ABSTRACT
Rabies is a highly fatal zoonotic disease caused by the rabies virus invading the central nervous system. When suspected of exposure to the rabies virus, post-exposure prophylaxis should be administered as soon as possible. Monoclonal antibodies (mAbs) neutralizing the rabies virus could be better in human rabies post-exposure prophylaxis than equine or human rabies immune globulin in terms of supply, cost, and efficacy. This article reviews anti-rabies mAbs produced by multiple techniques, and the results of clinical trials for anti-rabies mAbs cocktails recognizing non-overlapping epitopes are also discussed.
KEYWORDS: Rabies virus, glycoprotein, monoclonal antibody, antibody library, antibody cocktail
Introduction
Rabies virus (RABV), a single-stranded RNA virus of the genus Lyssavirus, family Rhabdoviridae, invades the central nervous system, causing acute and zoonotic natural foci disease worldwide.1 RABV was rod-shaped or bullet-shaped with a diameter of about 75 nm and a length of 100–300 nm under an electron microscope, which Louis Pasteur first isolated in 1885. The virus genomic RNA encodes five proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and polymerase protein (L). Glycoproteins are the major surface antigens to which neutralizing antibodies are bound. Glycoprotein antigen binds to acetylcholine receptors, leading to neurotransmitter release disorder and causing neurological symptoms.2,3 The glycoprotein molecule consists of three domains: an ectodomain (amino acid, a.a.1–439), a transmembrane domain (a.a.440–461), and a cytoplasmic domain (a.a.462–505). RABV glycoprotein has distinct antigenic sites (AS): I (a.a.226–231), II (IIa a.a.198–200, IIb a.a.34–42), III (a.a.330–338), IV (a single a.a.251), minor site a (a.a.342–343, otherwise referred to as G1), and G5 (a.a.261–264, also comprises AS VI, a single a.a.264).4,5 The positions of AS of the glycoprotein are shown in Figure 1. The vast majority of mAbs recognizes AS I and III of rabies glycoprotein.6 The literature has not reported the mAb candidates bound to the minor site a, AS IV, and AS VI.
Figure 1.
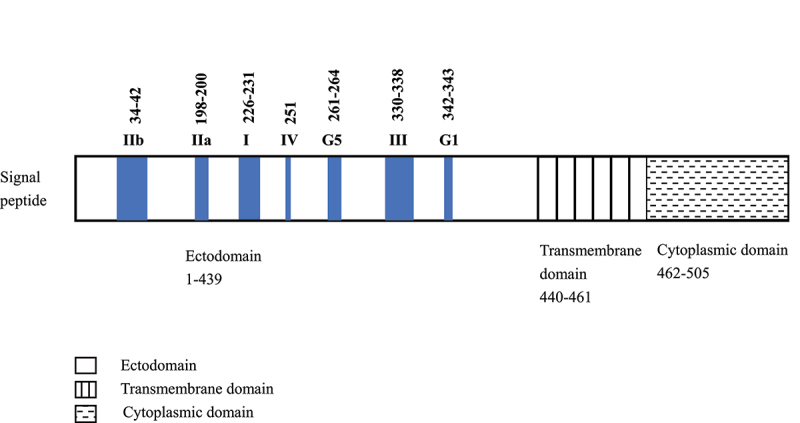
Antigenic sites of RABV glycoprotein. Numerals represent amino acid residue numbers.
RABV enters the human body through bites, scratches, or licks on damaged skin and mucous membranes of infected animals. Dogs, wolves, and cats are the main reservoirs and play a significant role in the process of carrying and spreading the virus.7 As a result of infected-animal bites, the virus moves from peripheral nerve endings to the spinal cord, then finally penetrates the brain. The incubation period of rabies is 1–3 months on average, mostly around 5 days to 1 week, rarely more than 1 year. The incubation period of rabies is related to the bite site. The closer the bite site is to the central nervous system and brain, the shorter the incubation period. Infected dog bites cause almost all cases of infection in humans. Effective dog and wildlife vaccines are critical in the reduction and even elimination of rabies in developed countries. However, the complexity of the animal-human interface of the virus makes elimination challenging. It requires significant investment in the administration of animal vaccination and efforts to reduce rabies virus infection. Despite successful control worldwide, rabies continues to result in tens of thousands of fatal cases each year, especially in Africa and Asia, where children under the age of 15 accounts for 40% of the fatal cases.8 There is an urgent need to ensure that suspected rabies exposures receive immediate treatment to prevent almost fatal clinical rabies.
Rabies post-exposure prophylaxis in human
The World Health Organization (WHO) has developed specific recommendations for rabies post-exposure prophylaxis (PEP) in humans. There are three categories of exposure to suspected rabid animals. Category I exposures only touch or feed animals that lick on intact skin. Category II exposure is defined as nibbling of uncovered skin, minor scratches, or abrasions without bleeding. Category III exposure involves single or multiple transdermal bites or scratches, contamination of mucous membranes or broken skin by saliva from animal licks, and exposure due to direct contact with bats.9 Thorough wound cleaning should be immediately administered for category I and category II exposures. Category II exposures should administer the rabies vaccine as soon as possible. Administration of rabies immunoglobulin (RIG) for wounds classified as category III exposure, is of utmost importance in wound management. RIG should be infiltrated around the wound as much as anatomically feasible and remaining RIG should be injected at an intramuscular site distant from that of vaccine inoculation. It takes some time for active immunity to be triggered by vaccines; thus, passive immunization with RIG can neutralize the rabies virus, while the active immune response develops. The rabies vaccine and RIG combination can almost wholly prevent disease development as soon as possible.10 It is estimated that nearly 29.2 million people worldwide undergo rabies PEP regimen annually,11 but only a tiny part of category III exposures actually receive RIG, which is a lifesaving treatment.
Currently, rabies RIGs used in treatment are mainly related to equine rabies immune globulin (ERIG) and human rabies immune globulin (HRIG).12,13 ERIG derived from the plasma of immunized horses may cause serious side effects and hypersensitivity reactions.14,15 Production was largely discontinued due to animal protection groups. HRIG derived from the serum of immunized humans is considered safer than ERIG, but it is hindered by its high cost and the insufficiency of donors.
Since rabies vaccines cannot provide comprehensive and timely protection, RIG is limited due to supply, cost, and safety. Rabies mAbs can be the best choice in rabies PEP. It has broad application prospects with the advantages of high neutralizing activity, strong specificity to the corresponding target, and easy to standardized production, and so on.16 Compared with polyclonal RIG (ERIG and HRIG) bound to different rabies virus epitopes, single mAb interacts with only one viral epitope. Thus, the ideal monoclonal antibody preparation used in urgent PEP should also meet the following requirements. First, the preparation must contain at least two different antibodies bound to nonoverlapping epitopes to neutralize the virus broadly. Second, the preparation must be safe, and the efficiency is not inferior to the existing HRIG. Last but not least, the cost of preparation must be acceptable for patients needing PEP, especially those in underdeveloped countries where fatal cases are most common.
Current licensed RABV mAbs
There are currently two licensed anti-rabies monoclonal antibodies in India, and three candidates have reached clinical trials (Table 1). The recombinant anti-rabies mAb preparation Rabishield (SII RMab)32 was licensed in India in December 2016; The mouse-derived monoclonal antibody preparation TwinrabTM (RabiMabs)33 developed by Crucell in the Netherlands and Zydus Research Center was licensed in India in September 2019.
Table 1.
Rabies mAbs licensed or in clinical development
| Name | Developer | Stage | Clinical trials registry number |
|---|---|---|---|
| Licensed | |||
| Rabishield (SII RMab) |
Serum Institute of India PVT. LTD. (SIIPL) |
Phase 1, 2/3 clinical trials completed and licensed in December 2016 | CTRI/2009/091/00046517 CTRI/2012/05/00270918 |
| TwinrabTM (RabiMabs) |
Zydus Cadila in India | Phase 1/2, 3 clinical trials completed and licensed in September 2019 | CTRI/2015/06/00583819 CTRI/2017/07/00903820 |
| Applying for licensure | |||
| rhRIG | NCPC (China) in collaboration with MITT (USA) | Phase 1, 2 and 3 clinical trials completed |
NCT0255992121 ChiCTR190002380922 ChiCTR190002378523 ChiCTR190002323624 ChiCTR190002147825 |
| In active clinical development | |||
| SYN023 (CTB011/CTB012) | Synermore Biologics in China | Phase 1 and 2 clinical trials completed and phase 3 clinical trials are planning | CTR2019028126 NCT0295674627 NCT0464448428 |
| Withdrawn from development | |||
| CL184 (CR57/CR4098) | Crucell in Netherlands | Phase 1 and 2 clinical trials completed | ISRCTN1866049329 ISRCTN1269323729 NCT0070808430 NCT0122838331 |
Rabishield (SII RMab)
The recombinant anti-rabies mAb Rabishield (SII RMab) is a human IgG monoclonal antibody that binds to a conformational epitope of rabies glycoprotein, including antigenic site III. SII RMab was developed by MassBiologics (Boston, Massachusetts, USA) under the name 17C7, then further developed under the name SII RMab. 17C7 derived initially from transgenic mice carrying human immunoglobulin genes. 17C7 was the most broadly neutralizing mAbs, neutralizing 92% of the 25 rabies virus isolates tested. In the hamster challenge experiment, SII RMab showed a higher neutralizing effect than ERIG or HRIG and did not interfere with the immune response of the rabies vaccine in the standard PEP regimen.34,35 These preclinical data support the initiation of clinical trials to demonstrate safety in humans. Gogtay et al.17 conducted a randomized, open-label, dose-escalating (1, 3,10, and 20 IU/kg) phase 1 clinical trial in healthy adults in India. SII RMab was well tolerated during the study. The geometric mean concentration (GMC) of rabies neutralizing antibodies was comparable to that of 20 IU/kg of HRIG when SII RMab at a dose of 10 IU/kg, and both cohorts combined with the vaccine (RabivaxTM, India). The GMC of rabies neutralizing antibody was 23.4 IU/ml vs. 15.3 IU/ml on day 14 evaluated by the rapid fluorescence focusing inhibition test(RFFIT), respectively, in the vaccine plus SII RMab cohort and in the vaccine plus HRIG cohort. Later, they conducted a phase two-thirds randomized, single-blind, non-inferiority study among 200 participants with suspected rabies category III exposure. Participants received SII RMab or HRIG (Imogam, Sanofi Pasteur) randomly in a ratio of 1:1. They all cleaned the wounds thoroughly and administered five doses of rabies vaccine (RabivaxTM, India) intramuscularly (0, 3, 7, 14, and 28 days). The GMC ratio of neutralizing antibodies in the SII RMab group to the HRIG group was 4.23 (96.9018% confidence interval [CI], 2.59–6.94) on the 14th day, with a GMC of 24.90 IU/mL for the SII RMab group and 5.88 IU/mL for the HRIG group. SII RMab was superior to HRIG rabies virus-neutralizing activity (RVNA) levels in suspected rabies category III exposures.18 A post-marketing safety study conducted in Bangalore included 397 category III exposures (142 in the SII RMab group, 243 in the ERIG group, and 12 in the HRIG group). All patients were treated according to the PEP regimen as recommended. About 8% subjects in the SII RMab group had delayed adverse reactions such as pain (4.2%), swelling (2.1%), and wound infection (0.7%) at the infiltration site, without any complications. There was no significant difference in safety evaluation among the three groups. The new human mAb was safe from post-exposure prophylaxis against rabies.36 There were potential concerns about the product. It contains only one monoclonal antibody shown to be unable to neutralize a rare rabies variant found in Peruvian bats in the Americas region.18,37 The WHO working group considered that as it was a first-licensed product and limited use of RIG, using it in specific areas and conducting post-marketing surveillance could help solve the above concern. They also figured that SII RMab was worth learning for future production and expected to be an effective alternative to RIG, especially in countries where most rabies cases occur.
TwinrabTM (RabiMabs)
This product combines two mouse monoclonal antibodies (M777-16-3 and 62–71-3) that bind to AS II and III of rabies glycoprotein, respectively. The two mAbs were donated by the WHO Collaborating Centers for Rabies (M777-16-3 from the Animal Diseases Research Institute, Canada, and 62–71-3 from the Centers of Disease Control and Prevention, USA). The binding of RabiMabs to two distinct antigenic sites provides adequate protection against a mutated rabies virus due to a mutation. The equipotent combination of 62–71-3 and M777-16-3 was efficacious in hamsters challenged with a lethal dose of rabies virus.38 Phases 1/2 and 3 clinical trials completed are shown on the Indian Clinical Trials Registry website. In the randomized, double-blind, placebo-controlled phase 1/2 study, RabiMabs was safe and well tolerated up to a dose of 40 IU/kg in conjunction with the rabies vaccine (Vaxirab N, Zydus Cadila).19 In the phase 3 trial, a total of 308 category III suspected rabies exposures were randomly assigned to RabiMabs (40 IU/kg) group and HRIG (20IU/kg) group in a 1:1 ratio. All exposures received wound cleaning and rabies vaccination (Vaxirab N, Zydus Cadila). The rabies virus neutralizing antibodies (RVNA) in the clinical samples was determined by RFFIT. This study confirmed that TwinrabTM was safe and non-inferior to HRIG, and that it could provide protection up to day 84 in combination with the rabies vaccine.20 This study was conducted in subjects with suspected rabies exposure rather than a confirmed infection. In other words, there was no evidence to prove rabies in the brain tissue of infected dogs.
Rabies mAbs obtained by different methods
Hybridoma technique
The hybridoma technology made mAbs possible to obtain, laying a theoretical foundation for future research. A somatic hybrid (hybridoma) between mouse myeloma cells immunized with rabies vaccine and spleen cells derived from BALB/c mice was produced by hybridoma technology in 1978. Hybridomas obtained by Wiktor et al.39 was able to protect experimental animals from lethal rabies virus infection. Muhamuda40 fused SP2/0 cells with spleen cells from BALB/C mice immunized with the rabies vaccine (Verorab, France) to obtain mAbs. The obtained mAbs was 2000 times more potent than commercial ERIG in terms of effective protein concentration and neutralizing titer. Cheng Wei41 fused the spleen cells of BALB/c mice immunized with the vaccine (Fort dodge, America) with SP2/0 myeloma cells. Two anti-RABV mAb were obtained, named 1G8 and 4G11. Both mAbs showed high neutralizing activity by indirect immunofluorescence assay (IFA). The two mAbs recognized non-overlapping epitopes were tested by the convenient enzyme-linked immunosorbent assay (ELISA) with an additivity index of 16.8%.
The murine mAbs had limitations on instability and inability to provide adequate protection owing to species differences. Human hybridomas was prepared from peripheral B lymphocytes from vaccines with the human rabies vaccine fused with somatic cells. One of the nine human mAbs, SO57, neutralizing a variety of RABV strains, was described by Dietzschold et al.42 NM57 is expressed and purified from a stable recombinant expression system in CHO cells. This is the prototype of rhRIG, a Chinese monoclonal antibody introduced later.43 Bakker et al.44 reformatted SO57 (renamed CR57) into their expression system for production in PER.C6 cells. CR57 showed high neutralizing potency in vitro and in vivo and recognized AS I by characterizing CR57 escape mutants.45 The above mAbs provide candidate antibodies for monoclonal antibody cocktails.
Antibody library technique
Antibody library technology aims to obtain mini-antibodies (single-chain antibody fragment, scFv) or Fab fragments of human antibodies through affinity screening from combinatorial libraries.46 We then construct full-size human immunoglobulins based on selected fragments.47 Phage and ribosomal display of combinatorial libraries are widely used to screen antibody fragments nowadays.
Phage display of combinatorial library technique
Kramer et al.48 isolated lymphocytes from the blood of rabies vaccinated volunteers conduct RABV phage display antibody libraries. The antibodies were selected using the inactivated virus and purified RABV glycoprotein. CR4098 was the best candidate bound to the AS III. Zhao et al.49 constructed a human phage antibody libraries of 7 × 108 clones. They successfully screened a new rabies mAb, named scFv-S12, bound explicitly to the rabies virus and had high affinity. A novel human single chain variable fragment of mAb (AR16) was obtained from a phage display library with a repertoire of approximately 108 clones, and AR16 recognized the G5 epitope of RABV specifically.50 In addition, a combinatorial scFv antibody phage library was constructed using a pttAL14 vector from the peripheral blood of immunized volunteers. Li Yu51 selected six mAbs with high affinity represented by high-level neutralizing activity using RFFIT. Fang and her team constructed a human scFv phage antibody library of 8.3 × 108 clones. They successfully verified 20 humanized anti-rabies mAbs with a neutralizing activity of more than 500 IU/mg.52
The Human Fab Library was constructed from peripheral blood lymphocytes of rabies vaccinated donors with 2 × 107 clones. The three selected mAbs could specifically bind to antigenic site III and have the ability to neutralize CVS-11 strain (challenge virus standard, GenBank number accession GQ918139.1). Purified Fab antibody fragments were considered more stable and had higher affinity than mini-antibodies (scFv).53 Sun et al.54 established a human phage Fab antibody library derived from vaccinated human donors. A panel of 11 mAbs specific for RABV glycoproteins were selected; five of them (RV01, RV03, RV05, RV08, and RV09) revealed high binding affinity and neutralizing activities by RFFIT in vitro. Epitope mapping and binding competition analysis showed that five human neutralizing antibodies recognized the antigenic site II of RABV glycoprotein.
Ribosomal display of combinatorial library technique
The scFv gene library was converted for phage display (constructed from the peripheral blood lymphocytes of three immunized donors described before)49 to ribosome display format by polymerase chain reaction (PCR). The mAbs was obtained using purified PCR products for in vitro transcription and translation after five selection rounds. These mAbs with high affinity showed binding activity to the rabies virus.55 Compared with phage display technology, the outstanding advantage of ribosome display technology is that the whole process of protein display and screening is performed entirely in vitro by a cell-free system, which overcomes the lack of cell dependence.56,57
B cells immortalization technique
Human immune B cells from vaccines selected for the presence of serum antibodies capable of broadly neutralizing RABV were immortalized by transformation with the Epstein-Barr virus. Culture supernatants neutralizing the CVS-11 strain were tested to select mAbs. It has become the prototype of antibody RVC-2058 and RVC-58 bound to AS I and AS III, respectively. Moreover, the combination of RVC20 and RVC58 protected hamsters from lethal RABV infection. The cocktail had the ability to neutralize all the RABV tested within a narrow range of concentrations compared with CR4098 and CR57.16 The characteristics of the above methods are shown in Table 2.
Table 2.
The characteristics of different methods to obtain monoclonal antibodies
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hybridoma technique | Long history and mature technology; Operation process visualization. |
Long production time; Antibodies need to be humanized; Unstable and poor reproducibility of hybridoma cells. |
| Antibody library technique | Low cost; Stable and strong specificity; Short production time and highly productive; Easy to humanize and restructure antibody structure. |
High technical requirements; Limited by the capacity of the antibody library; If library mutations, it may be immunogenic to humans. |
| B cells immortalization technique | Small workload and short screening cycle; Independent of viral infection and genetic modification in vitro culture. |
Insufficient numbers of B cells and antibodies; Short survival time and a limited extent in vitro culture. |
Rabies mAbs in clinical development
rhRIG
There are currently no rabies mAb licensed in China. Only recombinant human rabies immunoglobulin (rhRIG) is seeking licensure. The candidate consists of a mixture of human anti-rabies mAbs initially developed by Dr. Bernhard Dietzschold at Thomas Jefferson University. The North China Pharmaceutical Group Corporation (NCPC) has optimized the cell lines and processes for clinical material production. rhRIG could neutralize a wide range of rabies virus strains and provide protection in animal efficacy models.59 Limited information about the product could only be available on the MTTI website with the Google search. Information on the Clinical trials.gov website showed that a phase 2 clinical trial that evaluated the rabies virus neutralizing activity, safety, and tolerability of rhRIG vs. HRIG in combination with human rabies vaccine in 300 healthy adults has been completed.21 After conducting a targeted search at the Chinese Clinical Trial Registry (ChiCTR) website, two phase 1, one phase 2, and one phase 3 clinical trials were completed in Chinese subjects.22–25 Only one literature report found that a single injection with three doses (10, 20, 40IU/kg) of rhRIG was well tolerated in 48 healthy adults.43
SYN023
Among the currently known candidate antibodies for the alternative of RIG, we should first mention the SYN023. Murine hybridomas (3D11E3 and 7G11A3) was generated by the fusion of splenocytes with P3X63Ag8.653 mouse myeloma cells followed by humanized by CDR grafting, human germline sequences homologous to the variable regions of 3D11E3 and 7G11A3 were chosen as the acceptor for humanization. Humanized 3D11E3 and 7G11A3 were then transfected with CHO DG44 cells to obtain CTB011 and CTB012. The humanized mAbs consists of a high percentage of the human immunoglobulin, and only complementarity-determining regions (CDRs) are taken from the mouse antibody. A new humanized monoclonal antibody cocktail mixture SYN023, composed of CTB011 and CTB012 mixed 1:1. SYN023 showed the ability neutralize rabies isolated from China and North America and offered the same protection as HRIG (20 IU/kg) in the Syrian hamsters challenged model.60 They further assessed the efficacy of SYN023 in animal models, SYN023 afforded protection equal to standard HRIG (20 IU/kg) at 0.03 mg/kg in Syrian hamsters and 0.1 mg/kg in beagles. SYN023 at a dose of more than 0.1 mg/kg could reduce the neutralizing antibody titer similar to HRIG.61
An open, parallel, single-dose phase 1 study of healthy Chinese participants evaluated the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of SYN023 when administrated alone or in combination with the rabies vaccine (lot number: 201804088, Chengda Biotechnology). All of the adverse events were grade 1 or 2, transient, and self-limited without long-term sequelae. The participants achieved protective levels of the rabies virus neutralizing antibody (≥0.5 IU/mL) on day 3 after injection SYN023. Characterization of the PK in Chinese indicated that SYN023 displayed a typical and reliable PK, and SYN023 had similar PK/PD relationships in both Chinese and American participants.62 The American phase 2 trial compared the safety, PK and PD of a single dose of SYN023 and licensed rabies vaccines (Imovax, Sanofi Pasteur, RabAvert, GlaxoSmithKline) with one dose of HRIG (HyperRAB ST, Grifols) and licensed rabies vaccines (Imovax and RabAvert).26 SYN023 at dosages of 0.3 mg/kg was considered to be relatively safe, effective and had good PK/PD profiles. Another study compared the safety and neutralizing activity of SYN023 intramuscularly (0.3, 1.0, 2.0 mg/kg) and subcutaneously (0.3 mg/kg) administration in healthy adults. There was no significant difference in adverse event frequency or profile with increased dose or with administration route. At a dosage of 0.3 mg/kg by either the IM or SC route, RVNA levels exceeded the generally accepted protective concentration (≥0.5 IU/mL) by day 1 after injection.27 China is planning to start a phase 3 clinical trial on suspected rabies exposures.28
CL184
CL184 cocktail was a 1:1 protein mixture of two human mAbs (CR57/CR4098) described before.44,48 The hamster model was used to evaluate the ability of CL184 to prevent fatal infections with four selected distinct bat RABV variants: silver-haired bat (Ln RABV); western canyon bat (Ph RABV); big brown bat (Ef-w1 RABV), and Mexican free-tailed bat RABV (Tb RABV). It showed that 42–100% of the animals survived when CL184 was administered with the rabies vaccine. By comparison, 19–95% survivorship against bat RABV variants was observed when HRIG (20 IU/kg) was combined with rabies vaccine. CL184 represented an efficacious alternative for HRIG in this study. We think CL184 and HRIG should be tested separately without vaccine to see how early protective levels appear in blood.63 Bakker et al.29 presented the first clinical data for CL184. When CL184 was administered in conjunction with the rabies vaccine (RabipurTM, Lot No. 1415), less than 40% of the participants had pain at the injection site, no fever, local induration, redness, or swelling. The RVNA levels of all participants were higher than the standard level (0.5 IU/mL) from day 14 in combination with the rabies vaccine. CL184 can be used in lower volumes than HRIG or ERIG due to its high concentration, contributing to better local tolerability. Phase 2 clinical trials have been completed in adolescents and healthy adults,30,31 but no published results of this trial could be found yet. Crucell was acquired by Janssen Pharmaceutical Companies of Johnson & Johnson in 2011, and the product is no longer listed to be in development on its website.64
Discussion
Rabies mAbs can be obtained from large-scale culture directly after hybridization, and it is proved that hybridoma antibodies can protect animals against rabies virus attack. This is a milestone breakthrough in the development of rabies mAbs. The first generation of mAbs obtained by murine hybridoma had the same disadvantage as ERIG- the human anti-mouse antibody (HAMA) response. Due to species differences, the instability of some mouse hybridomas and the inability of animal mAbs to adequately activate protective immune functions in the human organism. Humanized murine hybridomas by CDR grafting and human hybridomas come into being. Chimeric and humanized antibodies have become more and more a thing of the past, which were replaced by human antibodies. The phage antibody library technology provides new ideas for obtaining human neutralizing antibodies. Phage displays allow the exposure of peptides and proteins to the surface of filamentous phage, which is eventually selected as antibodies with high affinity and strong specificity. This modern method is highly productive, but if the library mutations, it may be immunogenic to humans. In addition, other technologies such as B cells immortalization, have broad application prospects in the future. One of the best broadly neutralizing mAbs RVC20 was shown to neutralize all 35 tested RABV strains across the world. No matter, which method is used to prepare mAbs, only when mAbs are used in combination to recognize non-overlapping epitopes can they exert the broadest neutralizing efficiency. The application of human mAbs cocktail binding to non-overlapping epitopes of the glycoprotein has been confirmed to neutralize standard rabies strain in hamster challenge models. What’s more, the antibody cocktail must have a neutralizing effect on a broad range of natural RABV variants in the therapeutic antibodies used region.16,34,35 Lina Sun’s team developed a cocktail composed of CR5744 bound to AS I, RV0854 bound to AS II, and RV3A565 (improved CR4098 by replacing the light and heavy chains) bound to AS III. The cocktail could neutralize the CVS-11 strain and 10 rabies virus street strains. The preparation provided better protection with a survival rate of 100% against RABV and also protected all hamsters when combined with the vaccine against RABV.66 Further clinical trials should be conducted to determine the safety and effectiveness of the cocktail.
Despite rabies mAbs have been developing at home and abroad, fast clinical progress is challenging. Several of these mAbs have reached clinical trials with only two products gaining approval in India. Rabies mAbs was demonstrated to neutralize various rabies isolates in Syrian hamster challenge models, and other animal studies should also be conducted in preclinical studies. Early clinical studies in humans are relatively uncomplicated. The phase 1 study is main to explore the appropriate dose and safety evaluation. In phase 2 studies, pharmacokinetics, half-lives, neutralizing activity, and vaccine interaction/inhibition should be evaluated and compared with RIG. The sample size hindered the efficacy evaluation of rabies mAbs in phase 3 studies with category III exposures. That is, if dog bite incidence is low, it may take a long time to enroll adequate exposures to generate sufficient statistical power. The cost of production and conducting a trial in a large sample size, post-marketing surveillance may be too high to ensure return on investment for developers. Besides, all the phase 3 studies were conducted in category III suspected rabies exposures. The outcome of the suspected exposures should continue to be tracked to further determine the efficacy of the rabies mAbs. The efficacy of mAbs should be further confirmed in patients bitten by lab confirmed animals.
Conclusion
From serum polyclonal antibodies to hybridoma mAbs and from murine mAbs to human mAbs, considerable progress has been made in the urgent post-exposure prophylaxis of rabies. The efficiency of neutralizing mAbs cocktails has been confirmed in clinical trials. Developing a neutralizing rabies cocktail binding to the nonoverlapping epitopes of the glycoprotein is a grand challenge, and it also points out the direction for subsequent research. Safe, effective, and affordable rabies mAbs taking the place of HRIG in rabies PEP is just a matter of time.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
- RABV
Rabies virus
- mAbs
Monoclonal antibodies
- a.a.
Amino acid
- AS
Antigenic sites
- WHO
World Health Organization
- PEP
Post-exposure prophylaxis
- RIG
Rabies immune globulin
- ERIG
Equine rabies immune globulin
- HRIG
Human rabies immune globulin
- GMC
Geometric mean concentration
- CI
Confidence interval
- RFFIT
Rapid fluorescence focusing inhibition test
- RVNA
Rabies virus-neutralizing activity
- IFA
Indirect immunofluorescence assay
- ELISA
Enzyme-linked immunosorbent assay
- scFv
Single-chain antibody fragment
- CVS-11
Standard challenge rabies virus strain
- PCR
Polymerase chain reaction
- NCPC
North China Pharmaceutical Group Corporation
- MTTI
Molecular Targeting Technologies
- ChiCTR
Chinese Clinical Trial Registry
- PK
Pharmacokinetics
- PD
Pharmacodynamics
References
- 1.Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971;35(3):235–8. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang MH, Zhu HW, He MH, Wen YJ, Cheng SP. Advance in biological function of rabies virus glycoprotein. China Anim Husb Vet Med. 2016;43(12):3349–55. in Chinese. [Google Scholar]
- 3.Lv XJ, Ma XJ, Wang LH, Li H, Shen XX, Yu PC, Tang Q, Liang GD. Preparation and initial application of a monoclonal antibody specific for a newly discovered conserved linear epitope of rabies virus nucleoprotein. Biomed Environ Sci. 2012;25(1):98–103. doi: 10.3967/0895-3988.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Lafon M, Wiktor TJ, Macfarlan RI. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983;64(Pt 4):843–51. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- 5.Dietzschold B, Gore M, Casali P, Ueki Y, Rupprecht CE, Notkins AL, Koprowski H. Biological characterization of human monoclonal antibodies to rabies virus. J Virol. 1990;64(6):3087–90. doi: 10.1128/jvi.64.6.3087-3090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafon M, Edelman L, Bouvet JP, Lafage M, Montchâtre E. Human monoclonal antibodies specific for the rabies virus glycoprotein and N protein. J Gen Virol. 1990;71(Pt 8):1689–96. doi: 10.1099/0022-1317-71-8-1689. [DOI] [PubMed] [Google Scholar]
- 7.Yousaf MZ, Qasim M, Zia S, Khan M, Ashfaq UA, Khan S. Rabies molecular virology, diagnosis, prevention and treatment. Virol J. 2012;9:50. doi: 10.1186/1743-422X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkerson JA. Rabies update. Wilderness Environ Med. 2000;11(1):31–39. doi: 10.1580/1080-6032(2000)011[0031:RU]2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Rabies post-exposure prophylaxis. 2014. [accessed 2021 July 21]. https://apps.who.int/iris/bitstream/handle/10665/85346/9789245209829_chi.pdf;sequence=9.
- 10.Both L, Banyard AC, van Dolleweerd C, Horton DL, Ma JK, Fooks AR. Passive immunity in the prevention of rabies. Lancet Infect Dis. 2012;12(5):397–407. doi: 10.1016/S1473-3099(11)70340-1. [DOI] [PubMed] [Google Scholar]
- 11.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrieri ML, Peixoto ZM, Paciencia ML, Kotait I, Germano PM. Laboratory diagnosis of equine rabies and its implications for human postexposure prophylaxis. J Virol Methods. 2006;138(1–2):1–9. doi: 10.1016/j.jviromet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.de Kruif J, Bakker AB, Marissen WE, Kramer RA, Throsby M, Rupprecht CE, Goudsmit J. A human monoclonal antibody cocktail as a novel component of rabies postexposure prophylaxis. Annu Rev Med. 2007;58:359–68. doi: 10.1146/annurev.med.58.061705.145053. [DOI] [PubMed] [Google Scholar]
- 14.Satpathy DM, Sahu T, Behera TR. Equine rabies immunoglobulin: a study on its clinical safety. J Indian Med Assoc. 2005;103:238, 241–232. [PubMed] [Google Scholar]
- 15.Goudsmit J, Marissen WE, Weldon WC, Niezgoda M, Hanlon CA, Rice AB, Kruif J, Dietzschold B, Bakker AB, Rupprecht CE. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J Infect Dis. 2006;193(6):796–801. doi: 10.1086/500470. [DOI] [PubMed] [Google Scholar]
- 16.De Benedictis P, Minola A, Rota Nodari E, Aiello R, Zecchin B, Salomoni A, Foglierini M, Agatic G, Vanzetta F, Lavenir R, et al. Development of broad-spectrum human monoclonal antibodies for rabies post-exposure prophylaxis. EMBO Mol Med. 2016;8(4):407–21. doi: 10.15252/emmm.201505986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gogtay N, Thatte U, Kshirsagar N, Leav B, Molrine D, Cheslock P, Kapre SV, Kulkarni PS. Safety and pharmacokinetics of a human monoclonal antibody to rabies virus: a randomized, dose-escalation phase 1 study in adults. Vaccine. 2012;30(50):7315–20. doi: 10.1016/j.vaccine.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Gogtay NJ, Munshi R, Ashwath Narayana DH, Mahendra BJ, Kshirsagar V, Gunale B, Moore S, Cheslock P, Thaker S, Deshpande S, et al. Comparison of a novel human rabies monoclonal antibody to human rabies immunoglobulin for postexposure prophylaxis: a phase 2/3, randomized, single-blind, noninferiority, controlled study. Clin Infect Dis. 2018;66(3):387–95. doi: 10.1093/cid/cix791. [DOI] [PubMed] [Google Scholar]
- 19.The Clinical Trials Registry India (CTRI) . A randomized, double-blind, placebo-controlled phase I/II study to evaluate the safety, tolerability and neutralizing activity of Rabimabs (A murine anti-rabies monoclonal antibody cocktails) against rabies virus in healthy subjects. 2016. November 16 [accessed 2021 July 22]. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11852&EncHid=&userName=Rabimabs.
- 20.Kansagra K, Parmar D, Mendiratta SK, Patel J, Joshi S, Sharma N, Parihar A, Bhoge S, Patel H, Kalita P, et al. A phase 3, randomised, open-label, non-inferiority trial evaluating anti-rabies monoclonal antibody cocktail (TwinrabTM) against Human Rabies Immunoglobulin (HRIG). Clin Infect Dis. 2021;73(9):e2722–e2728. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov . Safety and immunogenicity study of recombinant human rabies immunoglobin (rhRIG) in combination with rabies vaccine for human use with human rabies immune globulin (HRIG) in combination with rabies vaccine for human use in healthy adult subjects. 2015. Sept 25 [accessed 2021 Sept 17]. https://clinicaltrials.gov/ct2/show/study/NCT02559921.
- 22.Chinese Clinical Trial Registry (ChiCTR) . Preliminary assessment of the dose tolerance, safety, pharmacokinetics and neutralizing activity of rhRIG. 2019. Jun13 [accessed 2021 Sept 15] http://www.chictr.org.cn/showproj.aspx?proj=39967.
- 23.Chinese Clinical Trial Registry (ChiCTR) . Assessment of the safety, pharmacokinetics, neutralizing activity and immunogenicity of rhRIG combined with rabies vaccine. 2019. Jun 11 [accessed 2021 Sept 15]. http://www.chictr.org.cn/showproj.aspx?proj=36532.
- 24.Chinese Clinical Trial Registry (ChiCTR) . Evaluation of the efficacy and safety of rhRIG combined with rabies vaccine and the HRIG combined with rabies vaccine. 2019. May 17 [accessed 2021 Sept 15]. http://www.chictr.org.cn/showproj.aspx?proj=36372.
- 25.Chinese Clinical Trial Registry (ChiCTR) . Evaluation of the efficacy and safety of rhRIG combined with rabies vaccine and the marketed HRIG combined with rabies vaccine: a randomized, blind, positive controlled trial. 2019. Feb 23 [accessed 2021 Sept 15]. http://www.chictr.org.cn/showproj.aspx?proj=36013.
- 26.ClinicalTrials.gov . A comparison of the safety, PD and PK of a single dose of SYN023 administered with licensed rabies vaccines. 2016. Nov 6. [accessed 2021 Sept 17]. https://clinicaltrials.gov/ct2/show/study/NCT02956746.
- 27.McClain JB, Chuang A, Moore SM, Tsao E. Safety, pharmacokinetics, and neutralizing activity of SYN023, a mixture of two novel antirabies monoclonal antibodies intended for use in postrabies exposure prophylaxis. Clin Pharmacol Drug Dev. 2021;10(7):807–17. doi: 10.1002/cpdd.917. [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov . A phase III clinical study to evaluate SYN023ʹs efficacy and safety. 2020. Nov 25 [accessed 2021 Sept 17]. https://clinicaltrials.gov/ct2/show/study/NCT04644484.
- 29.Bakker AB, Python C, Kissling CJ, Pandya P, Marissen WE, Brink MF, Lagerwerf F, Worst S, van Corven E, Kostense S, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine. 2008;26(47):5922–27. doi: 10.1016/j.vaccine.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 30.ClinicalTrials.gov . Randomized phase II trial on safety and neutralizing activity of CL184 and rabies vaccine versus human rabies immune globulin (HRIG) and rabies vaccine in children and adolescents. 2008. Jul 2 [accessed 2021 Sept 18]. https://clinicaltrials.gov/ct2/show/study/NCT00708084.
- 31.ClinicalTrials.gov . Rabies virus neutralizing activity and safety of CL184, a monoclonal antibody cocktail, in simulated rabies post exposure prophylaxis in healthy adults. 2010. Oct 26 [accessed 2021 Sept 18]. https://clinicaltrials.gov/ct2/show/study/NCT01228383.
- 32.Rabishield rabies human monoclonal antibody. Serum Institute of India PVT.LTD. 2016. [accessed 2021 Jul 19]. https://www.seruminstitute.com/product_ind_rabishield.php. [Google Scholar]
- 33.TwinrabTM (RabiMabs) rabies human monoclonal antibody. Zydus Cadila in India. 2019. [accessed 2021 Jul 19]. https://twinrab.com.
- 34.Sloan SE, Hanlon C, Weldon W, Niezgoda M, Blanton J, Self J, Rowley KJ, Mandell RB, Babcock GJ, Thomas WDJ, et al. Identification and characterization of a human monoclonal antibody that potently neutralizes a broad panel of rabies virus isolates. Vaccine. 2007;25(15):2800–10. doi: 10.1016/j.vaccine.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 35.McMichael JC, Fiske MJ, Fredenburg RA, Chakravarti DN, VanDerMeid KR, Barniak V, Caplan J, Bortell E, Baker S, Arumugham R, et al. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66(9):4374–81. doi: 10.1128/IAI.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anwith HS, Ravish HS; DHJIJoCH Ashwathnarayana . Safety of new indigenous human Rabies Monoclonal Antibody (RMAb) for post exposure prophylaxis corresponding author citation article cycle. Indian J. Community Health. 2018;30(3):196–201. [Google Scholar]
- 37.Kuzmina NA, Kuzmin IV, Ellison JA, Rupprecht CE. Conservation of binding epitopes for monoclonal antibodies on the rabies virus glycoprotein. J Antivir Antiretrovir. 2013;5:037–043. doi: 10.4172/jaa.1000061. [DOI] [Google Scholar]
- 38.Presentation from Zydus Cadila at the FDA workshop on rabies monoclonal antibody products as a component of rabies post-exposure prophylaxis. 2018. Jul 17 [accessed 5 Sept 2021]. https://www.fda.gov/downloads/Drugs/NewsEvents/UCM566898.pdf.
- 39.Wiktor TJ, Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. Proc Natl Acad Sci U S A. 1978;75(8):3938–42. doi: 10.1073/pnas.75.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muhamuda K, Madhusudana SN, Ravi V. Use of neutralizing murine monoclonal antibodies to rabies glycoprotein in passive immunotherapy against rabies. Hum Vaccin. 2007;3(5):192–95. doi: 10.4161/hv.3.5.4386. [DOI] [PubMed] [Google Scholar]
- 41.Cheng W, Yu H, Niu WB, Tang GQ. Preparation and identification of monoclonal antibodies against rabies virus glycoprotein. Vet Orientation. 2019;22:217–18. (in Chinese). [Google Scholar]
- 42.Ueki Y, Goldfarb IS, Harindranath N, Gore M, Koprowski H, Notkins AL, Casali P. Clonal analysis of a human antibody response. Quantitation of precursors of antibody-producing cells and generation and characterization of monoclonal IgM, IgG, and IgA to rabies virus. J Exp Med. 1990;171(1):19–34. doi: 10.1084/jem.171.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MX, Jia M, Jin M, Han J, Duan J, Wang LH, Jing RH, Li N, Yao JL, Li YF, et al. Safety of a single injection with recombinant human rabies immunoglobin at various dosages in humans. Chin J Biologicals. 2013;26(7):95–99. (in Chinese). [Google Scholar]
- 44.Marissen WE, Kramer RA, Rice A, Weldon WC, Niezgoda M, Faber M, Slootstra JW, Meloen RH, Clijsters-van der Horst M, Visser TJ, et al. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis. J Virol. 2005;79(8):4672–78. doi: 10.1128/JVI.79.8.4672-4678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakker AB, Marissen WE, Kramer RA, Rice AB, Weldon WC, Niezgoda M, Hanlon CA, Thijsse S, Backus HH, de Kruif J, et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J Virol. 2005;79(14):9062–68. doi: 10.1128/JVI.79.14.9062-9068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Z, Sun LN, Liang MF. Progress of therapeutic human anti-rabies antibodies. Chin Med Biotechnol. 2010;5(3):208–10. (in Chinese). [Google Scholar]
- 47.Vaughan TJ, Osbourn JK, Tempest PR. Human antibodies by design. Nat Biotechnol. 1998;16(6):535–39. doi: 10.1038/nbt0698-535. [DOI] [PubMed] [Google Scholar]
- 48.Kramer RA, Marissen WE, Goudsmit J, Visser TJ, Clijsters-van der Horst M, Bakker AQ, de Jong M, de Jongeneelen M, Thijsse S, Backus HH, et al. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur J Immunol. 2005;35(7):2131–45. doi: 10.1002/eji.200526134. [DOI] [PubMed] [Google Scholar]
- 49.Zhao XL, Yin J, Wang H, Jiang M, Hou XJ. Construction of human phage-displayed scFv library and selection of the ScFv against rabies virus. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004;20:243–47. [PubMed] [Google Scholar]
- 50.Zhao XL, Yin J, Chen WQ, Jiang M, Yang G, Yang ZH. Generation and characterization of human monoclonal antibodies to G5, a linear neutralization epitope on glycoprotein of rabies virus, by phage display technology. Microbiol Immunol. 2008;52(2):89–93. doi: 10.1111/j.1348-0421.2008.00016.x. [DOI] [PubMed] [Google Scholar]
- 51.YU L, Sun L, Jin J, Li C, Li DX, Liang MF. Generation of recombinant human ScFv antibodies against Rabies virus. Chinese J Exp Clin Virol. 2012;26(3):189–92. (in Chinese). [Google Scholar]
- 52.Fang SD, Zhao XR, Chen JJ, An C, Nan JJ, Mao XY. Construction and validation of humanized anti-rabies virus monoclonal antibodies. Prog Microbiol Immunol. 2019;47(4):7–13. (in Chinese). [Google Scholar]
- 53.Houimel M, Dellagi K. Isolation and characterization of human neutralizing antibodies to rabies virus derived from a recombinant immune antibody library. J Virol Methods. 2009;161(2):205–15. doi: 10.1016/j.jviromet.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Sun L, Chen Z, Yu L, Wei J, Li C, Jin J, Shen X, Lv X, Tang Q, Li D, et al. Generation and characterization of neutralizing human recombinant antibodies against antigenic site II of rabies virus glycoprotein. Appl Microbiol Biotechnol. 2012;96(2):357–66. doi: 10.1007/s00253-012-4171-4. [DOI] [PubMed] [Google Scholar]
- 55.Zhao XL, Chen WQ, H F, Shen CF, Ji Y, Li JM, Zhang SJ, Yang ZH. Preparation of human antibody fragments against rabies virus based on ribosome display technology. Prog Biochem Biophys. 2011;38(10):945–52. (in Chinese). doi: 10.3724/SP.J.1206.2010.00521. [DOI] [Google Scholar]
- 56.Groves M, Lane S, Douthwaite J, Lowne D, Rees DG, Edwards B, Jackson RH. Affinity maturation of phage display antibody populations using ribosome display. J Immunol Methods. 2006;313(1–2):129–39. doi: 10.1016/j.jim.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhao XL, Chen WQ, Yang ZH, Li JM, Zhang SJ, Tian LF. Selection and affinity maturation of human antibodies against rabies virus from a scFv gene library using ribosome display. J Biotechnol. 2009;144(4):253–58. doi: 10.1016/j.jbiotec.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 58.Hellert J, Buchrieser J, Larrous F, Minola A, de Melo GD, Soriaga L, England P, Haouz A, Telenti A, Schwartz O, et al. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat Commun. 2020;11(1):596. doi: 10.1038/s41467-020-14398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molecular Targeting Technologies . Rabies mAb product. [accessed 2021. Sept 23]. http://www.mtarget.com/mm5/pdfs/pipeline/RabiesMonoclonalAnti.pdf.
- 60.Chao TY, Ren S, Shen E, Moore S, Zhang SF, Chen L, Rupprecht CE, Tsao E. SYN023, a novel humanized monoclonal antibody cocktail, for post-exposure prophylaxis of rabies. PLoS Negl Trop Dis. 2017;11(12):e0006133. doi: 10.1371/journal.pntd.0006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chao TY, Zhang SF, Chen L, Tsao E, Rupprecht CE. In vivo efficacy of SYN023, an anti-rabies monoclonal antibody cocktail, in post-exposure prophylaxis animal models. Trop Med Infect Dis. 2020;5:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding Y, Wu M, Zhang H, Zhu X, Hu Y, Li X, Liu J, Tsao E, Liu M, Li C. Safety, pharmacokinetics and pharmacodynamics of SYN023 alone or in combination with a rabies vaccine: an open, parallel, single dose, phase 1 bridging study in healthy Chinese subjects. Antiviral Res. 2020;184:104956. doi: 10.1016/j.antiviral.2020.104956. [DOI] [PubMed] [Google Scholar]
- 63.Franka R, Carson WC, Ellison JA, Taylor ST, Smith TG, Kuzmina NA, Kuzmin IV, Marissen WE, Rupprecht CE. In vivo efficacy of a cocktail of human monoclonal antibodies (CL184) against diverse North American bat rabies virus variants. Trop Med Infect Dis. 2017;2:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sparrow E, Torvaldsen S, Newall AT, Wood JG, Sheikh M, Kieny MP, Abela-Ridder B. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: a review of the current status of the clinical development pipeline. Vaccine. 2019;37(Suppl 1):A132–a139. doi: 10.1016/j.vaccine.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Sun LN, Liu Y, Li C, Li DX, Liang MF. Generation of human scfv antibodies for antigenic site III of rabies virus glycoprotein from antibody-phage libraries by chain shuffling. Chin J Virol. 2016;32(4):393–98. (in Chinese). [PubMed] [Google Scholar]
- 66.Sun LN, Liu Y, Li C, Li DX, Liang MF. Development of recombinant human monoclonal antibody cocktail for post-exposure rabies prophylaxis. Chin J Virol. 2016;32(4):399–403. (in Chinese). [PubMed] [Google Scholar]


