Abstract
Ciprofloxacin, a fluoroquinolone antibiotic active against a wide variety of bacteria, is one of a few antibiotics which enters the human eye after oral administration. However, little is known about its pharmacokinetics in the human eye. One or two oral doses of 750 mg of ciprofloxacin (at a 12-h interval) were administered to 48 patients at various times prior to ocular surgery. Clotted blood, aqueous, and vitreous were collected at surgery, and the concentrations of ciprofloxacin were assayed by high-performance liquid chromatography. Our data were combined with those of others, and a population pharmacokinetic analysis was conducted. The concentrations of ciprofloxacin in both aqueous and vitreous were lower than those in serum and peaked at a later time. The pharmacokinetics of ciprofloxacin in aqueous and vitreous were fitted to a compartmental model in which the antibiotic was transferred into and out of the two compartments (aqueous and vitreous) by first-order processes. Population pharmacokinetic software, P-Pharm, was used to calculate the mean half-lives of the loss of ciprofloxacin from aqueous and vitreous, which were 3.5 and 5.3 h, respectively. At steady state, the mean ratios of then concentrations in aqueous and vitreous to the concentrations in serum were 23 and 17%, respectively. After the administration of one or two doses of 750 mg of ciprofloxacin, the concentrations in both aqueous and vitreous in a number of patients were lower than the MICs at which 90% of isolates are inhibited (0.5 mg/liter) for common intraocular bacterial pathogens. Simulations of concentrations in the eye after the administration of higher doses (1,500 mg of ciprofloxacin as a single dose, two doses of 750 mg 2 h apart, and 750 mg every 6 h) indicated that in approximately 20% of patients the concentrations would still be below 0.5 mg/liter. Although oral ciprofloxacin may be a beneficial adjunctive therapy, the use of oral ciprofloxacin alone may not be adequate for perioperative prophylaxis or for treatment of bacterial endophthalmitis.
Ciprofloxacin, a fluoroquinolone antibiotic, is active against a wide variety of bacteria. It is potentially a very useful antibiotic in ophthalmology because it is one of only a few antibiotics which enters the human eye after oral administration. Reported concentrations of ciprofloxacin varied from 0.1 to 0.65 mg/liter in aqueous and from 0.17 to 0.51 mg/liter in vitreous after the oral administration of various doses of the antibiotic to humans (4, 10, 11, 16, 17, 20, 21, 24, 28). However, few have provided an analysis of the pharmacokinetics of ciprofloxacin in the human eye.
In normal clinical practice, it is not possible to obtain a full concentration-time profile of a drug in the eye because of limited opportunities to sample ocular fluids. However, a population pharmacokinetic approach (1, 31) allows sparse observation data to be analyzed, so that even one observation can be used (2). Drusano et al. (8) used a population approach to examine the kinetics of ciprofloxacin using multiple plasma samples and single samples of vitreous from rabbits. An important aspect of the pharmacokinetics of any drug is understanding the interpatient variability in the pharmacokinetic parameters, and a feature of the population approach is that it allows the determination of the variability in the pharmacokinetic parameters from very limited data for each patient.
In the present study, the concentrations of ciprofloxacin in serum, aqueous, and vitreous were measured simultaneously after its oral administration to 48 patients who underwent intraocular surgery. The combination of our data together with those obtained from the literature (4, 10, 11, 16, 17, 20, 21, 24, 28) provided a substantial amount of information which enabled a population pharmacokinetic study of ciprofloxacin in the human eye. We were able not only to determine the interpatient variation in the pharmacokinetic parameters but also to use these differences to predict the extent of variation in the predicted time course of concentrations of ciprofloxacin in the two compartments of the eye. The kinetic parameters also permitted the prediction of the intraocular concentrations with various dosage schedules other than those used in the present study, thus allowing practical application of our pharmacokinetic analysis.
(This study was presented in part at the American Academy of Ophthalmology Meeting, San Francisco, Calif., November 1994.)
MATERIALS AND METHODS
Patients.
Following approval from the Ethics Committee of the Eastern Sydney Area Health Service, informed consent was obtained from 48 patients. A single dose of 750 mg of ciprofloxacin was administered to 19 patients at various times prior to cataract or glaucoma surgery, and 29 patients received two oral doses of 750 mg of ciprofloxacin, 12 h apart, at various times prior to vitrectomy or surgery for retinal detachment. Patients with ocular inflammation or with a clinically disturbed blood aqueous barrier (seen as an aqueous flare on the slit lamp) were excluded from the study. Ciprofloxacin was administered to fasting patients in the absence of any other agents, such as antacids, which may have interfered with the absorption of the antibiotic. There were 28 males and 20 females whose ages ranged from 15 to 83 years. None had significant impairment of renal function. Samples of clotted blood (10 ml), aqueous (0.1 ml), and, wherever possible, vitreous (0.1 ml) were taken simultaneously from each patient at the commencement of surgery. All samples were protected from light and were stored at −80°C until the concentrations of ciprofloxacin were measured.
Measurement of the concentrations of ciprofloxacin.
The concentrations of ciprofloxacin were measured by high-performance liquid chromatography (HPLC) with a C18 4μ Nova-Pak cartridge and a column (8 by 100 mm; Waters Millipore) (25). Ciprofloxacin was quantitated with a fluorescence detector, with the wavelengths of excitation and emission being 279 and 440 nm, respectively. The concentrations of ciprofloxacin were determined by comparing the peak areas for the samples with those for three standard preparations of the antibiotic which encompassed the expected range of measurement. The mobile phase consisted of 81% phosphate buffer (20 mM KH2PO4, 2 mM tetrabutylammonium hydroxide, 6 mM H3PO4 [pH 3.0]) with 5% acetonitrile and 14% methanol at a flow rate of 2.5 ml/min. The ciprofloxacin concentration in each sample of serum was determined after a 500-μl aliquot was added to 25 μl of perchloric acid and the mixture was vortexed for 30 s and centrifuged (Biofuge 13; Heraeus Sepatech) at 3,000 rpm for 30 min. A 25-μl aliquot of the supernatant was then injected into the column. The ciprofloxacin concentrations in aqueous and vitreous were determined after a 50-μl aliquot of each specimen was diluted with 200 μl of the mobile phase and centrifuged (Biofuge 13; Heraeus Sepatech) at 3,000 rpm for 10 min and 50 μl of the supernatant was injected into the column. There was an 8% analytical coefficient of variation over the range measured.
Population analysis.
A comprehensive search of the literature found several studies (10, 11, 17, 20, 21, 24, 28) in which the concentrations of ciprofloxacin were measured in samples of serum, aqueous, and vitreous which were collected simultaneously at various times after the oral administration of one or two doses of the antibiotic. In most cases ciprofloxacin was administered at doses of 750 mg. However, doses of 500, 1,000, and 1,500 mg were administered in two studies (11, 28) so 46 concentrations in serum and aqueous were normalized to a dose of 750 mg. These data were combined with our data for 48 samples of serum, 44 aqueous samples, and 23 vitreous samples (samples of both aqueous and vitreous were collected from some patients, but only one ocular humor sample was collected from the others). This produced a total of 161 serum samples, 108 samples of aqueous, and 66 vitreous samples. With the exception of the study by El Baba et al. (10), in which a microbiological assay was used, all other studies used HPLC to determine the concentrations of ciprofloxacin. In addition, four studies (4, 16, 21, 24) were found which included a total of 17 measurements of ciprofloxacin in the aqueous after intravenous administration of the antibiotic.
Pharmacokinetic modeling.
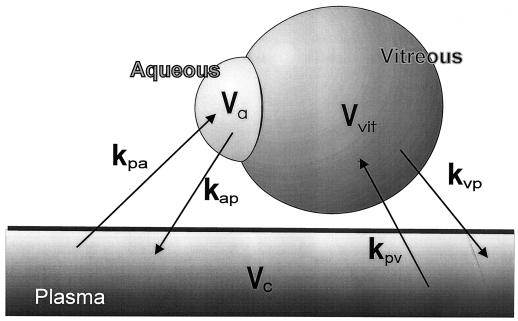
The time courses of the concentrations of ciprofloxacin in the various compartments (Fig. 1) were fitted by the general polyexponential equations of time assuming first-order transfer processes (see the Appendix).
FIG. 1.
Pharmacokinetic model used to describe the pharmacokinetics of ciprofloxacin in aqueous and vitreous. It was assumed that ciprofloxacin was transferred between plasma and aqueous or vitreous as separate compartments by first-order processes. Vc, Va, and Vvit are the volumes of the central, aqueous, and vitreous compartments, respectively. The rate constants kpa and kap and the rate constants kpv and kvp are the rate constants of absorption and loss for the aqueous and vitreous, respectively. In the final population analysis, the concentrations in aqueous humor and plasma (or serum) were fitted simultaneously by the model. The concentrations in vitreous and plasma were also fitted simultaneously but separately from the data for aqueous and plasma.
In general, the complex constant kpa · Vc/Va (where kpa is the first-order rate constant of transfer from the central compartment to the aqueous, Vc is the volume of the central compartment, and Va is the volume of the aqueous compartment) directly determines the concentrations at any time in the aqueous. If this complex constant is small (i.e., the corresponding half-life is long), then the concentrations in aqueous will tend to be low. By contrast, the rate constant of loss from aqueous (kap) determines the time taken to achieve the peak concentration in this compartment of the eye. If this constant is small, the peak concentration occurs later than in plasma but the concentrations in aqueous are more sustained. These considerations are analogous for the rate constants in vitreous.
The ratio of the mean concentration of ciprofloxacin in aqueous or vitreous to the mean concentration in serum can also be determined (15) (see the Appendix). The concentration ratio K (described previously as “the penetration” into aqueous [8]) is given by the ratio of the rate constants.
Population pharmacokinetic analysis.
The data analysis was conducted in two stages. (i) The concentrations in serum, aqueous, and vitreous were pooled for all subjects and were fitted by the model equations by using the nonlinear regression program Minim (R. D. Purves, University of Otago, Dunedin, New Zealand). This procedure is termed “naive pooling.”
(ii) By using the starting estimates of the pharmacokinetic parameters from naive pooling, the time courses of the concentrations in serum and aqueous were fitted simultaneously by a nonlinear mixed effect model with the P-Pharm program (version 1.3) (5, 12, 23). The pharmacokinetic parameters in serum and vitreous were similarly determined simultaneously but in a separate computation because the P-Pharm program does not readily allow the simultaneous estimation of the parameters in three fluids. The P-Pharm program uses an iterative process that involves computation of the maximum-likelihood estimates (EM-like algorithm) of the population parameters and their variability (12). Preliminary analysis of the pattern of residuals and reduction in the standard deviation of the estimates of the population parameters indicated that interpatient variability in the transfer rate constants kpa, kap, kpv, and kvp (where kpa, kap, kpv, and kvp are first-order rate constants of transfer from the central compartment to aqueous, aqueous to central compartment, central compartment to vitreous, and vitreous to central compartment, respectively) was best described by a log-normal distribution, while interpatient variability in the remaining parameters (volume of distribution [V], absorption rate constant [ka], and elimination rate constant [kel]) was described best by a normal distribution. Two error models available in P-Pharm were evaluated. These were a heteroscedastic error model, in which the residual error was inversely proportional to the squared value of the predicted value, and a homoscedastic error model, in which the residual error was constant. In the simultaneous analysis of the concentrations in serum and vitreous, the error with the heteroscedastic model was smaller (0.28 mg/liter) than that found with the homoscedastic model, with which the error was 0.47 mg/liter. The heteroscedastic model was also used in the analysis of the pharmacokinetics in the aqueous. Following the determination of the mean population pharmacokinetic parameters, the parameters that describe the pharmacokinetics in serum and in either aqueous or vitreous were subsequently obtained for each patient by using the population parameters and individual posterior Bayesian estimates based upon one ciprofloxacin concentration in each fluid.
Simulation study.
The concentration-time profiles of ciprofloxacin in aqueous and vitreous after different dose regimens were simulated by using the population means and standard deviations of the pharmacokinetic parameters described in Table 1. Pharmacokinetic parameters for 2,500 patients were generated by the procedure described by Upton (29). These parameters were used to calculate the mean and the upper and lower 68% confidence interval (±1 standard deviation) concentration-time profile following the administration of 1,500 mg of ciprofloxacin as a single dose, the administration of two doses of 750 mg ciprofloxacin given 2 h apart, or continuous dosing of 750 mg of ciprofloxacin every 6 h.
TABLE 1.
Pharmacokinetic parameters describing the time course of concentrations of ciprofloxacin in plasma and compartments of the eyea
| Compartment and parameterb | Initial estimate | Population mean | Coefficient of variationc | Distribution |
|---|---|---|---|---|
| Aqueous | ||||
| Vc (liters) | 175 | 174 | 4 | Normal |
| ka (h−1) | 1.99 | 2.06 | 39 | Normal |
| t1/2a (h) | 0.35 | 0.34 | ||
| kel (h−1) | 0.17 | 0.13 | 44 | Normal |
| t1/2el (h) | 4.1 | 5.3 | ||
| kpa · Vc/Va (h−1) | 0.052 | 0.045 | 14 | Log-normal |
| kap (h−1) | 0.22 | 0.2 | 34 | Log-normal |
| t1/2ap (h) | 3.2 | 3.5 | ||
| K | 0.24 | 0.23 | ||
| Vitreous | ||||
| Vc (liters) | 175 | 171 | 4 | Normal |
| ka (h−1) | 1.99 | 2.18 | 39 | Normal |
| t1/2a (h) | 0.35 | 0.32 | ||
| kel (h−1) | 0.17 | 0.14 | 39 | Normal |
| t1/2el (h) | 4.1 | 4.9 | ||
| kpv · Vc/Vvit (h−1) | 0.016 | 0.022 | 14 | Log-normal |
| kvp (h−1) | 0.054 | 0.13 | 34 | Log-normal |
| t1/2vp (h) | 13 | 5.3 | ||
| K | 0.3 | 0.17 |
Half-lives corresponding to the rate constants of the model are shown.
t1/2a, half-life of absorption; t1/2el, half-life of elimination; t1/2ap, half-life of loss from the aqueous; t1/2vp, half-life of loss from the vitreous. The other abbreviations are defined in the text.
Intersubject variability expressed as percent coefficient of variation of the population.
RESULTS
Profile of concentrations in serum, aqueous, and vitreous over time.
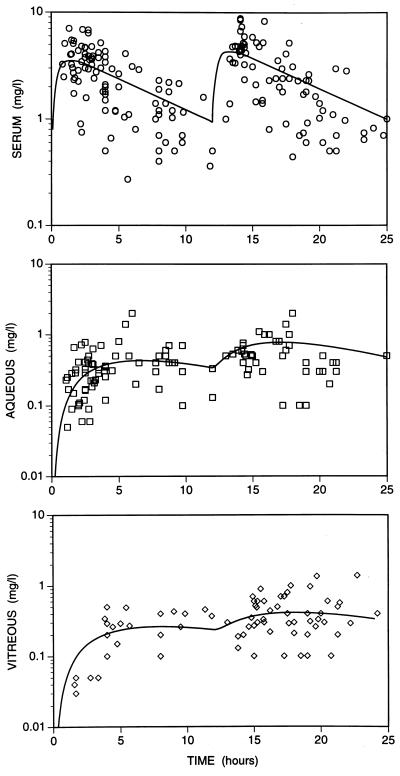
The concentrations of ciprofloxacin in serum showed considerable interpatient variation. The concentrations in serum reached a peak at approximately 1 to 2 h after administration of the initial dose of 750 mg of ciprofloxacin (Fig. 2). Concentrations in serum declined thereafter, until the second dose was administered 12 h after administration of the first dose. There was no significant accumulation of the antibiotic in serum, and the concentrations after administration of the second dose were very similar to the concentrations after administration of the first dose (Fig. 2).
FIG. 2.
Time courses of concentrations of ciprofloxacin in serum, aqueous, and vitreous after administration of one and two oral doses (750 mg) at 12-h intervals. In the modeling, the total concentrations after administration of the second dose were assumed to result from a second dose alone added to the concentrations remaining from the first dose. The curves are drawn from the mean population pharmacokinetic parameters (Table 1). Experimental values come from our data for 48 samples of serum, 44 samples of aqueous, and 23 vitreous samples, together with published data (10, 11, 17, 20, 21, 24, 28).
The concentrations of ciprofloxacin in aqueous and vitreous showed a pattern different from those in serum, and although variable, they were relatively constant from 4 h after administration of the first dose until 12 h after administration of the second dose (Fig. 2). In both compartments of the eye the concentrations were lower, but more sustained, than those in serum throughout the 24 h of the study. It is evident that ciprofloxacin is transferred slowly between serum and both compartments of the eye.
Pharmacokinetic modeling.
Although the time courses of the concentrations of ciprofloxacin in serum, aqueous, and vitreous were scattered (Fig. 2), the assumption of first-order absorption and elimination of ciprofloxacin together with first-order transfer into and out of the eye provided an adequate description of the concentration-time courses in the eye. The equations fitted to the data allowed the determination of the rate constants of the processes of ciprofloxacin distribution in the eye; however, the population analysis indicated that there was considerable interpatient variation in the kinetic parameters. The mean population kel was 0.135 h−1, which corresponded to a serum half-life of 5.1 h, which was in good agreement with the half-life (4 h) recorded in several previous studies (9, 13, 14). The smallest coefficient observed was V.
The mean pharmacokinetic parameters found by the population analysis were generally similar to the initial values found by the naive pooling method. Although naive pooling does not provide good estimates of the variability of any parameter, the only parameter which was altered to a large extent was kvp. For this parameter, the mean value from the population analysis was considerably smaller than the initial estimate from naive pooling.
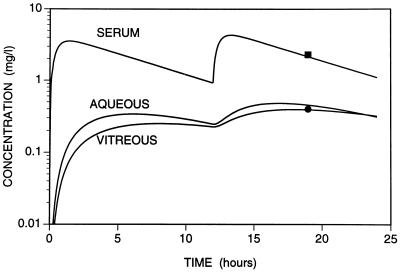
One advantage of the population approach is that the model can be used to predict the individual concentration-time profile expected in the plasma and the eye for any patient. By using one concentration in plasma and in a compartment of the eye, the population method allows the Bayesian estimate of the pharmacokinetic parameters for that individual. The predicted time course of concentrations can then be calculated from these parameters. An example is shown in Fig. 3.
FIG. 3.
Time course of concentrations of ciprofloxacin in serum, aqueous, and vitreous in one patient after administration of two doses of 750 mg of ciprofloxacin orally. Samples were collected at 19 h after administration of the first dose (7 h after administration of the second dose). The ciprofloxacin concentrations measured in aqueous and vitreous were both 0.4 mg/liter (●), and that measured in serum was 2.3 mg/liter (■). For this patient, the estimated pharmacokinetic parameters were as follows: Vc, 174.5 liters; ka, 2.05 h−1; kel, 0.134 h−1; kap, 0.233 h−1; kpa · Vc/Va, 0.039 h−1; kvp, 0.131 h−1; kpv · Vc/Vvit, 0.021 h−1.
The rate constants of transfer of ciprofloxacin into and out of the compartments of the eye (kpa, kap, kpv, kvp) were of the same order or longer than the rate constants of absorption and elimination from serum (ka, kel) determined from the time course of concentrations in serum (Table 1). The mean rate constants of elimination from aqueous (kap) and vitreous (kvp) were 0.20 and 0.13 h−1, respectively, corresponding to half-lives of 3.5 and 5.3 h, respectively. The predicted result from equations 1 and 5 in the Appendix is that the changes in the concentrations in both compartments of the eye lag behind the concentrations in serum, as was observed (Fig. 2 and 3).
The systemic pharmacokinetic parameters of ciprofloxacin were determined twice by the use of the P-Pharm program. The naive pooling method allows the simultaneous fitting to the concentrations in the three fluids, serum, aqueous, and vitreous, but P-Pharm allows only pair-wise simultaneous analysis. However, the systemic pharmacokinetic parameters (V, kel, and ka) fitted to serum and aqueous were very similar to those estimated from serum and vitreous (Table 1). For example, the mean half-lives of elimination from serum were 5.3 and 4.9 h from the aqueous and vitreous analyses, respectively. The similar systemic parameters produced by the two analyses support the validity of the pharmacokinetic modeling.
The rate constant of transfer of the drug into vitreous (kpv) was smaller than the rate constant of transfer into aqueous (kpa), this difference being consistent with the lower initial concentrations in vitreous. However at steady state, the mean concentration ratios (K; also termed “the penetration”) in the two compartments of the eye were similar (Table 1).
It is possible to make an estimate of the expected contribution of the eye in the distribution of ciprofloxacin in the eye from K (see equation 6 in the Appendix), the volumes of aqueous (Va) and vitreous (Vv) humors, and V. For example, the fractions of ciprofloxacin in the aqueous and vitreous humors are estimated by Ka · Va/V and Kv · Vv/V, respectively. Va and Vv are approximately 0.22 and 4 ml, respectively, making these factors approximately 0.0003 and 0.004, respectively. These small fractions are consistent with the assumption in the derivation of equation 5 in the Appendix, namely, that the amounts of ciprofloxacin in both aqueous and vitreous humors are small and do not influence the kinetics of the drug in plasma.
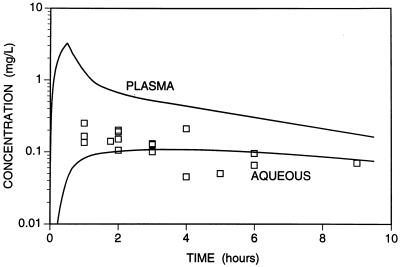
The mean kinetic parameters of transfer of ciprofloxacin into and out of aqueous were tested by using published data of the concentrations of the drug in aqueous following intravenous administration of the drug (4, 16, 20, 22). The ciprofloxacin time course in aqueous was predicted by using published kinetic parameters for serum (9, 13, 14) and the parameters determined in the present study. Although there was scatter of the ciprofloxacin concentrations in aqueous, these were close to the predicted pattern, providing some validation of our calculated mean rate constants of transfer of ciprofloxacin into and out of aqueous (Fig. 4). After 3 h, the concentrations in aqueous were similar to the predicted concentrations; however, the concentrations at prior times were higher than those predicted. Compared with oral administration, the smaller intravenous dose produced an earlier peak concentration, but this was not sustained and resulted in a lower concentration in aqueous.
FIG. 4.
Time course of predicted concentrations of ciprofloxacin in serum and aqueous following cessation of an intravenous infusion (200 mg over 30 min). The time course of concentrations in serum were modeled from the mean kinetic constants after intravenous administration (distribution phase constant = 2.9 h−1, elimination phase constant = 0.179 h−1) (9, 13, 14). The concentrations in aqueous were predicted by using these kinetic constants and the mean rate constants of transfer of ciprofloxacin into and out of aqueous determined in the present study. The datum points represent the actual concentration in previous studies after intravenous administration (4, 16, 21, 24).
DISCUSSION
The small number of previous pharmacokinetic studies of ciprofloxacin or related compounds in the eye were conducted mainly with rabbits (8, 22). However, one study with rabbits successfully demonstrated the use of a population modeling program to describe the pharmacokinetics with a limited amount of data (8). This rabbit model may be useful when examining the potential antibacterial activity of ciprofloxacin in the eye because the mean concentration in aqueous/concentration in plasma ratio for the rabbit was approximately 30% (22), which was similar to the mean ratio of 22.5% found in the present study for the human eye. However, the concentration in vitreous/concentration in plasma ratios for the rabbit were lower than those found in our study (mean values, 0.029 [9] and 0.065 [22] compared with 0.17 in the present study). A better penetration of ciprofloxacin into the vitreous of inflamed eyes was demonstrated in both the rabbit and the swine models (3). The higher ratio in the human eye in the present study may possibly reflect an abnormal blood-retina barrier due to disease in patients undergoing vitrectomy. However, patients with inflamed eyes and other clinical evidence of a grossly deficient blood-retina barrier were excluded from our study and from the other studies used in our analysis.
The modeling yields pharmacokinetic parameters that describe the handling of the drug in the body as a whole. V and the half-life of ciprofloxacin were very similar to those found previously, in which the V of ciprofloxacin ranged from means of 2.7 to 4.7 liters/kg (9, 13, 14, 30). In the present study the mean V's of 171 and 174 liters (Table 1) were approximately equivalent to the lower limit of the published range (30). Similarly, the mean values for half-life of elimination (4.9 and 5.3 h; Table 1) were within the published range of mean values (3 to 6.6 h) (9, 13, 14, 30).
The pharmacokinetic parameters of ciprofloxacin were calculated by assuming nonsaturable transfer between compartments. Saturation of any ciprofloxacin carrier-mediated processes may be possible, but the interpatient variation in the pharmacokinetics of the drug appear to be too great for our analyses to detect any saturation of significant systemic processes or any saturation of transfer of ciprofloxacin into or out of the eye.
While the concentrations of ciprofloxacin in the eye were relatively stable for at least 12 h after the administration of a second oral dose of 750 mg, the concentrations do not appear to be satisfactory for all patients. In 20% of patients who used this regimen, the intraocular concentrations of the antibiotic were lower than 0.5 mg/liter, which is below the MIC at which 90% of isolates are inhibited for commonly occurring intraocular pathogens (18). Consequently, we conducted simulations of the expected time courses of concentrations in both aqueous and vitreous using several other dosing schedules.
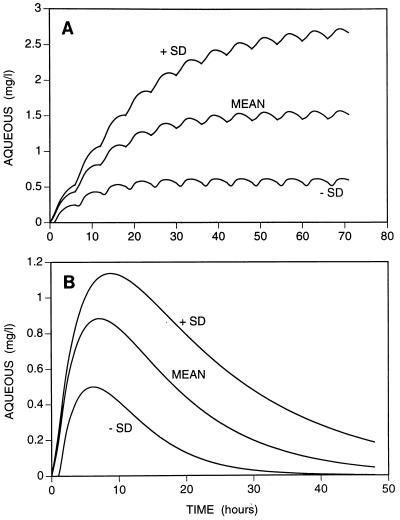
The first was the simulation of the concentrations in both compartments following administration of a single large oral dose of 1,500 mg of ciprofloxacin. The mean peak concentration in aqueous was above 0.5 mg/liter, but in approximately 20% of patients the peak was still below this level (Fig. 5). Although the peak predicted concentration in vitreous was less variable, it was lower (0.54 mg/liter) and in 16% of the patients it was still below 0.42 mg/liter. In view of the possible intolerance of such a large single dose of ciprofloxacin, the concentrations in the eye were simulated after administration of two oral 750-mg doses 2 h apart (data not shown). The concentrations in both compartments of the eye were similar to the concentrations after administration of a single oral dose of 1,500 mg.
FIG. 5.
Time courses of predicted concentrations in aqueous and vitreous following administration of 750 mg of ciprofloxacin every 6 h (A) and a single dose of 1,500 mg of ciprofloxacin (B). The mean ± 1 standard deviation is shown. Thus, 16% of all patients should have concentrations above the range shown and 16% should have concentrations below the range shown.
The remaining simulation was 750 mg every 6 h (Fig. 5). With this dose schedule, there was considerable accumulation of ciprofloxacin in both aqueous and vitreous. After 2 days the predicted mean concentration of ciprofloxacin in vitreous was approximately 1.0 mg/liter (±0.45 mg/liter). Despite the accumulation in the aqueous and vitreous, in approximately 15% of patients the concentrations were still below 0.5 mg/liter even after administration of several doses. However, it is possible that this proportion would be less in patients with inflamed eyes (3). It should also be noted that this dose is above that presently recommended and has not been evaluated for safety.
Topical application of ciprofloxacin appears to offer no advantage. Two studies found that application of 0.3% ciprofloxacin drops was insufficient to achieve a concentration in aqueous above 0.5 mg/liter (7, 19).
The aim of the present study was to examine the clinical utility of different doses of oral ciprofloxacin for ocular treatment and prophylaxis. Although there was a relatively constant concentration of ciprofloxacin in both compartments (particularly the vitreous) from about 5 h after administration of the first 750-mg dose, a stable concentration was best achieved after administration of an equivalent second dose. The model predicts some accumulation after administration of the second dose (Fig. 5), and furthermore, the absorption phase is less significant after administration of the second dose. If ciprofloxacin is used for ocular surgical prophylaxis, we recommend administration of at least two 750-mg tablets 12 h preoperatively. Surgery could commence at any time within 12 h after administration of the second dose.
In general, systemic ciprofloxacin should not be relied upon as a sole prophylactic agent before ocular surgery or in the treatment of suspected intraocular infections, especially as one study found that for 27% of the organisms isolated the MIC of ciprofloxacin at which 90% of isolates are inhibited was >0.5 mg/liter (18). However, the results from the present study indicate that an appropriate oral dose of ciprofloxacin may still be a useful adjunct for ocular therapy and prophylaxis.
ACKNOWLEDGMENT
The assistance of M. R. Lesk, who supplied details of his results, is gratefully acknowledged.
Appendix
The time (t) course of concentrations (C) in plasma was fitted to polyexponential equations of the general form
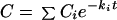 |
1 |
where Ci and ki are concentration and disposition rate constants, respectively.
In the present study, the time courses of concentrations in plasma after oral and intravenous dosing were described by biexponential equations 2 and 4, respectively:
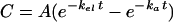 |
2 |
where kel and ka are the rate constants of elimination and absorption, respectively, and A is a complex concentration constant:
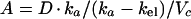 |
3 |
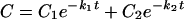 |
4 |
where C1 and C2 are concentration constants, k1 and k2 are rate constants of disposition, and D is the dose.
Assuming that ciprofloxacin is transferred between plasma and aqueous or vitreous by first-order processes and that the amounts transferred do not alter significantly the concentrations in plasma (6, 26), then the concentrations in these fluids should follow the general equation
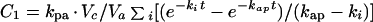 |
5 |
where kpa is the first-order rate constant of transfer from the central compartment to the aqueous, while Vc and Va are the volumes of the central compartment and aqueous, respectively. The rate constant of loss from aqueous is kap (Fig. 1).
The constants A, kel, and ka were determined from the plasma ciprofloxacin concentrations (equation 2); the rate constant kpa and the complex constant kpa · Vc/Va were then determined by fitting equation 5 to the time course of concentrations in the aqueous.
The model equations 1 to 5 are applicable after administration of a single dose. After administration of the second dose, it was assumed that the principle of superposition was followed. Thus, the concentrations in plasma and aqueous were the sums of those remaining from the first dose and those produced from the second dose.
An equation analogous to equation 5 can be written for the time course of concentrations in vitreous, and the rate constants of transfer can be determined.
The concentration ratio K (described previously [8] as “the penetration” into aqueous) is given by the ratio of the rate constants. Thus:
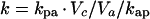 |
6 |
A similar equation can be written for the ratio of the mean concentration in vitreous and plasma.
REFERENCES
- 1.Aarons L. Population pharmacokinetics: theory and practice. Br J Clin Pharmacol. 1991;32:669–670. [PMC free article] [PubMed] [Google Scholar]
- 2.Aarons L. Sparse data analysis. Eur J Drug Metab Pharmacokinet. 1993;18:97–100. doi: 10.1007/BF03220012. [DOI] [PubMed] [Google Scholar]
- 3.Alfaro D V, Hudson S J, Rafanan M M, Moss S T, Levy S-D P. The effect of trauma on the ocular penetration of intravenous ciprofloxacin. Am J Ophthalmol. 1996;122:678–683. doi: 10.1016/s0002-9394(14)70486-6. [DOI] [PubMed] [Google Scholar]
- 4.Behrens-Baumann W, Martell J. Ciprofloxacin concentrations in human aqueous following intravenous administration. Chemotherapy (Basel) 1987;33:328–330. doi: 10.1159/000238517. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J E, Wakefield J C. A comparison of a Bayesian population method with two methods as implemented in commercially available software. J Pharmacokinet Biopharm. 1996;24:403–432. doi: 10.1007/BF02353520. [DOI] [PubMed] [Google Scholar]
- 6.Coburn W A. Simultaneous pharmacokinetic and pharmacodynamic modelling. J Pharmacokinet Biopharm. 1981;9:367–388. doi: 10.1007/BF01059272. [DOI] [PubMed] [Google Scholar]
- 7.Donnenfeld E D, Schrier A, Perry T, Aulicino T, Gombert M E, Snyder R. Penetration of topically applied ciprofloxacin, norfloxacin and ofloxacin into the aqueous. Ophthalmology. 1994;101:902–905. doi: 10.1016/s0161-6420(13)31248-2. [DOI] [PubMed] [Google Scholar]
- 8.Drusano G L, Liu W, Perkins R, Madhu A, Madhu C, Mayers M, Miller M H. Determination of robust ocular pharmacokinetic parameters in serum and vitreous of albino rabbits following systemic administration of ciprofloxacin from sparse data sets by using IT2, a population pharmacokinetic modeling program. Antimicrob Agents Chemother. 1995;39:1683–1687. doi: 10.1128/aac.39.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drusano G L, Plaisance K I, Forrest A, Standiford H C. Dose ranging study and constant infusion evaluation of ciprofloxacin. Antimicrob Agents Chemother. 1986;30:440–443. doi: 10.1128/aac.30.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Baba F Z, Trousdale M D, Gauderman J, Wagner D G, Liggett P E. Intravitreal penetration of oral ciprofloxacin in humans. Ophthalmology. 1992;99:483–486. doi: 10.1016/s0161-6420(92)31943-8. [DOI] [PubMed] [Google Scholar]
- 11.Fern A I, Sweeney G, Doig M, Lindsay G. Penetration of ciprofloxacin into aqueous. Trans Ophthalmol Soc. 1986;105:650–652. [PubMed] [Google Scholar]
- 12.Gomeni R, Pineau G, Ment F. Population kinetics and conditional assessment of the optimal dosage regimen using P-PHARM software package. Anticancer Res. 1994;14:2321–2326. [PubMed] [Google Scholar]
- 13.Gonzalez M A, Moranchel A H, Duran S, Pichardo A, Magana J L, Painter B, Drusano G L. Multiple-dose ciprofloxacin dose ranging and kinetics. Clin Pharmacol Ther. 1985;37:633–637. doi: 10.1038/clpt.1985.102. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez M A, Moranchel A H, Duran S, Pichardo A, Magana J L, Painter B, Forrest A, Drusano G L. Multiple dose pharmacokinetics of ciprofloxacin administered to normal volunteers. Antimicrob Agents Chemother. 1985;28:235–239. doi: 10.1128/aac.28.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham G. Kinetics of non-steroidal anti-inflammatory drugs in synovial fluid. Agents Actions. 1987;24(Suppl.):66–75. doi: 10.1007/978-3-0348-9160-8_6. [DOI] [PubMed] [Google Scholar]
- 16.Gumbel H, Schnaudigel O E, Heider W, Shah P M. Penetration von ciprofloxacin in das Kammerwasser nach parenteraler Anwendung. Fac Frotschr Antimikrob Antiepolast Chemother. 1989;8:61–64. [Google Scholar]
- 17.Keren G, Alhalel A, Bartov E, Kitzes-Cohen R, Rubinstein E, Segev S, Treister G. The intravitreal penetration of orally administered ciprofloxacin in humans. Investig Ophthalmol Vis Sci. 1991;32:2388–2392. [PubMed] [Google Scholar]
- 18.Kowalski R P, Karenchak L M, Eller A W. The role of ciprofloxacin in endophthalmitis therapy. Am J Ophthalmol. 1993;116:695–699. doi: 10.1016/s0002-9394(14)73468-3. [DOI] [PubMed] [Google Scholar]
- 19.Leeming J P, Diamond J P, Trigg R, White L, Hoh H B, Easty D L. Ocular penetration of topical ciprofloxacin and norfloxacin drops and their effect upon eyelid flora. Br J Ophthalmol. 1994;78:546–548. doi: 10.1136/bjo.78.7.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesk M R, Ammann H, Marcil G, Vinet B, Lamer L, Sebag M. The penetration of oral ciprofloxacin into the aqueous, vitreous and subretinal fluid of humans. Am J Ophthalmol. 1993;115:623–628. doi: 10.1016/s0002-9394(14)71460-6. [DOI] [PubMed] [Google Scholar]
- 21.Luthy R, Joos B, Gassmann F. The 1st International Ciprofloxacin Workshop. Amsterdam, The Netherlands: Excerpta Medica; 1986. pp. 192–196. [Google Scholar]
- 22.Madu A A, Mayers M, Perkins R, Liu W, Drusano G L, Aswani R, Madu C N, Miller M H. Aqueous and vitreous penetration of ciprofloxacin following different modes of systemic administration. Exp Eye Res. 1996;63:129–136. doi: 10.1006/exer.1996.0101. [DOI] [PubMed] [Google Scholar]
- 23.McLachlan A J, Tett S E. Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. Br J Clin Pharmacol. 1996;41:291–298. doi: 10.1046/j.1365-2125.1996.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mounier M, Adenis J P, Denis F. Intraocular penetration of ciprofloxacin after infusion and oral administration. Pathol Biol. 1988;36:724–727. [PubMed] [Google Scholar]
- 25.Nix D E, DeVito J M, Schentag J J. Liquid chromatographic determination of ciprofloxacin in serum. Clin Chem. 1985;31:684–686. [PubMed] [Google Scholar]
- 26.Noe D A, Kumor K M. Drug kinetics in low flux (small) anatomic compartments. J Pharm Sci. 1983;72:718–719. doi: 10.1002/jps.2600720637. [DOI] [PubMed] [Google Scholar]
- 27.Phillips I, King A, Shannon K. In vitro properties of the quinolones. In: Andriole V T, editor. The quinolones. London, United Kingdom: Academic Press; 1988. pp. 83–117. [Google Scholar]
- 28.Sweeney G, Fern A I, Lindsay G, Doig M W. Penetration of ciprofloxacin into the uninflamed human eye after oral administration. J Antimicrob Chemother. 1990;26:99–105. doi: 10.1093/jac/26.1.99. [DOI] [PubMed] [Google Scholar]
- 29.Upton R N. An analysis of errors arising from the direct use of mass balance principles to describe regional drug uptake and elution. J Pharmacokinet Biopharm. 1994;22:309–321. doi: 10.1007/BF02353624. [DOI] [PubMed] [Google Scholar]
- 30.Vance-Bryan K, Guay D R P, Rotschafer J C. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet. 1990;19:434–461. doi: 10.2165/00003088-199019060-00003. [DOI] [PubMed] [Google Scholar]
- 31.Whiting B, Kelman A W, Grevel J. Population pharmacokinetics: theory and clinical application. Clin Pharmacokinet. 1985;11:387–401. doi: 10.2165/00003088-198611050-00004. [DOI] [PubMed] [Google Scholar]