ABSTRACT
This study comprehensively evaluated and compared three human rabies vaccines. Seven electronic databases were systematically searched. The Cochrane Handbook v5.1.0 was used to assess the risk of bias. A random-effects model was used to combine individual rates, and network meta-analysis was used for pairwise comparisons. Twenty-seven articles were included, with a total of 18,630 participants. The pooled incidence of the total adverse reaction to HDCV was significantly lower than that of PCECV. HDCV administration resulted in a lower incidence of local pain, fever, and weakness than purified Vero cell vaccine. HDCV caused a lower incidence of local pain and fever than PCECV. No significant difference was observed in terms of the seroconversion rate on day 7 or the rabies virus-neutralizing antibody titer on day 14. HDCV demonstrated superiority in terms of safety compared with the other two rabies vaccines, while the same was not observed in terms of immunogenicity.
KEYWORDS: Rabies vaccines, post-exposure prophylaxis, immunogenicity, adverse reaction, meta-analysis
Introduction
Rabies remains one of the deadliest zoonotic diseases worldwide and is caused by the rabies virus, with diffuse encephalomyelitis being the main pathological characteristic. Rabid dogs are one of the main sources of infection. People of different ages are susceptible to rabies infection, with a case fatality rate approaching 100% following the appearance of clinical symptoms.1,2 Globally, canine-transmitted rabies causes approximately 59,000 (95% confidence intervals (CI): 25–159,000) deaths each year and up to $8.6 billion (95% CI: $2.9–21.5 billion) economic losses.3 Although there is currently no effective treatment for rabies, potential infections can be prevented using a series of clinical methods. Rabies exposure is divided into three categories according to the World Health Organization (WHO). For category II exposure and the above, in addition to thorough wound management, victims should also be vaccinated with a qualified rabies vaccine following the prescribed vaccination regimen. Rabies immune globulin (RIG) must be administered via injection to those under category III.4 According to the vaccination site (intramuscular or intradermal) and the injection frequency, there are various vaccination regimens, including the five-site Essen regimen, four-site Zagreb regimen, and eight-site Thai Red Cross regimen. Among them, as the gold standard, the Essen regimen (intramuscular injection) has been widely applied worldwide.5
The first rabies vaccine was developed by Louis Pasteur in 1885 using the dried spinal cord of rabbits suffering from rabies.6 With the continuous improvement and innovation of vaccine production technology, most rabies vaccines produced worldwide are now cell-culture vaccines, including human diploid cell vaccine (HDCV), purified Vero cell vaccine (PVRV), and purified chick embryo cell vaccine (PCECV).7 Persistence of immunity after administration of the three rabies vaccines is satisfactory. According to the results of a recent randomized controlled trial (RCT) in China, the mean seroconversion rates are > 98% even after 10 years post the initial vaccination in both the HDCV and PVRV groups.8 The seroconversion rate can also reach 95% one year after initial vaccination using PCECV.9 As recommend by the WHO, the three rabies vaccines are widely used in Asia. PCECV has the lowest cost and is widely used in developing countries, especially India. Compared to HDCV, PVRV has been increasingly used in China in recent years due to higher cost of the former. Compared with HDCV, PVRV, and PCECV, rabies vaccines produced in animal cells are easier to manufacture and store; hence, they have a greater yield and are widely used in developing countries. However, HDCV is a vaccine cultured and manufactured using healthy human embryonic lung fibroblasts as a matrix. It is often used as a reference vaccine because it has no potential tumor-causing DNA residues or risk of foreign protein allergens, and is theoretically safer.10 Hence, vaccine safety and immunogenicity may be influenced by the cell types used for their production in vitro because of differences in the composition of rabies vaccines produced in different cell-type cultures.
To date, there have been several studies comparing different types of rabies vaccines, but no study has compared HDCV, PVRV and PCECV simultaneously. In particular, a large proportion of studies were based on healthy individuals,11–13 which cannot represent the real efficacy of vaccines in exposed populations. Therefore, we focused on post-exposure populations because post-exposure prophylaxis (PEP) is more common than pre-exposure prophylaxis (PrEP) in daily life. Data were collected from related studies, and the safety and immunogenicity of HDCV, PVRV, and PCECV were quantitatively evaluated and compared simultaneously through meta-analysis. Moreover, this study considered the adverse reaction (AR) rate as a measurable variable for safety, in addition to determination of seroconversion rates and rabies virus neutralizing antibody (RVNA) titer or concentration for immunogenicity.
Materials and methods
Search strategy
Chinese and English electronic databases were systematically searched from inception to November 30, 2021; the databases included the China National Knowledge Infrastructure, the Wanfang database, Sinomed, VIP, Web of Science, PubMed, and the Cochrane Library databases. The following search terms were used: “rabies,” “vaccine,” “effectiveness,” “safety,” “immunogenicity,” “adverse reactions,” “side effects,” “RCT,” “observational research.” There were no limitations on language. To avoid selection bias caused by the reviewer as much as possible, the above retrieval process and the literature screening and subsequent full-text reading process were independently completed and cross-checked by two reviewers. If there was a disagreement, an agreed consensus was arrived at after discussion or referencing a third-party opinion.
Study selection
Studies that met the following criteria were included in the review: (1) Types of rabies vaccines: research used HDCV and/or PVRV and/or PCECV to induce immunity; (2) Subjects: the subjects had a clear history of exposure to confirmed or suspected rabid animals and no history of vaccination; (3) Vaccination regimen: all subjects were vaccinated by the five-site Essen regimen viz. vaccinated intramuscularly on days 0, 3, 7, 14, and 28 or 30; (4) Study design: randomized controlled trial, prospective observational study, or retrospective study; (5) Results: study provided the outcome data, such as AR and/or seroconversion rates. Studies were excluded if (1) the subjects were special populations, such as the people with HIV/AIDS, infants, and pregnant women; (2) they were cell or animal experiments; and (4) they primarily focus on vaccine development and production technology.
Data extraction
Two researchers independently extracted data from the literature which was included in the meta-analysis. The data extracted include first author’s name, year of publication, country/region, study duration, inclusion and exclusion criteria for subjects, number of subjects, age distribution, sex ratio, vaccine type, exposure type, administration of rabies immunoglobulin (yes or no), and information related to the safety and immunogenicity of rabies vaccines. The measurement variables involving safety specifically included the incidence of total ARs and the incidence of some common local and systemic solicited ARs, including local pain, erythema, pruritus, edema, induration, headache, fever, myalgia, and weakness. Geometric mean titer or concentration (GMT or GMC) of RVNA in serum, seroconversion rates and their corresponding 95% CIs were metrics for immunogenicity.
Risk of bias assessment
Two reviewers independently assessed the risk of bias for each included article, according to the Cochrane Handbook V5.1.0. Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases are the seven domains of risk of bias. Each domain was judged as “low risk,” “uncertainty,” or “high risk” for each study.
Data analysis
Endnote X9 software was used for document screening and management. The extracted raw data were recorded in Excel 2016, and then it was imported into R v4.0.3 software for statistical analysis after a simple arrangement. First, a single-rate meta-analysis was conducted to evaluate the pooled total AR rates and seroconversion rates of HDCV, PVRV, and PCECV. Then, when we performed multiple comparisons among the three rabies vaccines, the method of adjusted indirect comparison was adopted, which is also known as network meta-analysis (NMA). The single-rate meta-analysis was conducted based on the “meta” package in R software. NMA was performed based on the Bayesian framework and was implemented by calling OpenBUGS v3.2.2 software through the “R2OpenBUGS” package in the R software. Relative risk (RR) was calculated for qualitative data, and the weighted mean difference (WMD) was calculated for quantitative data as the effect sizes. Statistical tests were based on 95% CIs. When the 95% CI contained 1 for RR, or the 95% CI included 0 for WMD, it indicated that there was no significant difference between the two rabies vaccines. Considering the universal heterogeneity of clinical and statistical methods between studies, we used random-effects models in both single-rate meta-analysis and NMA to ensure the rationality and universality of the pooled results. Begg’s test was used to determine whether there was publication bias. When the p-value was < 0.05, publication bias existed.
Results
Literature search
A total of 1,591 articles, including 19 manually retrieved reports, were retrieved from the electronic databases. After removing duplicate entries, 1,105 articles were screened based on the title and abstract. Furthermore, 125 articles that met the screening criteria had entered the stage of “Full-text articles assessed for eligibility.” Finally, 27 qualified articles were included in the systematic review and meta-analysis, including which 17 RCTs, nine prospective observational studies and one retrospective study. The literature screening process is illustrated in Figure 1.
Figure 1.
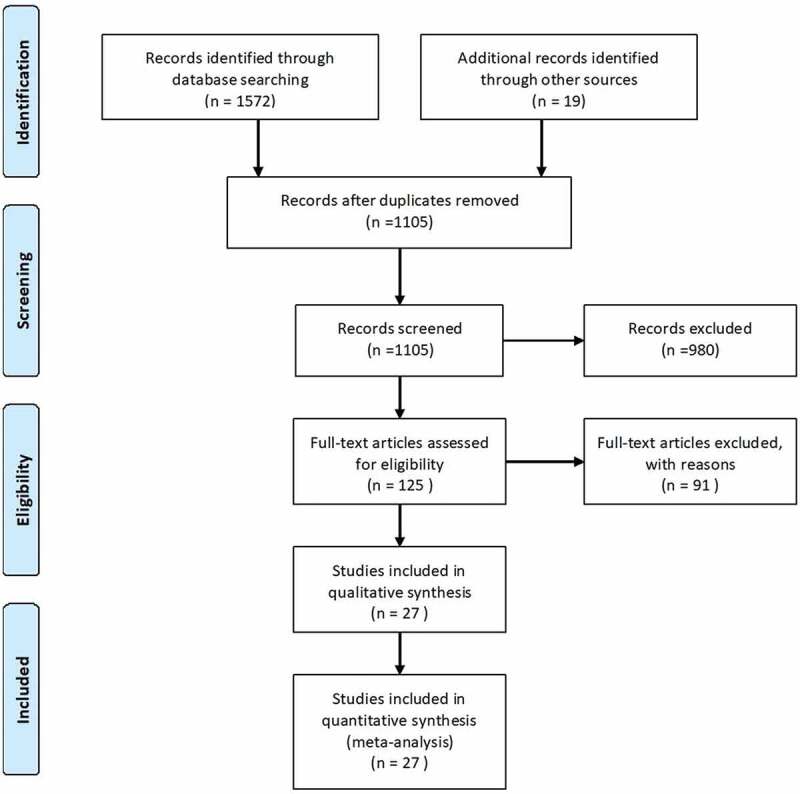
Flowchart of literature selection.
Research characteristics
Most of the studies included in the systematic review and meta-analysis were published over the past 20 years and were mainly distributed in Asia (China, India, Thailand, Iran, Pakistan, and the Philippines) and North America (the United States). A total of 18,630 subjects reported in the 27 documents14–40 were included in the meta-analysis, most of whom were between 10 and 60 years old, and all had different categories of rabies exposure. Rabies exposure was mostly categorized as category II or above. The characteristics of individual studies are presented in Table 1. Details of exposure categories and use of RIG are displayed in Stable 1, but most were not reported.
Table 1.
Study characteristics
| Study | Year | Country | Study designa | Number | Sex ratio (Male/Female) |
Age | Categories of exposure | Vaccine name/manufacturer | RIGb | Immunogenicityc | Safetyc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PVRVe | |||||||||||
| Li14 | 2020 | China | RCT | 150 | 0.92 | 10–60 | NRd | NR | - | √ | √ |
| Fan15 | 2019 | China | RCT | 200 | 0.92 | 40 ± 26.7 | NR | Guangzhou NuoCheng | NR | × | √ |
| Chen16 | 2018 | China | RCT | 200 | 1.17 | 2–68 | I & II | Guangzhou NuoCheng | - | × | √ |
| Peng17 | 2016 | China | RCT | 869 | NR | >2 | II | NR | NR | × | √ |
| Peng17 | 2016 | China | RCT | 941 | NR | >2 | II | NR | NR | × | √ |
| Peng17 | 2016 | China | RCT | 881 | NR | >2 | II | NR | NR | × | √ |
| Bose18 | 2016 | India | RCT | 60 | 3 | 5–77 | II & III | Rabivax-S | ± | √ | √ |
| Ramezankhani19 | 2016 | Iran | RCT | 702 | 3.74 | 26.8 ± 13.1 | II & III | Verorab | NR | × | √ |
| Fang20 | 2014 | China | RCT | 28 | NR | 19–60 | II | Liaoning ChengDa | NR | √ | √ |
| Wang21 | 2011 | China | RCT | 200 | 0.63 | 6–67 | NR | Speeda | NR | √ | × |
| Wang21 | 2011 | China | RCT | 50 | 1.27 | 16–78 | NR | Verorab | NR | √ | × |
| Liu22 | 2011 | China | RCT | 30 | 0.88 | 22–57 | NR | SPEEDA | NR | √ | √ |
| Ashwathnarayana23 | 2009 | India | RCT | 50 | 5.25 | 8–55 | II & III | Verorab | ± | √ | √ |
| Shu24 | 2007 | China | RCT | 300 | NR | >2 | II & III | Liaoning ShengWu | - | × | √ |
| Shu24 | 2007 | China | RCT | 300 | NR | >2 | II & III | Changchun ChangSheng | - | × | √ |
| Cao25 | 2007 | China | RCT | 1250 | 0.69 | 2–80 | I & II | Liaoning ChengDa | - | × | √ |
| Cao25 | 2007 | China | RCT | 1180 | 0.97 | 2–80 | I & II | Verorab | - | × | √ |
| Sampath26 | 2005 | Pakistan | RCT | 75 | NR | NR | II | Abhayrab | - | √ | × |
| Sampath26 | 2005 | Pakistan | RCT | 67 | NR | NR | III | Abhayrab | - | √ | × |
| Sampath26 | 2005 | Pakistan | RCT | 88 | NR | NR | III | Abhayrab | + | √ | × |
| Huang27 | 2018 | China | P. O. | 58 | 2.22 | NR | NR | NR | NR | × | √ |
| Liu28 | 2012 | China | P. O. | 398 | 1.17 | 2–67 | NR | Verorab | - | √ | √ |
| Lu29 | 2010 | China | P. O. | 300 | 0.99 | 3–65 | II | Verorab | - | × | √ |
| Niu30 | 2019 | China | R. O. | 5347 | NR | NR | NR | Changchun ChangSheng | NR | × | √ |
| PCECVf | |||||||||||
| Peng17 | 2016 | China | RCT | 813 | NR | >2 | II | NR | NR | × | √ |
| Bose18 | 2016 | India | RCT | 60 | 2.75 | 5–77 | II & III | Rabipur | ± | √ | √ |
| Ramezankhani19 | 2016 | Iran | RCT | 747 | 4.7 | 27.4 ± 13.9 | II & III | Rabipur | NR | × | √ |
| Fang20 | 2014 | China | RCT | 28 | NR | 19–60 | II | Rabipur | NR | √ | √ |
| Shao31 | 2013 | China | RCT | 400 | NR | 18–59 | II & III | Rabipur | NR | √ | √ |
| Ashwathnarayana23 | 2009 | India | RCT | 50 | 4 | 7–48 | II & III | Rabipur | ± | √ | √ |
| D.J. Briggs32 | 2000 | Thailand | RCT | 57 | 0.97 | 5–66 | II & III | NR | ± | √ | √ |
| Benjavongkulchai33 | 1997 | Thailand | RCT | 17 | NR | NR | I & II | Kaketsuken | - | √ | √ |
| Benjavongkulchai33 | 1997 | Thailand | RCT | 21 | NR | NR | III | Kaketsuken | + | √ | √ |
| Benjavongkulchai33 | 1997 | Thailand | RCT | 21 | NR | NR | III | Kaketsuken | + | √ | √ |
| Sirikun34 | 2018 | Thailand | P. O. | 29 | 0.45 | 19–73 | III | Rabipur | + | √ | √ |
| Narayana35 | 2014 | India | P. O. | 129 | 3.61 | 18–55 | II & III | Vaxirab-N | ± | √ | √ |
| Lu29 | 2010 | China | P. O. | 300 | 0.95 | 3–65 | II | NuoHua | - | × | √ |
| HDCVg | |||||||||||
| Li14 | 2020 | China | RCT | 150 | 0.86 | 12–60 | NR | NR | - | √ | √ |
| Fan15 | 2019 | China | RCT | 200 | 0.56 | 43 ± 28.9 | NR | Chendu KangHua | - | × | √ |
| Chen16 | 2018 | China | RCT | 200 | 1.3 | 2–68 | I & II | Kanghua | - | × | √ |
| Sudarshan36 | 2008 | India | RCT | 29 | 4 | 15–55 | II & III | Rabivax | ± | √ | √ |
| Sudarshan36 | 2008 | India | RCT | 16 | 4 | 15–55 | II & III | MIRV | ± | √ | √ |
| Sudarshan36 | 2008 | India | RCT | 148 | 3.11 | 5–55 | II & III | Rabivax | ± | √ | √ |
| Benjavongkulchai33 | 1997 | Thailand | RCT | 39 | NR | NR | III | NR | + | √ | √ |
| Yan37 | 2018 | China | P. O. | 700 | NR | 7–60 | NR | Kanghua | ± | × | √ |
| Huang27 | 2018 | China | P. O. | 53 | 1.79 | NR | NR | NR | NR | × | √ |
| Wilde38 | 1995 | Thailand | P. O. | 100 | 2.13 | 2–60 | III | NR | ± | √ | √ |
| Anderson39 | 1980 | America | P. O. | 90 | 1.5 | 1–83 | NR | Wyeth Laboratories | + | √ | √ |
| Bahmanyar40 | 1976 | Iran | P. O. | 45 | 3.1 | 3–90 | NR | Institute Merieux | ± | √ | × |
| Niu30 | 2019 | China | R. O. | 464 | NR | NR | NR | Kanghua | NR | × | √ |
a: RCT represents randomized controlled trial; P. O. represents reprospective observational; R. O. represents retrospective observational. b: RIG represents rabies immunoglobulin; ”-” represents no injection of RIG; ”+” represents injection of RIG; ”±” represents injection of RIG for part of the subjects. c: ”√” represents the study provide corresponding data; ”×” represents not. d: NR represent not reported. e: PVRV represents purified Vero cell vaccine. f: PCECV represents purified chick embryo cell vaccine. g: HDCV represents human diploid cell vaccine.
In the included studies, selection bias, performance bias, detection bias, and attrition bias were considered to be low risk for those studies that clearly introduced the use of randomized, controlled, and blinded methods without loss of follow-up. Otherwise, they were considered to be at high risk or unclear. The risk of other biases is unclear in studies that were not designed and conducted following the principles of randomized, controlled, and blinded methods. There was no reporting bias in any of the included studies. The bias assessment results are summarized in Figure 2.
Figure 2.
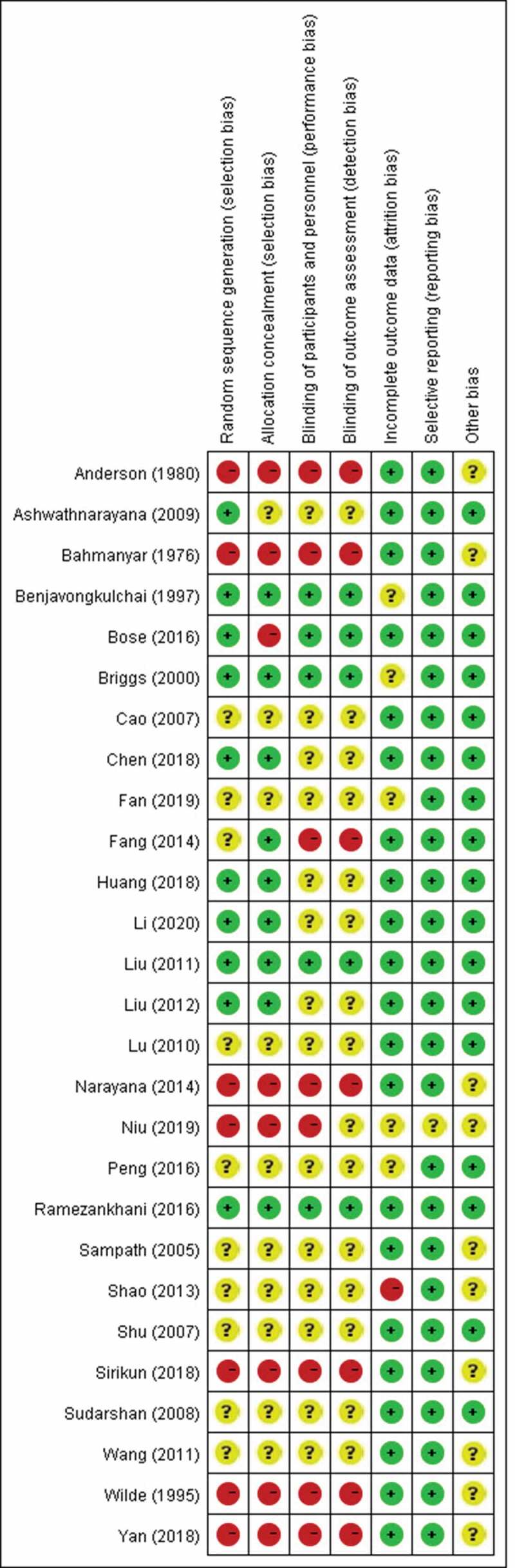
Quality assessment of included studies. “+” represents low risk of bias; “?” represents unclear risk of bias; “-” represents high risk of bias.
Safety
To describe the incidence of total ARs of the HDCV, PVRV, and PCECV in general, we first conducted a single-rate meta-analysis. The results are shown in Figure 3. Meta-analysis of single-rate data requires that the rate distribution follow a normal distribution as much as possible; hence, we performed an arcsine transformation of the original data that did not meet the normality. Data regarding the incidence of total ARs for HDCV, PVRV, and PCECV were obtained from seven,15,27,30,36–39 three,17,32,35 and seven15,17,24,25,27,28,30 articles, respectively. The results showed that the pooled incidence of total AR of HDCV was 3.2% (95% CI: 0.9%–10.9%), that of PVRV was 11.7% (95% CI: 5.2%–24.1%), and that of PCECV was 26.0% (95% CI: 16.4%–38.7%). The pooled incidence of total ARs was 8.4% (95%CI: 4.1%–16.3%) for the combination of the three rabies vaccines.
Figure 3.
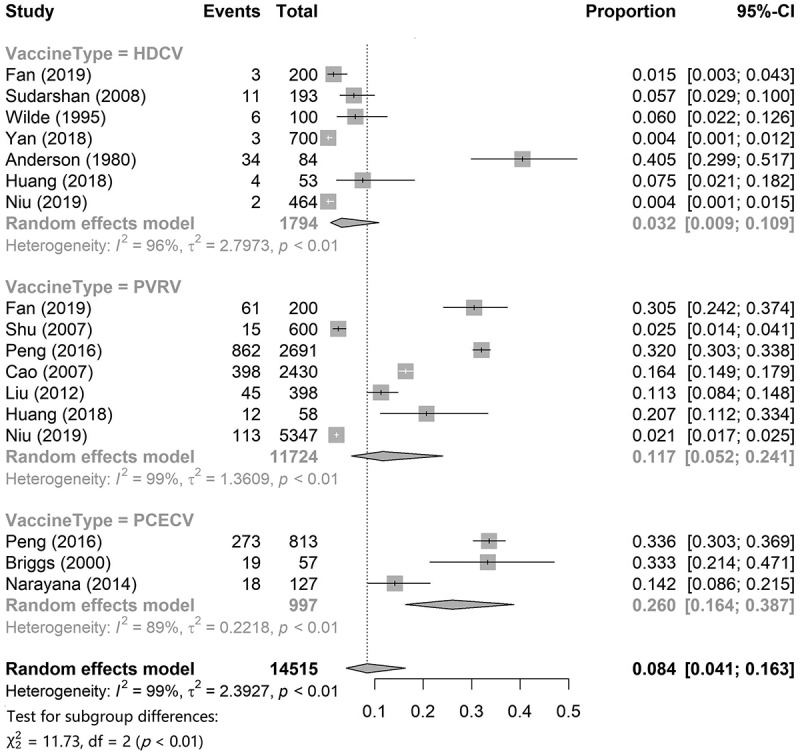
The pooled incidence of total ARs of HDCV, PVRV and PCECV.
Subsequently, we compared the incidence of solicited symptoms in local and systemic ARs. The results are shown in Table 2. The data used for conducting NMA were obtained from the studies by Bose,18 Benjavongkulchai,33 Fang,20 Ashwathnarayana,23 Ramezankhani,19 Li,14 Chen,16 Lu,29 and Huang.27 Comparing the HDCV and PVRV vaccines, we found that there was a statistically significant difference in the incidence of one local symptom and two systemic symptoms: local pain (RR = 0.51, 95% CI: 0.21–0.98), fever (RR = 0.20, 95% CI: 0.03–0.64), and weakness (RR = 0.25, 95% CI: 0.06–0.73). Thus, the incidence of local pain, fever, and weakness for HDCV were about one-half, one-fifth, and one-quarter of that for PVRV, respectively. Comparing HDCV and PCECV vaccines, we found statistically significant differences in the incidence of one local symptom and one systemic symptom: local pain (RR = 0.49, 95% CI: 0.20–0.95) and fever (RR = 0.27, 95% CI: 0.03–0.92). The incidence of local pain and fever for HDCV was approximately one-half and one-quarter that of PCECV, respectively. Comparing PVRV and PCECV, there was no significant difference in the incidence of any local and systemic symptoms, which indicated that the safety of the two was equivalent.
Table 2.
Multiple comparisons of 3 rabies vaccines about safety and immunogenicity (Case/Total)
| Study | Vaccine type | Safety |
Immunogenicity (D14)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local AR |
|
Systemic AR |
Sero conversion |
RVNA titer [GMT(95%CI) /Subjects, IU/ml] |
||||||||||
| Local pain | Erythema | Pruritus | Edema | Induration | Headache | Fever | Myalgia | Weakness | ||||||
| Benjavongkulchai (1997) | PCECV | 13/72 | 1/72 | 0/72 | 0/72 | 0/72 | 1/72 | 16/72 | 15/72 | 0/72 | 55/55 | 1.86(NR)/22 | ||
| Benjavongkulchai (1997) | HDCV | 3/40 | 1/40 | 0/40 | 0/40 | 0/40 | 1/40 | 0/40 | 0/40 | 0/40 | 38/39 | 3.10(NR)/39 | ||
| Fang (2014) | PVRV | 0/28 | 0/28 | 0/28 | 1/28 | 0/28 | 0/28 | 1/28 | 0/28 | 0/28 | NRb | NR | ||
| Fang (2014) | PCECV | 2/33 | 0/33 | 0/33 | 1/33 | 0/33 | 0/33 | 1/33 | 0/33 | 0/33 | NR | NR | ||
| Bose (2016), 1 | PVRV | 20/30 | 3/30 | 2/30 | 2/30 | 5/30 | 7/30 | 4/30 | 3/30 | 11/30 | 27/27 | 20.57(17.03 ~ 24.84)/27 |
||
| Bose (2016), 1 | PCECV | 21/31 | 0/31 | 3/31 | 3/31 | 4/31 | 5/31 | 2/31 | 5/31 | 9/31 | 29/29 | 16.01(12.46 ~ 20.57)/29 |
||
| Bose (2016), 2 | PVRV | 13/30 | 0/30 | 1/30 | 1/30 | 1/30 | 5/30 | 1/30 | 2/30 | 7/30 | 27/27 | 16.47(13.39 ~ 20.26)/27 |
||
| Bose (2016), 2 | PCECV | 9/31 | 0/31 | 0/31 | 1/31 | 1/31 | 2/31 | 0/31 | 2/31 | 4/31 | 29/29 | 14.13(11.42 ~ 17.47)/29 |
||
| Ashwathnarayana (2009) | PCECV | 1/50 | 0/50 | 1/50 | 0/50 | 0/50 | 0/50 | 0/50 | 0/50 | 0/50 | 50/50 | 6.88(6.11 ~ 7.75)/50 |
||
| Ashwathnarayana (2009) | PVRV | 1/50 | 0/50 | 1/50 | 0/50 | 0/50 | 0/50 | 0/50 | 0/50 | 0/50 | 48/48 | 6.65(5.91 ~ 7.49)/48 |
||
| Ramezankhani (2016) | PVRV | 27/702 | 9/702 | 7/702 | 4/702 | 0/702 | 8/702 | 11/702 | 6/702 | 5/702 | NR | NR | ||
| Ramezankhani (2016) | PCECV | 28/747 | 8/747 | 1/747 | 2/747 | 0/747 | 16/747 | 10/747 | 5/747 | 11/747 | NR | NR | ||
| Li (2020) | HDCV | 6/150 | 0/150 | 2/150 | 3/150 | 2/150 | 4/150 | 3/150 | 1/150 | 0/150 | 149/150 | 21.47(18.91 ~ 24.36)/150 |
||
| Li (2020) | PVRV | 7/150 | 0/150 | 1/150 | 3/150 | 1/150 | 4/150 | 3/150 | 0/150 | 1/150 | 148/150 | 20.78(18.21 ~ 23.71)/150 |
||
| Chen (2018) | HDCV | 7/200 | 2/200 | 2/200 | 2/200 | 5/200 | 1/200 | 3/200 | 0/200 | 5/200 | NR | NR | ||
| Chen (2018) | PVRV | 11/200 | 5/200 | 6/200 | 5/200 | 4/200 | 6/200 | 25/200 | 1/200 | 26/200 | NR | NR | ||
| Lu (2010) | PCECV | 19/300 | 1/300 | 11/300 | 1/300 | 4/300 | 4/300 | 3/300 | 0/300 | 8/300 | NR | NR | ||
| Lu (2010) | PVRV | 15/300 | 1/300 | 13/300 | 1/300 | 4/300 | 5/300 | 4/300 | 0/300 | 9/300 | NR | NR | ||
| Huang (2018) | HDCV | 1/53 | 0/53 | 0/53 | 0/53 | 0/53 | 1/53 | 1/53 | 0/53 | 1/53 | NR | NR | ||
| Huang (2018) | PVRV | 5/58 | 0/58 | 0/58 | 0/58 | 0/58 | 1/58 | 2/58 | 0/58 | 3/58 | NR | NR | ||
| Pooled PVRV | 99/1548 | 18/1548 | 31/1548 | 17/1548 | 15/1548 | 36/1548 | 51/1548 | 12/1548 | 62/1548 | 250/252 | - | |||
| Pooled PCECV | 93/1264 | 10/1264 | 16/1264 | 8/1264 | 9/1264 | 28/1264 | 32/1264 | 27/1264 | 32/1264 | 163/168 | - | |||
| Pooled HDCV | 17/443 | 3/443 | 4/443 | 5/443 | 7/443 | 7/443 | 7/443 | 1/443 | 6/443 | 263/267 | - | |||
| RR, 95%CI (HDCV vs. PVRV) | 0.51 (0.20 ~ 0.98)* |
0.79 (0.12 ~ 2.63) |
1.15 (0.17 ~ 3.72) |
1.04 (0.26 ~ 2.83) |
1.82 (0.41 ~ 5.19) |
0.74 (0.15 ~ 2.36) |
0.20 (0.03 ~ 0.64)* |
0.46 (0.02 ~ 2.10) |
0.25 (0.06 ~ 0.73)* |
1.00 (0.75 ~ 1.34) |
0.03 (−0.26 ~ 0.32) |
|||
| RR, 95%CI (HDCV vs. PCECV) | 0.49 (0.20 ~ 0.95)* |
1.58 (0.19 ~ 5.79) |
2.35 (0.24 ~ 9.99) |
1.33 (0.20 ~ 4.73) |
2.24 (0.38 ~ 6.86) |
1.60 (0.24 ~ 5.73) |
0.27 (0.03 ~ 0.92)* |
0.32 (0.01 ~ 1.36) |
0.48 (0.08 ~ 1.65) |
1.00 (0.68 ~ 1.46) |
0.16 (−0.17 ~ 0.49) |
|||
| RR, 95%CI (PVRV vs. PCECV) | 1.08 (0.65 ~ 1.87) |
0.71 (0.19 ~ 1.67) |
0.67 (0.18 ~ 1.66) |
1.07 (0.29 ~ 2.62) |
0.98 (0.34 ~ 2.25) |
0.57 (0.17 ~ 1.35) |
1.07 (0.27 ~ 2.91) |
1.82 (0.41 ~ 5.85) |
0.61 (0.24 ~ 1.21) |
1.00 (0.71 ~ 1.39) |
0.13 (−0.03 ~ 0.28) |
|||
a: Immunogenicity data were obtained 14 days after the first vaccination dose. b: NR represents not reported. *: Difference was statistically significant.
Immunogenicity
This study mainly focused on the seroconversion rate on day 7 and the RVNA titer or concentration on day 14. The data of seroconversion on day 7 of HDCV, PVRV, and PCECV were obtained from five,14,33,38–40 six,14,18,20–22,26 and five18,20,31,33,34 articles, respectively. Single-rate meta-analysis results (Figure 4) showed that the pooled seroconversion rate of HDCV was 35.7% (95% CI: 8.6%–69.3%), that of PVRV was 55.6% (95% CI: 22.6%–86.2%), and that of PCECV was 58.3% (95% CI: 15.0%–94.7%). The pooled seroconversion rate for the combination of the three rabies vaccines was 50.4% (95% CI: 29.6%–71.1%) for the combination of the three rabies vaccines. A total of three studies provided comparative results for RVNA titer or concentration for two rabies vaccines, among which Bose18 and Ashwathnarayana23 compared PVRV and PCECV, while Li14 compared HDCV and PVRV. The results of the adjusted indirect comparisons showed that there were no significant differences between HDCV and PVRV (WMD = 0.03, 95% CI = −0.26–0.32), HDCV and PCECV (WMD = 0.16, 95% CI = −0.17–0.49), and PVRV and PCECV (WMD = 0.13, 95% CI = −0.03–0.28).
Figure 4.
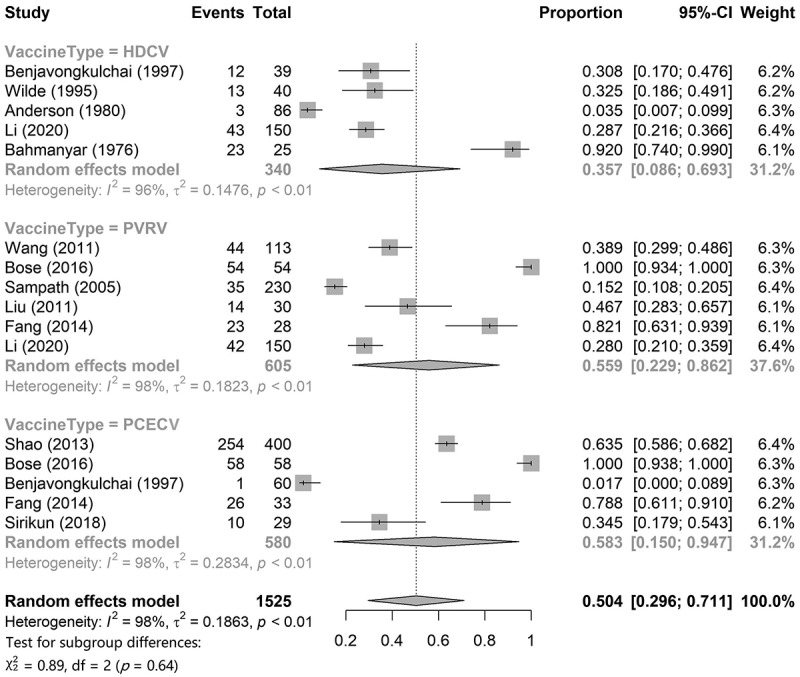
Pooled seroconversion rates on day 7 of HDCV, PVRV and PCECV.
Assessment of publication bias
Begg’s test was performed to identify whether there was publication bias or not in the studies used for conducting single-rate meta-analysis and NMA. All the p values were > 0.05, which indicating that the null hypothesis of funnel plot symmetry was not rejected. In other words, there was no publication bias.
Discussion
In this study, we performed a meta-analysis of immunogenicity and safety of three rabies vaccines widely administered to humans for immunization against rabies. The purpose and time of rabies vaccination can be divided into PrEP and PEP. PrEP is often applied to occupational populations or travelers with a high risk of rabies infection, or in epidemic areas of a rabies outbreaks.41 However, PEP is more common in daily life. Unlike for that in PrEP, vaccination is possibly the only life-saving option for post-exposure rabies populations. Therefore, although vaccination is only required for rabies exposures of category II and above according to WHO recommendations,4 it is not uncommon for people to receive rabies vaccination unnecessarily. In addition, both PrEP and PEP use rabies vaccine to induce fundamental immunity, whereas the vaccination regimens are entirely different. Based on the above considerations, we paid more attention to the post-exposure populations and excluded the studies based on healthy populations. Moreover, studies42,43 have shown that if individuals with a history of rabies vaccination are vaccinated again, the induced immune response levels are different from that of the initial vaccination. Therefore, individuals with a history of rabies vaccination were excluded from this study to minimize clinical heterogeneity between studies.
Immunogenicity and safety are two essential aspects for evaluating the merits and demerits of rabies vaccines. Factors affecting the immunogenicity and safety of rabies vaccines mainly include the nature of the vaccine itself, the characteristics of the individual vaccinated, and the vaccination regimen. A meta-analysis study previously compared differences in immunogenicity and safety between different vaccination regimens.5 However, no systemic review or meta-analysis compares the differences in immunogenicity and safety between different cell-culture rabies vaccines. The first rabies vaccine was developed more than a 100 years ago and since then different kinds of rabies vaccines have been released. The earliest neural tissue-derived vaccines (NTV) represented by the Pasteur vaccine have been eliminated because of their high incidence of neurological complications, long immune cycle, frequent injections, slow immune responses, and poor protective efficacy.44 Subsequently, most embryo-culture vaccines were also eliminated because of high AR rates and poor protective effects; only some of them are still produced because of their more straightforward manufacturing processes and lower costs, such as PCECV.45 Currently, most countries and regions have adopted cell-culture rabies vaccines represented by PVRV and HDCV. Theoretically, the advantage of cell-culture vaccines is that rabies viruses from cell culture are easy to purify because the culture system has a single cell type and fewer impurities.46 Therefore, the cell-culture vaccines have the advantages of high potency, good immune effects, and low price. However, although these three rabies vaccines have been applied clinically for decades, to date, no research has summarized and compared their safety and immunogenicity. Hence, we have evaluated the safety and immunogenicity of three currently marketed rabies vaccines used in PEP, with the aim of providing a scientific basis for policy formulation and clinical practice. In addition, studies47–49 have shown that RIG does not affect the safety and immunogenicity of rabies vaccines; thus, we did not conduct a subgroup analysis according to RIG.
Single-rate meta-analysis is a simple combination of the incidence of outcomes of interest, which has the advantage of being more flexible in terms of study types. Although it is not possible to directly provide an estimate of RR, we can make full use of existing data to initially compare the safety and immunogenicity of the three rabies vaccines with the help of the single-rate meta-analysis method.50 Furthermore, direct and indirect evidence were combined by NMA in the research network to compare the safety and immunogenicity of the three rabies vaccines in a single analysis. The advantage of NMA is that it can estimate the relative effect of any two interventions in the network, which is usually more accurate than a single direct or indirect estimate. It also allows for an estimate of the level and level of intervention.51
In terms of safety, the results of a single-rate meta-analysis showed that the pooled incidence of total ARs to HDCV was 5.8% (95% CI: 1.3%–12.5%), which was significantly lower than that of PCECV, but not significantly different from PVRV. HDCV is cultured from human diploid cells, while both PVRV and PCECV are cultured from animal cells. From the perspective of the principles of vaccine manufacture and immunological theories, HDCV contains fewer allogeneic substances and does not contain allogeneic acids, resulting in fewer ARs after vaccination.52 Compared with PCECV, although PVRV was lower than PCECV in the point estimates, the results of the two interval estimates were overlapped, and there was no factor which indicated that the pooled incidence of total ARs to PVRV was significantly lower than that of PCECV. A sample size of 11,724 was used to calculate the pooled incidence of total ARs to PVRV, while HDCV and PCECV comprised only 1,794 and 997, respectively. Therefore, it will be necessary to increase the HDCV and PCECV participant sample size to obtain more stable results. To further explore which specific AR symptoms were different, we compared the differences in five local symptoms and four systemic ones among the three vaccines by NMA. The results showed no significant differences in AR symptoms between the three vaccines, except for local pain, fever, and weakness. The incidence of local pain and fever were significantly lower in HDCV than in PVRV and PCECV. As for the incidence of weakness, HDCV was significantly lower than PVRV, but the difference between PCECV and HDCV was not statistically significant. Therefore, in terms of AR symptoms, HDCV was safer than the other two rabies vaccines, which was primarily reflected in the lower incidence of local pain, fever, and weakness after vaccination.
It is notable that no deaths from rabies were reported in the studies included in this review. With RVNA ≥ 0.5 IU/ mL representing the standard for seropositivity, the seroconversion rates were very close to 100% after day 7 in almost every study (STable 2), indicating that the protective rate of the three types of rabies vaccines can reach nearly 100% below the premise of scientifically and effectively delivering rabies vaccines. The seroconversion rate on day 7 and RVNA titer or concentration on day 14 were used as measurable variables to compare the immunogenicity of the three rabies vaccines. For this reason, complete data concerning immunogenicity after a full five-dose vaccination series were not often easy to obtain because of poor compliance; thus, most vaccine studies selected day 14 (i.e. after the third dose and before the fourth dose) as a crucial timepoint for immunogenicity assessment, for both PEP and PrEP.18,36,53 In addition, short-term immunogenicity of rabies vaccine is more important for PEP compared with PrEP, because exposed people must reach a sufficiently high RVNA level in a short time to neutralize the rabies virus. Hence, we thought that discussing the immune effect of a full five-dose vaccine was not significant for the three rabies vaccines; instead, it was much more noteworthy to discuss the short-term immunogenicity of the three rabies vaccines on days 7 and 14. The results showed that the 95% CIs for pooled seroconversion rates on day 7 for the three rabies vaccines overlapped with each other, and a significant differences were not observed. The same results were obtained when performing NMA for RVNA titer or concentration on day 14 as the measurement variable. Therefore, HDCV, PVRV, and PCECV effectively prevented rabies, and their immunogenicity levels were similar. However, the broad range of 95% CIs of pairwise comparisons suggested that an insufficient sample size might lead to false-negative results in such comparisons. Hence, further studies are required that can address this limitation.
In conclusion, HDCV, which exhibited good safety and immunogenicity is worthy of being recommended first for PEP. Although the immunogenicity of PVRV and PCECV is not significantly different from that of HDCV, there are elevated risks of local pain and fever after vaccination, and greater caution should be exercised in actual applications. For a prolonged time, HDCV has had limited use in developing countries owing to technical difficulties and high prices.54 In recent years, the perfusion bioreactor based on microcarrier technology created by Chengda Biotechnology Co. has greatly reduced the cultivation period of HDC and increased the production capacity of HDCVs. It is believed that with the launch and large-scale production of domestic HDCV in China, the cost of HDCV applications on a global scale will be significantly reduced, enabling further promotion and application of this safe and effective vaccine.
However, the current study had certain limitations. First, immunogenicity data were only collected and analyzed for days 7 and 14 after the first dose. More immunogenicity data at other time points are needed, especially for RVNA titer, based on more PEP research for the three vaccines in the future. Notwithstanding the insufficient long-term immunogenicity data in studies meeting our inclusion and exclusion criteria, substantial clinical practices have demonstrated the satisfying long-term effectiveness of all the three vaccines. Second, the variety of measurable variables and the lack of documentation have in a shortage of subjects included in the NMA. Furthermore, due to the small number of studies used for multiple comparisons, the assessment results of publication bias may be unreliable. Third, rapid fluorescence focus inhibition tests (RFFITs) were used in most studies, while other methods were used in a small number of studies, such as the mouse neutralization tests (MNTs) and enzyme-linked immunosorbent assays (ELASAs), which might lead to heterogeneity.
Supplementary Material
Funding Statement
This study was funded by the Natural Science Foundation of Jiangsu Province [grant number: BK20190357].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2027714.
References
- 1.Banyard AC, McElhinney LM, Johnson N, Fooks AR.. Introduction history of rabies control by vaccination. Introduction history of rabies control by vaccination. Rev Sci Tech. 2018;37(2):305–11. doi: 10.20506/rst.37.2.2804. [DOI] [PubMed] [Google Scholar]
- 2.Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, Müller T, Nadin-Davis S, Picard-Meyer E, Wilde H, et al. Rabies. Nat Rev Dis Primers Published 2017 Nov 30. 2017;3:17091. doi: 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 3.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Correction: estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(5):e0003786. Published 2015 May 11. doi: 10.1371/journal.pntd.0003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO expert consultation on rabies: third report. World Health Organization; 2018. [accessed 2021 Dec 1]. https://apps.who.int/iris/handle/10665/272364. [Google Scholar]
- 5.Li T, Wang X, Cheng H. Meta-analysis of immunogenicity and safety of human rabies vaccination under Zagreb and Essen regimens. J Comp Eff Res. 2020;9(7):459–68. doi: 10.2217/cer-2019-0202. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CR, Schnell MJ. New developments in rabies vaccination. New developments in rabies vaccination. Rev Sci Tech. 2018;37(2):657–72. doi: 10.20506/rst.37.2.2831. [DOI] [PubMed] [Google Scholar]
- 7.Toovey S. Preventing rabies with the Verorab vaccine: 1985-2005 Twenty years of clinical experience. Travel Med Infect Dis. 2007;5(6):327–48. doi: 10.1016/j.tmaid.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Wang S, Zhou R, Liu H, Gan X, Wei M, Zhu F, Meng F, Hou W. Long-term immunity and the effect of one or two booster doses with a lyophilized human rabies vaccine (human diploid cells) at 10 years post primary vaccination in China. Hum Vaccin Immunother. 2021;17(9):3162–68. doi: 10.1080/21645515.2021.1906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother. 2014;10(6):1645–49. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayaz A, Simani S, Janani A, Farahtaj F, Biglari P, Howeizi N, Eslami N. Antibody persistence, 32 years after post-exposure prophylaxis with human diploid cell rabies vaccine (HDCV). Vaccine. 2011;29(21):3742–45. doi: 10.1016/j.vaccine.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Wang Z, Yang B, Cai K, Jiang C, Xie R, Xiao H, Ren Q, Qi Z, Li J, et al. Immunogenicity and safety of purified vero cell-cultured rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in the healthy Chinese subjects: a randomized, double-blind, positive controlled phase 3 clinical trial. Hum Vaccin Immunother. 2021;17(2):351–57. doi: 10.1080/21645515.2020.1778408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheiermann N, Baer J, Hilfenhaus J, Marcus I, Zoulek G. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC)-rabies vaccine in man. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;265(3–4):439–50. doi: 10.1016/s0176-6724(87)80263-8. [DOI] [PubMed] [Google Scholar]
- 13.Arora A, Moeller L, Froeschle J. Safety and immunogenicity of a new chromatographically purified rabies vaccine in comparison to the human diploid cell vaccine. J Travel Med. 2004;11(4):195–99. doi: 10.2310/7060.2004.19001. [DOI] [PubMed] [Google Scholar]
- 14.Li R. Safety and immunogenicity of domestic lyophilized rabies vaccine for human use. Forum Primary Med. 2020;24(10):1459–60. doi: 10.19435/j.1672-1721.2020.10.078. [DOI] [Google Scholar]
- 15.Fan B, Qiao FT, Zhang XH, Lei XK, Wang N, Tang HS, Miao YL, Guo JH, et al. Comparative analysis of safety between human diploid rabies vaccine and Vero cell purified rabies vaccine. Chin J Appl Med. 2019;14(36):143–44. doi: 10.14163/j.cnki.11-5547/r.2019.36.078. [DOI] [Google Scholar]
- 16.Chen XL, Lu CT. Observation on adverse reaction and nursing effect of 400 cases inoculated with freeze-dried human rabies vaccine. Int J Med Health Rev. 2018;24:1720–23. [Google Scholar]
- 17.Peng J, Lu S, Zhu Z, Zhang M, Hu Q, Fang Y. Safety comparison of four types of rabies vaccines in patients with WHO category II animal exposure: an observation based on different age groups. Medicine (Baltimore). 2016;95(47):e5049. doi: 10.1097/MD.0000000000005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose A, Munshi R, Tripathy RM, Madhusudana SN, Harish BR, Thaker S, Mahendra BJ, Gunale B, Gogtay NJ, Thatte UM, et al. A randomized non-inferiority clinical study to assess post-exposure prophylaxis by a new purified vero cell rabies vaccine (Rabivax-S) administered by intramuscular and intradermal routes. Vaccine. 2016;34(40):4820–26. doi: 10.1016/j.vaccine.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Ramezankhani R, Shirzadi MR, Ramezankhani A, Poor Mozafary J. A comparative study on the adverse reactions of purified chick embryo cell vaccine (PCECV) and purified vero cell rabies vaccine (PVRV). Arch Iran Med. 2016;19:502–07. [PubMed] [Google Scholar]
- 20.Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. PLoS Negl Trop Dis. 2014;8(12):e3412. doi: 10.1371/journal.pntd.0003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LY, Sun MP, Zhang XC, Suo L-D, Xu R-H, Zou Y-J, Zuo L-B, Qi H. Safety and immunogenicity of two freeze-dried Vero cell rabies vaccines for human use in post-exposure prophylaxis. Vaccine. 2011;29(15):2679–81. doi: 10.1016/j.vaccine.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M, et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin. 2011;7(2):220–24. doi: 10.4161/hv.7.2.14003. [DOI] [PubMed] [Google Scholar]
- 23.Ashwathnarayana DH, Madhusudana SN, Sampath G, Sathpathy DM, Mankeshwar R, Ravish HHS, Ullas PT, Behra TR, Sudarshan MK, Manjula Shamanna G. A comparative study on the safety and immunogenicity of Purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purifed vero cell rabies vaccine (PVRV, Verorab) [published correction appears in vaccine. 2010 Mar 16;28(13):2575]. Vaccine. 2009;28(1):148–51. doi: 10.1016/j.vaccine.2009.09.090. [DOI] [PubMed] [Google Scholar]
- 24.Shu HP, Zhou CE, Liu YB, Xiong L. Immune effect analysis of freeze-dried purified rabies vaccine (Vero cells). Chin J Public Health Manage. 2007;01:47–48. doi: 10.19568/j.cnki.23-1318.2007.01.030. [DOI] [Google Scholar]
- 25.Cao Q, Huang GH, Wen SY, Pan H, Zhang DM. Clinical safety observation of 2430 cases of Chengda and Weibo rabies vaccine. J Trop Med. 2007;7:693–94. [Google Scholar]
- 26.Sampath G, Reddy SV, Rao ML, Rao YU, Palaniappan C. An immunogenicity study of a newly introduced purified Vero cell rabies vaccine (Abhayrab) manufactured in India. Vaccine. 2005;23(7):897–900. doi: 10.1016/j.vaccine.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang WB, Wang P. Analysis and evaluation of clinical application of human diploid rabies vaccine in 53 cases. Chin J Drug Appl. 2018;12(15):102–04. doi: 10.14164/j.cnki.cn11-5581/r.2018.15.058. [DOI] [Google Scholar]
- 28.Liu XB, Hu P, Wang L. Comparison of immunization program of rabies vaccine inoculation with 5 injections and 2-1-1 4 injections. Occup Health Inj. 2012;27:233–34. [Google Scholar]
- 29.Lu S, Zhu ZG, Fu YA. Safety observation of human rabies vaccine with purified chicken embryo cells (freeze-dried). Public Health Prevent Med. 2010;21:20–22. [Google Scholar]
- 30.Niu HQ, Yue WL, Zhao HB. Analysis of adverse reactions after rabies vaccination. Henan J Prevent Med. 2019;30(3):188–90. doi: 10.13515/j.cnki.hnjpm.1006-8414.2019.03.009. [DOI] [Google Scholar]
- 31.Shao W, Zheng YS, Zhang J, Jiang YZ, Jiang XY. Observation on the efficacy and safety of ”2-1-1” program of post-exposure immunization against rabies vaccine. Chin J General Pract. 2013;11(11):1768–69. doi: 10.16766/j.cnki.1674-4152.2013.11.019. [DOI] [Google Scholar]
- 32.Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S, Wasi C. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ. 2000;78:693–98. [PMC free article] [PubMed] [Google Scholar]
- 33.Benjavongkulchai M, Kositprapa C, Limsuwun K, Khawplod P, Thipkong P, Chomchey P, Yountong P, Naraporn N, Ayuthya AB, Raksakate S, et al. An immunogenicity and efficacy study of purified chick embryo cell culture rabies vaccine manufactured in Japan. Vaccine. 1997;15(17–18):1816–19. doi: 10.1016/s0264-410x(97)00143-6. [DOI] [PubMed] [Google Scholar]
- 34.Sirikun J, Suputtamongkol Y, Rattanachinakorn P, Primsirikunawut A. Immunogenic response in obese patients undergoing rabies post-exposure prophylaxis with combined equine rabies immunoglobulin and rabies vaccination. Vaccine. 2018;36(2):285–91. doi: 10.1016/j.vaccine.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 35.Ashwath Narayana DH, Madhusudana SN, Sampath G, Tripathy RM, Sudarshan MK, Gangaboraiah, Ravish HS, Satapathy DM, Gowda G, Holla R, et al. Safety and immunogenicity study of a new purified chick embryo cell rabies vaccine Vaxirab-N (Pitman-Moore strain) manufactured in India. Hum Vaccin Immunother. 2014;10(1):120–25. doi: 10.4161/hv.26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudarshan MK, Bhardwaj S, Mahendra BJ, Sharma H, Sanjay TV, Ashwathnarayana DH, Bilagumba G. An immunogenicity, safety and post-marketing surveillance of a novel adsorbed human diploid cell rabies vaccine (Rabivax®) in Indian subjects. Hum Vaccin. 2008;4(4):275–79. doi: 10.4161/hv.4.4.5588. [DOI] [PubMed] [Google Scholar]
- 37.Yan YY, Du XY, Liu JQ, Zhou L, Zhang X. Study on safety of human diploid rabies vaccine after inoculation. J Prevent Med Intell. 2018;34:636–41. [Google Scholar]
- 38.Wilde H, Glueck R, Khawplod P, Cryz SJ, Tantawichien T, Thipkong P, Chomchey P, Prakongsri S, Benjavongkulchai M, Sumboonanondha A, et al. Efficacy study of a new albumin-free human diploid cell rabies vaccine (Lyssavac-HDC, Berna) in 100 severely rabies-exposed Thai patients. Vaccine. 1995;13(6):593–96. doi: 10.1016/0264-410x(94)00049-s. [DOI] [PubMed] [Google Scholar]
- 39.Anderson LJ, Sikes RK, Langkop CW, Mann JM, Smith JS, Winkler WG, Deitch MW. Postexposure trial of a human diploid cell strain rabies vaccine. J Infect Dis. 1980;142(2):133–38. doi: 10.1093/infdis/142.2.133. [DOI] [PubMed] [Google Scholar]
- 40.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection. Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. 1976. Wilderness Environ Med. 2000;11(1):42–46. doi: 10.1580/1080-6032(2000)011. [DOI] [PubMed] [Google Scholar]
- 41.Sibunruang S, Tepsumethanon S, Raksakhet N, Tantawichien T. Rabies immunization of travelers in a canine rabies endemic area. J Travel Med. 2013;20(3):159–64. doi: 10.1111/jtm.12023. [DOI] [PubMed] [Google Scholar]
- 42.Tantawichien T, Benjavongkulchai M, Limsuwan K, Khawplod P, Kaewchompoo W, Chomchey P, Sitprija V. Antibody response after a four-site intradermal booster vaccination with cell-culture rabies vaccine. Clin Infect Dis. 1999;28(5):1100–03. doi: 10.1086/514737. [DOI] [PubMed] [Google Scholar]
- 43.Langedijk AC, De Pijper CA, Spijker R, Holman R, Grobusch MP, Stijnis C. Rabies antibody response after booster immunization: a systematic review and meta-analysis. Clin Infect Dis. 2018;67(12):1932–47. doi: 10.1093/cid/ciy420. [DOI] [PubMed] [Google Scholar]
- 44.Tan SN, Zhang FY. Research on rabies and human rabies vaccine. Med Inf. 2011;24:2841–43. [Google Scholar]
- 45.Sun CQ. Current situation and progress of rabies virus vaccine production. Int J Med. 1997;03:79–82. [Google Scholar]
- 46.Li YY, Dai BX, Tan LX, Zhang CQ, Li HJ, Zhang WT. Research progress of rabies vaccine. Biotechnology. 2017;01:100–04. [Google Scholar]
- 47.Nicholson KG, Turner GS. Studies with human diploid cell strain rabies vaccine and human antirabies immunoglobulin in man. Dev Biol Stand. 1978;40:115–20. [PubMed] [Google Scholar]
- 48.Wu WC. Effect of systematic injection of rabies immunoglobulin on the immune effect of rabies vaccine [dissertation]. Dalian (CN): Biological Engineering; 2017. [Google Scholar]
- 49.Deng SP, Yang KM, Liu XX, Zhan FQ, Tong QH. Observation on the immune effect of rabies vaccine and rabies immunoglobulin. Chin J Trop Med. 2007;08:1412–13. [Google Scholar]
- 50.Ai CL, Xie YM, Li MQ, Wang LX, Liao X. Incidence rate of adverse reaction/event by Qingkailing injection: a meta-analysis of single rate. Zhongguo Zhong Yao Za Zhi. 2015;40:4770–78. [PubMed] [Google Scholar]
- 51.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, Lu Q, Zhu XP, Wang G, Zhou XY. Meta-analysis of the incidence of adverse reactions to human diploid rabies vaccine. Chin J Emergency Resuscitation Disaster Med. 2019;14:548–52. doi: 10.3969/j.1673-6966.2019.06.015. [DOI] [Google Scholar]
- 53.Wang J, Luo F, Feng Z, Li L, Bai Y, Ai X, Ma J, Zhang Z, Shi N. Immunogenicity and safety of purified vero cell rabies vaccine (PVRV) produced by Liaoning Cheng Da Co. under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese adults aged 50 and above. Hum Vaccin Immunother. 2017;13(1):144–50. doi: 10.1080/21645515.2016.1230260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YB. Development of modern rabies vaccine production technology. Paper presented at: 2nd Annual Conference of Chinese Preventive Medical Association and 2nd Annual Conference of Global Chinese Public Health Association; 2006. Nov; Xianghe, Hebei, China. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


