated with only minimal side effects that were typically gastrointestinal in nature. The drug appears to be distributed widely throughout the body but is not orally absorbed to an appreciable degree. A reversible leukocytosis, maximally just below three times the baseline, is possibly related to interference with leukocyte-endothelial cell interactions. Clinical studies with HIV-infected patients are necessary and warranted to establish the antiviral effect, safety, and tolerability of AMD-3100 in that patient population.
ACKNOWLEDGMENTS
AnorMED, Inc., provided partial funding for this study. Charles Flexner serves as a consultant to AnorMed. The terms of this arrangement are being managed by The Johns Hopkins University. This work was partially supported by NIH General Clinical Research Center grant RR00052-38.
We recognize the significant contributions to the study by Elizabeth Cornell and by the Johns Hopkins General Clinical Research Center staff.
REFERENCES
- 1.Aiuti A, Webb I J, Bleul C, Springer T, Gutierrez-Ramos J C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan S Y, Speck R F, Power C, Gaffen S L, Chesebro B, Goldsmith M A. V3 recombinants indicate a central role for CCR5 as a coreceptor in tissue infection by human immunodeficiency virus type 1. J Virol. 1999;73:2350–2358. doi: 10.1128/jvi.73.3.2350-2358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1alpha and MIP-1beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 7.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datema R, Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune J M. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E. Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. J Med Chem. 1995;38:2491–2517. doi: 10.1021/jm00014a001. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, Thornton D, Skerlj R, Gaul F, Padmanabhan S, Bridger G, Henson G, Abrams M. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38:668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Este J A, Cabrere C, De Clercq E, Struyf S, Van Damme J, Bridger G, Skerlj R T, Abrams M J, Henson G, Gutierrez A, Clotet B, Schols D. Activity of Different bicyclam derivatives against human immunodeficiency virus depends on their interaction with the CXCR4 chemokine receptor. Mol Pharmacol. 1999;55:65–73. doi: 10.1124/mol.55.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Fenyo E M, Morfeldt-Manson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Asjo B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrielson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis. Stockholm, Sweden: Swedish Pharmaceutical Press; 1994. [Google Scholar]
- 17.Gupta S K, Lysko P G, Pillarisetti K, Ohlstein E, Stadel J M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 18.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 19.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1alphaα is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 20.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, De Clercq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1041. [PMC free article] [PubMed] [Google Scholar]
- 22.Locati M, Murphy P M. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–440. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 23.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 24.Ponath P D. Chemokine receptor antagonists: novel therapeutics for inflammation and AIDS. Exp Opin Investig Drugs. 1998;7:1–18. doi: 10.1517/13543784.7.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Schols D, Struyf S, Damme J V, Este J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schols D, Este J A, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res. 1997;35:147–156. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 27.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, De Goede R E, Van Steenwijk R P, Lange J M A, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tersmette M, de Goede R E Y, Al B J, Winkel I, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Van Gelder J, Witvrouw M, Pannecouque C, Henson G, Bridger G, Naesens L, De Clercq E, Annaert P, Shafiee M, Van den Mooter G, Kinget R, Augustijns P. Evaluation of the potential of ion pair formation to improve the oral absorption of two potent antiviral compounds, AMD3100 and PMPA. Int J Pharm. 1999;186:127–136. doi: 10.1016/s0378-5173(99)00150-7. [DOI] [PubMed] [Google Scholar]
- 30.van't Wout A B, Ran L J, Kuiken C L, Kootstra N A, Pals S T, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viardot A, Kronenwett R, Deichmann M, Haas R. The human immunodeficiency virus (HIV)-type 1 coreceptor CXCR-4 (fusin) is preferentially expressed on the more immature CD34+ hematopoietic stem cells. Ann Hematol. 1998;77:193–197. doi: 10.1007/s002770050442. [DOI] [PubMed] [Google Scholar]
- 32.Volin M V, Joseph L, Shockley M S, Davies P F. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 33.Wong J K, Ignacio C C, Torriani F, Havlir D, Fitch N J S, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]