Abstract
Lysosomes contribute to cellular homeostasis via processes including macromolecule degradation, nutrient sensing, and autophagy. Defective proteins related to lysosomal macromolecule catabolism are known to cause a range of lysosomal storage diseases; however, it is unclear whether mutations in proteins involved in homeostatic nutrient sensing mechanisms cause syndromic sensory disease. Here, we show that SLC7A14, a transporter protein mediating lysosomal uptake of cationic amino acids, is evolutionarily conserved in vertebrate mechanosensory hair cells and highly expressed in lysosomes of mammalian cochlear inner hair cells (IHCs) and retinal photoreceptors. Autosomal recessive mutation of SLC7A14 caused loss of IHCs and photoreceptors, leading to presynaptic auditory neuropathy and retinitis pigmentosa in mice and humans. Loss-of-function mutation altered protein trafficking and increased basal autophagy, leading to progressive cell degeneration. This study implicates autophagy-lysosomal dysfunction in syndromic hearing and vision loss in mice and humans.
Disruption of SLC7A14-mediated lysosomal homeostasis causes syndromic hearing loss.
INTRODUCTION
Lysosomes are acidic organelles that serve as catabolic centers in the cell, degrading macromolecules with intraluminal, pH-dependent hydrolases into building blocks to be reused in biosynthesis (1, 2). Lysosomes also function as a signaling hub integrating nutrient sensing mechanisms and signaling pathways to regulate biogenesis, trafficking, and repair to maintain cellular homeostasis (2, 3). Mutations in genes encoding lysosomal enzymes or transporters lead to accumulation of macromolecules, causing multisystem syndromic disorders classified as lysosomal storage diseases (LSDs); to date, more than 70 LSDs have been found (4). Lysosomal dysfunction can also trigger complex cellular pathogenic cascades, disrupt autophagy, and ultimately lead to cell death. While several LSDs manifest with neurological symptoms, it is unclear whether lysosomal dysfunction due to defective genes encoding proteins not associated with LSDs but rather with nutrient sensing mechanisms causes syndromic diseases involving sensory systems such as vision and hearing in humans. Identification of new disease-related genes and their underlying molecular mechanisms is essential for diagnosis, curative therapies, and preventative measures.
The mammalian auditory sensory epithelium, the organ of Corti, contains inner and outer hair cells (IHCs and OHCs), each with distinct morphology and function (5). IHCs are the true sensory receptor cells that transmit information to the brain, while the OHCs are a mammalian innovation with the unique capability of changing length in response to changes in receptor potential (6–9). Our previous hair cell type–specific transcriptomic analyses showed that Slc7a14, encoding solute carrier family 7 member 14 (SLC7A14), is differentially up-regulated in IHCs compared to OHCs (10–12). SLC7A14 is a cationic amino acid transporter that mediates lysosomal uptake of arginine (13, 14). Slc7a14 is also expressed in retinal photoreceptor cells and subcortical regions of the brain (13, 15, 16). Mutations in SLC7A14 are associated with autosomal recessive retinitis pigmentosa (RP) in humans, and deletion of Slc7a14 in adult mice led to decreased retinal thickness and abnormal electroretinography response (15). Knockdown of slc7a14 in zebrafish showed retinal degeneration and visual defects (15, 17).
In this study, we investigated the function of Slc7a14 in the mammalian inner ear since Slc7a14 is distinctly expressed in hair cells. We questioned whether SLC7A14 is essential for hair cell differentiation, function, and survival and whether the point mutation (c.988G>A; p.Gly330Arg) identified in patients with RP causes hearing loss. Our study shows that while SLC7A14 was not essential for hair cell differentiation and development, deletion or mutation of Slc7a14 led to progressive IHC loss in adult mice without affecting OHC function, a condition known as auditory neuropathy. Auditory function tests in patients with SLC7A14 mutation showed progressive, sensorineural hearing loss in addition to an RP phenotype. Loss-of-function p.Gly330Arg mutation also caused thinning of the photoreceptor layer in the mouse retina, a retinopathy indicative of RP. To elucidate the pathogenic mechanisms underlying IHC degeneration, we examined subcellular localization and function of SLC7A14 in vitro and in vivo. We showed that SLC7A14 was expressed in the lysosomal membrane and trafficked there by a direct intracellular route. The missense mutation SLC7A14 p.Gly330Arg decreased lysosomal membrane localization and likely disrupted SLC7A14-mediated uptake of arginine into the lysosome. Disruption of lysosomal homeostasis after knockdown of SLC7A14 resulted in up-regulation of basal autophagy, causing a significant increase in autophagosome formation and cellular degeneration. Therefore, SLC7A14 is a gene whose mutation can cause hearing loss, in addition to vision impairment, as a result of lysosomal dysfunction. This study also shows that a defective lysosomal transporter can cause syndromic sensory degeneration in mice and humans that is unrelated to LSD.
RESULTS
Expression of SLC7A14 is distinctive in mature IHCs
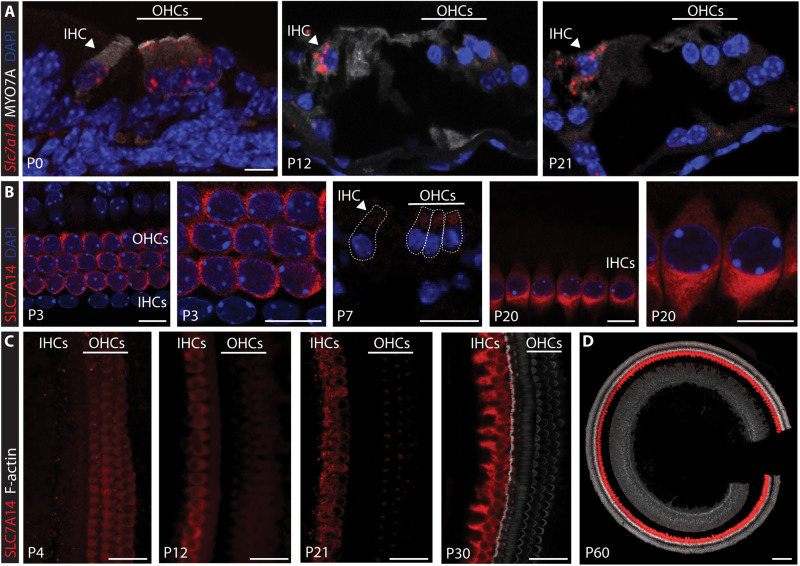
We first used RNAscope-based small-molecule fluorescent in situ hybridization (smFISH) and immunofluorescence to examine the expression of Slc7a14/SLC7A14 in developing and adult hair cells of mouse (C57BL/6) cochleae. As shown in Fig. 1A, Slc7a14 was expressed in both IHCs and OHCs at postnatal day 0 (P0). However, at P7, there was a distinctive down-regulation in OHCs, while expression continued to increase in IHCs (Fig. 1, A and B). IHC-specific expression of SLC7A14 increased from P12, and strong expression was observed in P30 and P60 IHCs along the entire length of the cochlea (Fig. 1, B to D, and fig. S1A). Expression of Slc7a14 mRNA was observed in the spiral ganglion at P0; however, no protein expression was detected at any postnatal or adult ages examined (fig. S1, B and C). Expression of SLC7A14 increased in the developing mouse retina and was restricted to rod and cone photoreceptors and ganglion neurons in the adult retina, consistent with previous observations (fig. S1D) (15). In addition to expression in specialized sensory receptors in the cochlea and retina, SLC7A14 is expressed in distinct neuronal populations in the brain including the hippocampus (fig. S1, E and F). Mild gene level expression was observed in vestibular hair cells at embryonic day 18.5; however, no mRNA or protein expression was detected in postnatal vestibular hair cells (fig. S2, A to D).
Fig. 1. Expression of Slc7a14/SLC7A14 in developing mouse organ of Corti.
(A) RNAscope smFISH of Slc7a14 (red probe) in developing mouse organ of Corti, with IHCs and OHCs identified on the basis of MYO7A expression and indicated labels. Scale bar, 10 μm. (B) SLC7A14 expression in mouse (C57BL/6) OHCs observed at P3 and P7 and distinctive expression in IHCs at P20. Scale bars, 10 μm. (C) Immunofluorescent labeling of SLC7A14 in wild-type IHCs and OHCs from P4 to P30. Scale bars, 20 μm. (D) Expression of SLC7A14 observed in mature P60 IHCs. Scale bar, 100 μm.
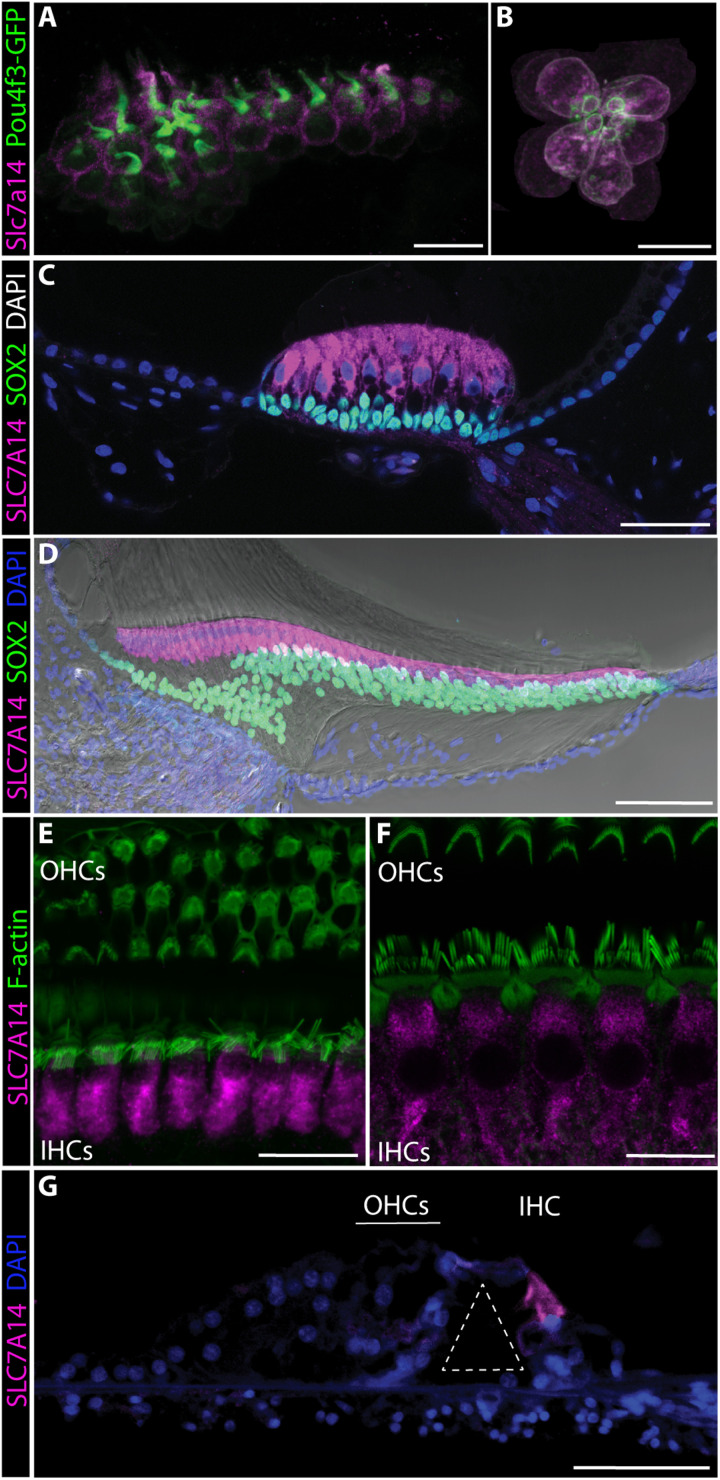
Immunofluorescence was also used to examine cell type–specific expression of SLC7A14 orthologs in mature vertebrate auditory end organs to demonstrate conservation of this relatively unknown SLC protein. Slc7a14(b) expression was observed in zebrafish inner ear hair cells in the saccule and utricle, as well as the lateral line neuromasts, consistent with our previous RNA sequencing data (Fig. 2, A and B) (18). Similarly, hair cells in the turtle auditory papilla and chicken basilar papilla exhibited high-level expression of Slc7a14 (Fig. 2, C and D). Expression of SLC7A14 in the mammalian cochlea was observed to be IHC specific in mouse and rat and, most importantly, in the human cochlea (Fig. 2, E to G). Thus, SLC7A14 is highly conserved through evolution, which is evidenced by homologous protein expression in the sensory hair cells across vertebrate species and, more specifically, in mammalian IHCs.
Fig. 2. Conserved expression of SLC7A14 orthologs among vertebrate sensory hair cells.
SLC7A14 labeled by immunofluorescence in (A) zebrafish saccule and (B) neuromast hair cells (72 hours postfertilization; scale bars, 10 μm); (C) turtle auditory papilla hair cells (adult; scale bar, 20 μm); (D) chicken basilar papilla hair cells (7 days posthatch; scale bar, 50 μm); (E) mouse cochlear hair cells (P60; scale bar, 20 μm); (F) rat cochlear hair cells (P20; scale bar, 20 μm); and (G) adult human cochlear IHC (tunnel of Corti identified by dashed white line) (scale bar, 50 μm).
Loss of SLC7A14 function causes progressive hearing loss in adult mice
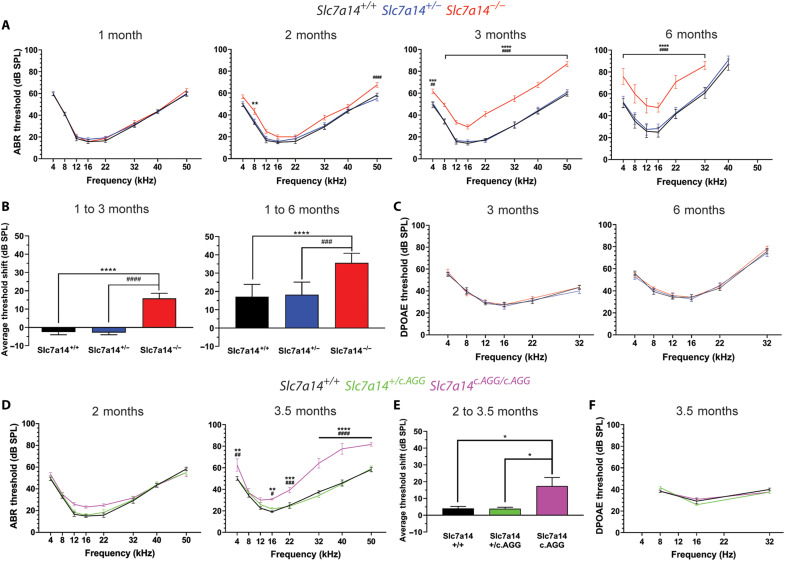
The progressive loss of photoreceptors and visual dysfunction, indicative of RP, was previously observed in Slc7a14 knockout (KO) mice (15). We sought to determine whether loss of SLC7A14 function would also lead to hearing loss in Slc7a14 KO mice, which lack SLC7A14 expression in the IHCs (fig. S3). Auditory function in mice was characterized by measuring auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) thresholds. ABRs are electrical signals evoked from the brainstem during presentation of an acoustic signal, while DPOAEs are generated by the cochlear amplifier and reflect OHC function (6, 7). Figure 3 shows the ABR thresholds of homozygous KO (Slc7a14−/−), heterozygous (Slc7a14+/−), and wild-type (WT) (Slc7a14+/+) mice at 1, 2, 3, and 6 months of age. At 1 month, ABR thresholds of KO mice were comparable to that of wild-type and heterozygous mice, suggesting that mechanotransduction, amplification, and synaptic transmission of hair cells are normal at this stage (Fig. 3A). Thus, deletion of Slc7a14 did not affect differentiation and development of IHCs or OHCs. At 3 months, the ABR thresholds of KO mice were significantly elevated at all frequencies tested. Thresholds were further elevated at 6 months, suggesting progressive hearing loss in the KO mice (Fig. 3, A and B). Notably, the ABR thresholds of heterozygous mice remained comparable to that of wild-type mice at all ages, indicating that a single functional allele provides sufficient protein for IHC function. We also measured DPOAE in these mice to assess the motor activity of OHCs. Contrary to the ABR results, there was no measurable difference in DPOAE thresholds in KO, heterozygous, and wild-type mice at 3 and 6 months (Fig. 3C), suggesting that OHCs are spared despite deletion of this gene.
Fig. 3. Measurement of auditory function in Slc7a14 KO and KI mice.
(A) ABR threshold measurements of Slc7a14+/+ (black line), Slc7a14+/− (blue line), and Slc7a14−/− (KO, red line) mice at 1, 2, 3, and 6 months of age (n = 6). (B) ABR threshold shifts at 3 and 6 months, compared to 1-month baseline. (C) DPOAE threshold measurements of Slc7a14+/+, Slc7a14+/−, and Slc7a14−/− mice at 3 and 6 months (n = 6). (D) ABR thresholds of Slc7a14+/+ (black line), Slc7a14+/c.AGG (green), and Slc7a14c.AGG/c.AGG (KI, magenta line) mice at 2 months (n = 6) and 3.5 months of age (n = 6). (E) ABR threshold shifts in KI mice at 3.5 months compared to 2-month baseline. (F) DPOAE threshold measurements in Slc7a14+/+, Slc7a14+/ c.AGG, and Slc7a14c.AGG/c.AGG mice at 3.5 months (n WT = 6 and KI = 6). Means from the indicated number of biological repeats were statistically compared by two-way analysis of variance (ANOVA) with repeated measures, followed by post hoc analysis with Tukey’s multiple comparisons test (α = 0.05). Symbols: Wild type to KO/KI, *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001; heterozygous to KO/KI, #P < 0.05, ##P < 0.005, ###P < 0.0005, and ####P < 0.0001; error bars, ±SE. SPL, sound pressure level.
Previously, we reported that SLC7A14 mutations were linked to autosomal recessive RP, including the missense variant c.988G>A (15). To demonstrate that the SLC7A14 p.Gly330Arg mutation would lead to hearing loss in mice, we generated Slc7a14 knock-in (KI) mice using CRISPR-Cas9 nuclease and homology-driven repair to replace exon six with a sequence containing the missense codon substitution (c.AGG) (fig. S3). The KI variant allele encodes a mutation of amino acid residue 330 (Gly>Arg), which resides in the intraluminal loop domain between transmembrane domains 7 and 8 of the monomeric transporter protein (fig. S3E). Immunostaining with anti-SLC7A14 antibody showed positive albeit weaker expression of SLC7A14 in the IHCs of homozygous KI mice (fig. S3, H and I). Auditory function was examined in wild-type, heterozygous (Slc7a14+/c.AGG), and KI (Slc7a14c.AGG/c.AGG) mice at 2 and 3.5 months of age. There was no significant difference in ABR thresholds among the three genotypes at 2 months (Fig. 3D). At 3.5 months, ABR threshold was significantly elevated at 4, 32, and 40 kHz in KI mice compared to wild-type and heterozygous mice (Fig. 3, D and E). Similar to heterozygous KO mice, the auditory threshold of heterozygous KI mice remained comparable to that of the wild-type mice. In addition, DPOAE thresholds at 3.5 months showed no difference among the three genotypes, consistent with that observed in the KO mice (Fig. 3F). The KO and KI mice showed comparable (~15 dB) auditory threshold shifts (Fig. 3, B and E). Collectively, these findings demonstrate that loss of function of SLC7A14 causes progressive, sensorineural hearing loss in mice.
Degeneration of IHCs was observed in Slc7a14 KO and KI mice
Scanning electron microscopy (SEM) was used to examine hair bundle morphology in KO and KI mice. Figure 4 shows sample images of IHC and OHC stereocilia bundles in the mid-cochlear region of wild-type, KO, and KI (3-month-old) mouse cochleae. As shown, KO and KI IHC stereocilia exhibited signs of fusion and partial loss (Fig. 4, B to D and F to H). In addition to IHC degeneration, complete loss of IHC stereocilia bundles was also observed (Fig. 4, marked by yellow arrows), indicative of IHC loss. There was no region-specific pattern of IHC degeneration as signs of sporadic IHC stereocilia bundle degeneration and loss were observed along the length of the cochlea. Not unexpectedly, OHC bundles of the KO and KI mice appeared similar to wild-type bundles with a V-shaped appearance and no clear signs of degeneration or loss (Fig. 4, A, E, and I). Confocal microscopy was also used to examine hair cell survival. Immunostaining of 3-month-old KI mouse cochlea showed absent IHCs in the apical and low apical cochlear regions (Fig. 4, J and K). IHCs have ribbon synapses in the basolateral membrane, which are essential for transduction of auditory stimuli. To examine whether SLC7A14 loss-of-function mutation altered presynaptic density, we compared the number of synaptic ribbons in 3.5-month-old KI and wild-type IHCs using anti-CtBP2 antibodies. CtBP2 is a major component of synaptic ribbons, and immunostaining against CtBP2 is often used to quantify presynaptic ribbons. Quantitative data of CtBP2-positive puncta in IHCs, compared by independent t test, revealed no measurable difference between the KI and wild-type IHCs in the apical (KI = 8.61 ± 1.70; WT = 8.46 ± 2.32; P = 0.83) or low apical (KI = 15.7 ± 0.87; WT = 14.2 ± 1.19; P = 0.21) cochlear turns. We also examined stereocilia morphology in the utricle to determine whether loss of SLC7A14 would lead to degeneration. The stereocilia bundles of utricle hair cells appeared normal at 12 months in the KO mouse sensory macula (fig. S2, E and F), consistent with the lack of SLC7A14 expression in vestibular hair cells.
Fig. 4. Morphology of KO and KI mouse cochlear hair cells.
SEM of 3-month-old cochleae (low apical turn). (A) Wild-type IHC and OHC bundles with no signs of degeneration or absent bundles. Scale bars, 50, 10, and 5 μm. (B and C) Slc7a14−/− (KO) cochlea; yellow arrows indicate absent IHC bundles. Scale bars, 50 and 10 μm. (D) Representative images of KO IHC and (E) OHC stereocilia bundles. Scale bars, 5 μm. (F and G) Slc7a14c.AGG/c.AGG (KI) cochlea; yellow arrows indicate absent IHC bundles. Scale bars, 50 and 10 μm. (H) Representative images of KI IHC and (I) OHC stereocilia bundles. Scale bars, 5μm. Immunofluorescence of wild-type and KI mouse cochlea at 2 and 3 months. (J) Low apical turn region. Scale bars, 20 μm. (K) Whole-mount images of low apical turn hair cells; white arrows indicate absent IHCs. Scale bars, 10 μm.
SLC7A14 mutation causes syndromic disease
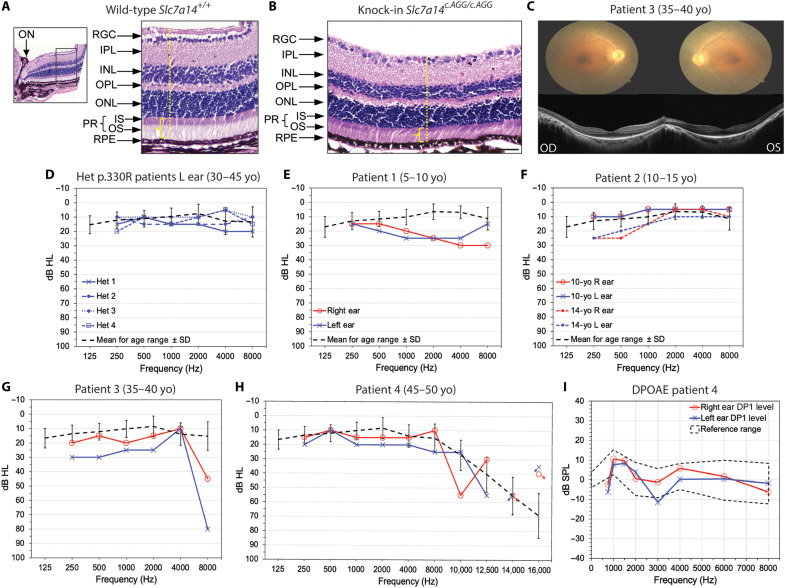
Retina morphology and function associated with KO of SLC7A14 in mice were described previously (15). To examine the syndromic phenotype associated with a loss-of-function mutation of SLC7A14, we conducted histochemical analysis of cross sections of KI mouse retinas. The KI retina was thinner compared to the age- and location-matched wild-type retina, with a visible difference in the thickness of the photoreceptor layer (Fig. 5, A and B). For comparison, we included representative diagnostic exams from homozygous SLC7A14 (c.988G>A) patients with RP. Consistent with previous data (15), patients with RP had bone-spicule pigment deposits in the peripheral retina. Optical coherence tomography (OCT) showed marked disruption of the photoreceptor layer of the retina with preservation of the ellipsoid zone in the fovea, and OCT scans revealed loss of photoreceptor layer outside the macula and bilateral macular edema (Fig. 5C and fig. S4A). Last, representative electroretinography showed severe decreased scotopic and photopic wave amplitude (fig. S4B). These examinations corroborate previous diagnosis of progressive, bilateral retinal degeneration in these patients.
Fig. 5. Syndromic phenotype associated with SLC7A14 loss-of-function mutation.
(A and B) Hematoxylin and eosin–stained retinal cross sections from 3.5-month-old wild-type and homozygous KI mice. Inset in (A) shows location of images, medial to the optic nerve (ON). Decreased retinal thickness (yellow dotted line) and thinning of the photoreceptor layer (yellow bracket) were observed in KI mouse retina. Retinal layers: RGC, retinal ganglion cells; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PR, photoreceptor layer; IS and OS, inner segment and outer segment of rods and cones; RPE, retinal pigment epithelium. Scale bars, 50 μm. (C) Fundoscopic images and OCT scans of the retinas of patient 3, previously diagnosed with RP. (D) Audiogram measurements (left ear) of four heterozygous SLC7A14 c.988G<A patients aged 30 to 45 years. Threshold levels are shown in decibels in hearing level (dB HL), with the age-matched mean dB HL shown for comparison (±SD). (E to H) Audiogram measurements of four probands with homozygous SLC7A14 c.988G<A mutation. Threshold levels are shown in dB HL, with the age-matched mean dB HL shown for comparison (±SD). (E) Patent 1: 5 to 10 years old (yo). (F) Patient 2: 10 to 15 years old; audiogram measurements at two time points, 4 years apart, are shown. (G) Patient 3: 35 to 40 years old. (H) Patient 4: 45 to 50 years old; disconnected data points with arrow indicate no response. (I) Representative DPOAE measurement (patient 4). F1 level, 65 dB; F2 level, 55 dB; normal range outlined in black. L, left; R, right.
To support the hypothesis that loss of function of SLC7A14 causes syndromic disease in humans, we examined whether SLC7A14 mutations cause hearing loss in humans by measuring auditory function in four proband patients previously diagnosed with autosomal recessive RP due to the homozygous mutation SLC7A14 (c.988G>A) (15). For comparison, auditory thresholds of four heterozygous SLC7A14 (c.988G>A) patients were measured (Fig. 5D). The patients live in rural communities and did not have any reported history of job-related exposure to noise. After tympanometry, Weber and Rinne exams were performed to measure middle ear function and exclude conductive hearing loss, and each patient was tested with pure-tone audiometry. Heterozygous (c.988G>A) patients tested within the normal auditory threshold range bilaterally (Fig. 5D and fig. S4). Homozygous patient 1, a 5- to 10-year-old male, had mild bilateral hearing loss between 1 and 8 kHz (Fig. 5E). Progressive bilateral hearing loss (10 to 15 dB) was observed in patient 2, a 10- to 15-year-old male, tested at two time points 4 years apart (Fig. 5F). Patient 3, a 35- to 40-year-old male, displayed moderate to severe bilateral hearing loss at 4 and 8 kHz (Fig. 5G). The fourth patient, a 45- to 50-year-old female, showed normal hearing between 1 and 8 kHz; however, extended-frequency testing showed moderate to severe bilateral hearing loss between 9 and 13 kHz (Fig. 5H). In addition, patients were subjected to DPOAE measurements between 1 and 8 kHz to assess OHC function. The otoacoustic emissions measured were diagnosed as present and normal; a representative example is presented in Fig. 5I. These results suggest that patients homozygous for the SLC7A14 (c.988G>A) variant have mild to moderate sensorineural hearing loss with normal OHC function. Collectively, our data suggest that mutation of SLC7A14 can cause syndromic disease.
SLC7A14 is trafficked by direct intracellular routes to the lysosomal membrane
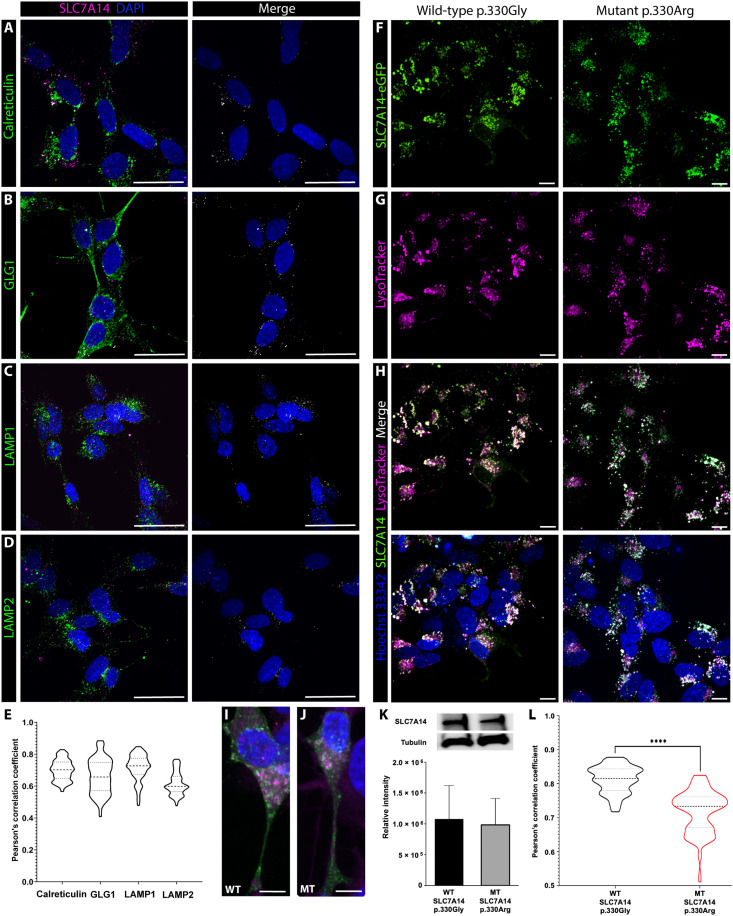
While SLC7A14 is highly and specifically expressed in IHCs, the transporter’s subcellular localization and function in sensory IHCs are unknown. Previous in vitro studies showed that SLC7A14 localizes to the membranes of intracellular vesicles, including lysosomes (14, 15). We used two approaches to examine the subcellular location of SLC7A14 expression. We first used anti-SLC7A14 antibodies and colabeled different intracellular organelles [i.e., endoplasmic reticulum with anti-calreticulin, Golgi apparatus with anti-GLG1 (Golgi apparatus protein 1), and lysosomes with anti-LAMP1 (Lysosomal-associated membrane protein) or anti-LAMP2] to examine trafficking and subcellular localization of SLC7A14 in SH-SY5Y neuroblastoma cells, which have moderate endogenous expression of the protein. Colocalization analysis did not include mitochondria as SLC7A14 was neither predicted nor previously shown to localize to the mitochondrial membrane (14, 15). Confocal microscopy and colocalization analyses showed that SLC7A14 expression colocalized with the expression of LAMP1 or LAMP2 (Fig. 6), suggesting its localization to lysosomes; however, there was also labeling of SLC7A14 that did not coincide with the lysosome. SLC7A14 exhibited perinuclear expression with calreticulin and GLG1, indicating that the protein also localizes to the endoplasmic reticulum and Golgi (Fig. 6). Nikon NIS-Elements software was used to measure the Pearson’s correlation coefficient (PCC), which quantifies the overlap of each respective fluorophore-labeled protein (e.g., LAMP1 or calreticulin) and SLC7A14 (19). The mean PCC values ranged from 0.61 to 0.71, with the highest overlap between lysosomes expressing SLC7A14 and LAMP1 (Fig. 6E). The lower, albeit still significant, correlation between SLC7A14 and GLG1 suggests that the protein is trafficked from the endoplasmic reticulum, where it is produced, to the Golgi for processing and eventually to its destination, the lysosome. In addition, SLC7A14 was not expressed on the plasma membrane in vitro or in vivo, suggesting that the membrane-bound transporter is trafficked by a direct intracellular route to the lysosomal membrane (20).
Fig. 6. Localization of wild-type and mutant SLC7A14 in SH-SY5Y cells.
Immunofluorescence of SH-SY5Y cells shows colocalization of endogenous SLC7A14 and (A) calreticulin to label the endoplasmic reticulum, (B) GLG1 to label the Golgi body, and (C) LAMP1 and (D) LAMP2 to label lysosomes. Scale bars, 10 μm. (E) Violin plot of colocalization of each respective organelle membrane label and SLC7A14 quantified by PCC. Mean individual PCC values for >100 z-stack images (n = 3 technical replicates per label) were compared by one-way ANOVA, and no statistically significant difference among samples was detected (P > 0.05). (F to J) Live SH-SY5Y cells were transfected with (F) wild-type or mutant p.330Gly>Arg SLC7A14-eGFP plasmids and then incubated with (G) LysoTrackerRed99 to label lysosomes. Scale bars, 10μm. (H) Colocalization between SLC7A14-eGFP and LysoTracker shown in white; nuclei labeled with Hoechst 33342. Magnified images of cells expressing (I) wild-type or (J) mutant SLC7A14-eGFP. Scale bars, 10 μm. (K) SLC7A14 protein expression in cells transfected with wild-type or mutant plasmids. Normalized intensity, relative to α-tubulin, quantified by Western blot showed no significant difference in SLC7A14 expression between wild-type and mutant samples (n = 3), unpaired, two-tailed t test (α = 0.05). (L) Violin plots of measured overlap of green and red channels based on PCC. The mean PCC values for >125 z-stack images (n = 3 WT or MT transfected technical replicates) were analyzed by unpaired, two-tailed t test (α = 0.05); ****P < 0.00001.
Mutant SLC7A14 protein showed aberrant subcellular localization
Membrane targeting is essential for the function of the membrane-bound SLC protein. We questioned whether the pathogenicity of the missense mutation SLC7A14 p.Gly330Arg, which alters the primary protein structure, could result in the reduction or loss of lysosomal membrane expression. To demonstrate whether mutant SLC7A14 shows aberrant cellular localization, SH-SY5Y cells were transfected with pcDNA3.1 plasmids encoding either the human wild-type or mutant (p.Gly330Arg) SLC7A14 protein with a fused enhanced green fluorescent protein (eGFP) tag at the C terminus. Following transfection, live cells were labeled with LysoTracker Red and observed for localization of SLC7A14-eGFP to lysosomes (Fig. 6, F to J). There was no difference in SLC7A14-eGFP expression in wild-type or mutant transfected cells (Fig. 6K). Post hoc analysis of corrected total cell fluorescence (CTCF) of the LysoTracker label showed no significant difference in lysosome density between the wild-type and mutant transfected cells (n = 10; unpaired t test, P = 0.757). The eGFP tag did not alter normal trafficking of the wild-type protein to the lysosome. However, quantification of PCC showed that there was a significant decrease in localization of the SLC7A14 p.Gly330Arg-eGFP protein to the lysosome (Fig. 6L).
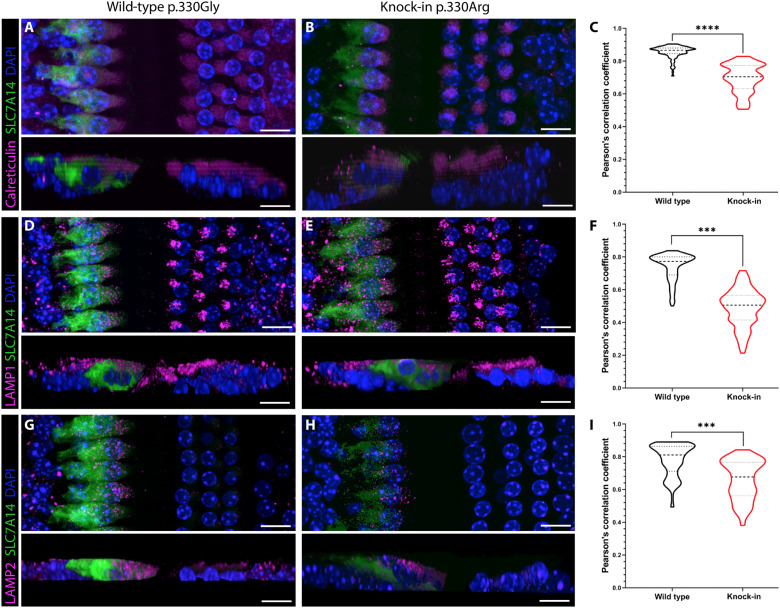
Subcellular localization of the mutant SLC7A14 p.Gly330Arg protein was next examined in the KI mouse cochleae. Similarly, immunostaining showed that colocalization of SLC7A14 p.Gly330Arg with each respective organelle label was significantly decreased in the KI IHCs compared to the wild-type IHCs (Fig. 7). The aberrant localization and diffuse cytosolic expression suggested that the modified residue in the loop domain between transmembrane domains 7 and 8 (fig. S3E) may disrupt trafficking to the lysosome. The abnormal localization and decreased expression of the lysosomal transporter resulting from the missense mutation provide some explanation for the functional deficit seen in KI mice and patients with autosomal recessive RP.
Fig. 7. Localization of SLC7A14 in wild-type and KI mouse IHCs.
Immunofluorescent labeling of SLC7A14 and respective organelle labels: (A and B) endoplasmic reticulum marker calreticulin, (D and E) lysosome label LAMP1, and (G and H) lysosome label LAMP2. Scale bars, 10 μm. (C, F, and I) Violin plots of PCC showing quantified colocalization of each organelle label in wild-type and KI IHCs. Statistical differences between mean PCC values from >85 z-stack images (n = 3 technical replicates per genotype) were determined by unpaired t test (α = 0.05). ***P < 0.0001; ****P < 0.00001.
The SLC7A14 mutation results in dysregulation of basal autophagy
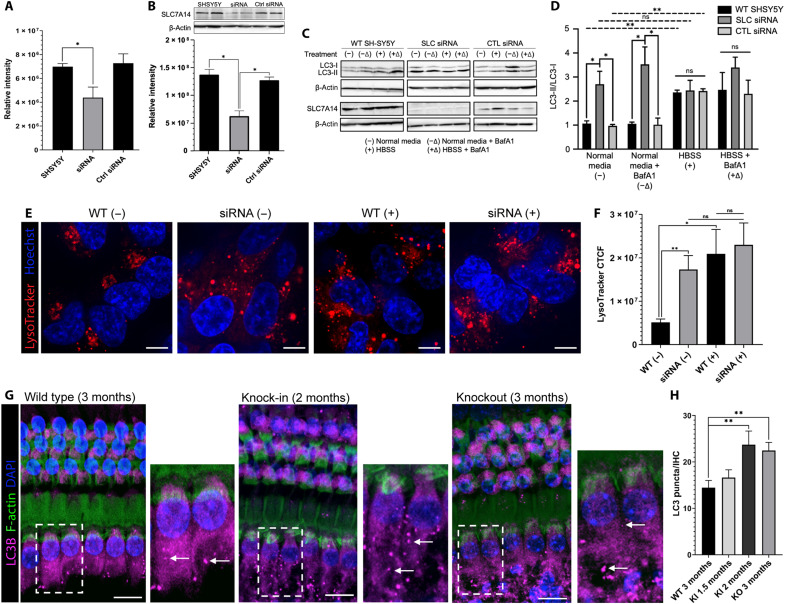
Lysosomal SLC transporters have frequently been implicated in disease; their dysfunction can result in impairment of autophagy or lysosomal storage disorders (21, 22). The activity of SLC7A14 in the lysosomal uptake of arginine suggests that the transporter may regulate metabolic homeostasis and autophagy (14, 23). In postmitotic cells, such as photoreceptors, hair cells, and neurons, basal autophagy is an essential mechanism for cell survival, and dysregulation of autophagy in these cell populations can lead to cell degeneration and death (24). For this reason, we first investigated the role of SLC7A14 in regulating autophagy by examining autophagosome formation and autophagy activity in SH-SY5Y cells following small interfering RNA (siRNA)–mediated knockdown of SLC7A14. Cultured SH-SY5Y cells were transfected with SLC7A14 siRNA, control siRNA, or incubated in transfection media alone. Following gene knockdown, cells were incubated under normal serum or autophagy-inducing starvation conditions, with or without an autophagy inhibitor. Quantitative polymerase chain reaction (PCR) and Western blot were used to measure mRNA and protein expression, respectively. The SLC7A14 siRNA–treated cells had significantly decreased protein expression compared to untreated and control siRNA–treated cells (Fig. 8, A and B). The autophagy marker microtubule-associated protein 1 light chain kinase (LC3) was used to assess autophagy activity. Normally, LC3 is in the cytosol as LC3-I, but upon autophagic activation, LC3-I conjugates to form the autophagosome-associated LC3-II, which appears as punctate rather than diffuse throughout the cytoplasm (25). There was a significant increase in the LC3-II–to–LC3-I ratio in both wild-type SH-SY5Y and control siRNA–treated cells after starvation, indicative of increased autophagosome formation and autophagy activity (Fig. 8, C and D). However, the SLC7A14 siRNA–treated cells had a significant increase in LC3-II–to–LC3-I ratio under normal serum conditions, compared to wild-type and control siRNA–treated cells. These results suggest that a significant decrease in SLC7A14 expression induced an autophagic response, increasing basal autophagy. Samples were also incubated with bafilomycin A1 (BafA1), an inhibitor of autolysosome acidification and autophagosome-lysosome fusion (26), to examine whether the observed elevated autophagosome formation was due to autophagic flux. Under starvation conditions, in the presence of BafA1, both wild-type and control siRNA–treated cells showed elevated LC3-II–to–LC3-I ratios, evidencing induction of autophagy and accumulation of autophagosomes (Fig. 8, C and D). Furthermore, in the presence of BafA1, the SLC7A14 siRNA–treated cells had elevated ratios, indicating an increase in autophagosome formation with inhibition of autophagosome-lysosome–mediated degradation.
Fig. 8. Autophagy activity in SH-SY5Y cell line and cochlear IHCs.
(A) SLC7A14 mRNA expression quantified by RT-PCR after gene knockdown in untreated, SLC7A14 siRNA–, or control siRNA–treated SH-SY5Y cells. Relative intensity normalized to GAPDH control. Statistical differences in mean intensity (n = 3) determined by unpaired t test (α = 0.05). (B) SLC7A14 protein expression in untreated, siRNA-, and control siRNA–treated SH-SY5Y cells, following a 96-hour incubation, calculated as mean intensity (normalized to β-actin) (n = 3). Statistical differences determined by two-way ANOVA (α = 0.05). Representative Western blot image shown. (C) Following SLC7A14 protein knockdown, untreated, siRNA, and control siRNA samples were incubated in normal medium (−), normal medium plus inhibitor BafA1 (−Δ), HBSS starvation media (+), or HBSS starvation media plus inhibitor BafA1 (+Δ). Representative Western blot image from treated samples. Mean intensity normalized to β-actin (n = 3). (D) Calculated LC3-II/LC3-I ratios (n = 3) from quantified samples in (C) compared by two-way ANOVA with repeated measures, followed by post hoc analysis with Tukey’s multiple comparisons test (α = 0.05). (E) Live-cell confocal microscopy of untreated (WT) and siRNA-treated cells labeled with LysoTracker and Hoechst. Scale bars, 5 μm. (F) CTCF of LysoTracker label in samples represented in (E). Fluorescence quantified with ImageJ (n = 10). Statistical differences determined by unpaired, two-tailed t test (α = 0.05). (G) Representative images of mouse cochleae depicting autophagy activity, indicated by LC3 puncta (white arrows). Scale bars, 10 μm. (H) Mean LC3B puncta in wild-type, KI, and KO IHCs (n = 18 IHCs per genotype from two cochlea; low apical turn region) compared by unpaired, two-tailed t test (α = 0.05). P values shown as follows: *P < 0.05; **P < 0.005; ns, not statistically significant; error bars, ±SE.
We questioned whether the increased basal autophagy in SLC7A14 siRNA–treated cells was due to increased lysosomal activity. Wild-type and siRNA-treated cells, under normal medium or starvation conditions, were labeled with LysoTracker and imaged (Fig. 8E). Under starvation conditions, there was a significant increase in CTCF of LysoTracker-labeled lysosomes in the wild-type cells, consistent with observed elevated autophagy activity (Fig. 8, D and F). However, a similar increase in lysosomal label fluorescence was present in the SLC7A14 siRNA–treated cells, independent of nutrient status, suggesting dysfunction of lysosome-mediated metabolic homeostasis mechanisms.
To investigate whether loss-of-function mutation similarly altered basal autophagy in vivo, LC3-I to LC3-II conversion was examined in KI and KO mouse cochleae (aged 1.5 to 3 months). The number of LC3 puncta in IHCs, marking active autolysosome formation, was significantly higher in KI and KO mice, compared to wild-type animals (Fig. 8, G and H). Disruption of autophagy in mature hair cells, due to loss of normal SLC7A14 function, suggests a pathological mechanism leading to progressive IHC degeneration and subsequent hearing loss.
DISCUSSION
In this study, we investigated the function of Slc7a14 in cochlear hair cells. Unlike other SLC proteins that are ubiquitously expressed in many tissue types, expression of SLC7A14 and corresponding homologs is highly conserved and restricted to distinct neurosensory cell populations including vertebrate inner ear sensory hair cells and retinal photoreceptors. SLC7A14 can serve as a new, highly specific marker for all hair cells in nonmammals and mature IHCs in mammals. Slc7a14 was detected in developing vestibular hair cells and neonatal OHCs, but no protein expression was observed in adults. This transient expression during development is not unusual as other SLC family members such as Slc17a8 (vGLUT3), Slc1a3 (GLAST), and Slc26a5 (Prestin) are expressed in both IHCs and OHCs early in development but show differential expression in mature IHCs and OHCs (gEAR Database, https://umgear.org/). Note that although high expression of Slc7a14 was observed in early postnatal spiral ganglion neurons, expression decreased during maturation, and no positive staining of SLC7A14 protein was detected in neonate or adult cochlea, suggesting no functional role of SLC7A14 in these cochlear neurons. In addition, we confirmed SLC7A14 expression in mature retinal photoreceptors and subcortical regions of the brain, including high-level expression in the hippocampus.
Although SLC7A14 was detected in developing IHCs and OHCs, KO or loss of function did not affect differentiation and development of hair cells; hearing thresholds of 1-month-old KO and KI mice were comparable to their wild-type counterpart. However, KO of SLC7A14 caused late-onset hearing loss progressing from mild/moderate loss at 3 months to severe hearing loss by 6 months. Elevated hearing thresholds were observed across all frequencies, consistent with the expression pattern of SLC7A14 in all cochlear IHCs. Similar auditory phenotypes were exhibited in KI mice at a comparable age, with significantly elevated thresholds at higher frequencies. The auditory phenotypes seen in KO and KI mice were dissimilar to the pattern of progressive hearing loss observed in mouse models with mutations in other functional hair cell genes, such as CDH23, KCNQ4, and Eps8L2, which often proceeds from high to low frequencies (27–29). DPOAE thresholds of KO and KI mice did not significantly differ from those of wild-type littermates, likely due to the lack of SLC7A14 expression in adult OHCs. Morphological examination of Slc7a14 KO and KI mouse cochleae with SEM and confocal microscopy confirmed degeneration of IHC stereocilia bundles and loss of IHCs, while OHCs remained normal. Thus, mutation of Slc7a14 results in progressive hearing loss due to loss of IHC function. This distinctive type of sensorineural hearing loss, known as auditory neuropathy, is a condition that affects neuronal processing of auditory stimuli due to dysfunction of IHCs, ganglion neurons, or synaptic transmission, while OHC function is sustained (30). Other IHC-specific transporters have also been implicated in presynaptic auditory neuropathy including SLC19A2 (high-affinity thiamine transporter), SLC17A8 (vesicular glutamate transporter VGLUT3) (31), OTOF (synaptic vesicular release) (32, 33), and CACNA1D (voltage-gated L-type calcium channel) (34). However, SLC7A14 is the first intracellular, lysosomal transporter implicated in presynaptic, sensorineural hearing loss.
The pathophysiological evidence from KI mice suggested that the SLC7A14 p.Gly330Arg mutation, identified in patients with RP, can cause syndromic disease, with progressive hearing and vision impairment. Audiometric testing in four patients with SLC7A14 p.Gly330Arg mutation showed mild to moderate bilateral sensorineural hearing loss, supporting the conclusion from mouse experiments that mutation of SLC7A14 can cause syndromic disease. Although the rate of progression and severity of the auditory and visual phenotypes varied among patients, it is conceivable that compounding variables such as genetic variants, sex, and environment may play a role in pathogenesis. It is interesting that the auditory phenotype is more pronounced in mice than in humans, although it should be noted that variation of auditory phenotype and onset also depends on the function of the gene or protein and the presence of other compensating mechanisms. Mutations of those molecules critical for hair cell development (e.g., MIR96) and specialization (such as TMC1 and USH1C) tend to cause hearing loss more quickly and consistently among patients, whereas mutations of other proteins (KCNQ4) often exhibit large variations in phenotype and onset (35–38). It is feasible that another cationic amino acid transporter can partially compensate for the loss of SLC7A14-mediated functions. Alternatively, there may be a mechanism in human IHCs that can somewhat maintain hair cell homeostasis and promote cell survival when SLC7A14 function is disrupted. Most patients tested exhibited differences in auditory thresholds bilaterally, which is not uncommon in sensorineural hearing loss. Similarly, bilateral variation in retinal pathology was also observed in these patients with RP (15).
A distinct feature shared between hair cells and photoreceptor cells is that they both have ribbon synapses that allow the specialized receptor cells to transduce sensory stimuli into an electrical signal that is graded in response to stimulus intensity (39, 40). A ribbon synapse is characterized by the presence of a large electron-dense presynaptic organelle, the synaptic ribbon, that tethers glutamate-containing synaptic vesicles near the active zone. The cell type–specific expression of the lysosomal-associated protein SLC7A14 may cause one to speculate that it is involved in the molecular or structural tuning of ribbon synapses via lysosome-mediated vesicle breakdown or signaling. Our study does not support this speculation for two reasons. First, vestibular hair cells also have ribbon synapses with glutamate as a neurotransmitter; however, SLC7A14 is not expressed in vestibular hair cells. Second, immunostaining and confocal microscopy showed no measurable decrease in CtBP2 count in Slc7a14 KI IHCs, suggesting that loss of transporter function does not affect presynaptic ribbon structure or vesicle recycling.
To investigate why loss of function caused IHC degeneration, we examined intracellular trafficking and localization of wild-type and mutant SLC7A14. Using colocalization assays and confocal microscopy, we showed that the SLC7A14 transporter is localized to intracellular membranes, including the endoplasmic reticulum and Golgi, and trafficked through direct intracellular routes to the lysosome. These results corroborate previous in silico predictions based on subcellular localization motifs and colabeling analyses that the membrane-bound transporter localizes to the lysosome (13–15). While expression of SLC7A14 in the endoplasmic reticulum and Golgi might be transient, on its route to the lysosome, it is unknown whether the protein may also have a functional role in these organelles. The mutant SLC7A14 p.Gly330Arg showed reduced membrane targeting, both in transfected cells and KI IHCs, suggesting that the loss-of-function variant likely altered protein stability and trafficking. Aberrant protein localization disrupts lysosomal function and may also cause cell stress due to cytosolic accumulation of protein. Lysosomes are important regulator of metabolic homeostasis, and dysfunction of lysosomal proteins can trigger complex cellular cascades, disrupt autophagy, and ultimately lead to cell death (41). Normal localization of SLC7A14 to the lysosome membrane and its function in the uptake of arginine provide an important clue about the transporter. Its role in maintaining cellular homeostasis or facilitating nutrient sensing mechanisms merits further exploration. Other lysosomal arginine transporters expressed broadly across tissue types, such as SLC38A9, have been shown to act as arginine sensors regulating autophagy and metabolic homeostasis (41–43). Disruption of autophagy in postmitotic cells, whether through prevention of autophagosome formation, loss of lysosomal function, or other associated mechanisms, has been implicated in the pathogenesis of neurodegenerative disease (44). We showed that decreased expression of SLC7A14 caused an increase in lysosomal activity and basal autophagy in vitro. Furthermore, the KO and KI IHCs both showed an increase in autophagosome formation compared to age-matched wild-type IHCs. These results hint at autophagy disruption, resulting from SLC7A14 mutation, as a pathophysiological mechanism that leads to progressive photoreceptor and IHC loss (45, 46). Disruption of autophagy has been shown to cause loss of photoreceptors (45). The IHC-specific expression of SLC7A14 in the cochlea may underlie differential autophagy mechanisms in IHCs to maintain cellular homeostasis and promote cell survival, compared to the functionally distinct and more vulnerable OHCs. The heterogeneity of lysosomes in IHCs and OHCs, including both the IHC-specific SLC7A14 expression and differential expression of other lysosomal proteins, including LAMP2, suggests both common and distinctive lysosome functions in cochlear hair cells.
Investigation of the pathogenesis of LSDs has elucidated more diverse functions of lysosomes, now considered to be a key cellular signaling organelle regulating processes from metabolic homeostasis to membrane repair, immune response, and cell survival (22). The majority of more than 70 LSDs present as progressive neurological diseases associated with accumulation of a variety of macromolecules due to dysfunction of lysosomal enzymes or transporters (4). While LSDs with a distinctive auditory phenotype are rare, studies from two LSD mouse models with mucopolysaccharidosis type I or type VII showed progressive hearing loss from 2 months of age (47, 48). However, this hearing loss was not due to degeneration and loss of hair cells but rather alterations in mass and stiffness of the cochlear structures. A recent study showed that mice lacking expression of two lysosomal cation channels, mucolipins 1 and 3, displayed accelerated age-related hearing loss due to OHC degeneration (49). Unlike our observations in SLC7A14 KO hair cells, there was no observed increase in lysosome-mediated autophagy activity (LC3); rather, the absence of both mucolipins caused swelling and increased permeabilization of lysosomes, which led to cell death. While these studies also underscore the importance of lysosome function in hair cell survival, the current study is the first to show that lysosomal-autophagy dysfunction causes syndromic hearing and vision impairment in mice and humans.
More than 120 genes have been associated with hearing loss with some related to syndromic disease including Usher, Alport, and Stickler syndromes (50, 51). While mutations of SLC7A14 cause hearing loss and RP, similar to Usher syndrome, there are some differences between them. First, all 10 genes (CDH23, CIB2, CLRN1, DFNB31, GPR98, MY07A, PCHD15, USH1C, USH1G, and USH2A) identified in Usher syndrome are expressed in both IHCs and OHCs and encode proteins associated with structure and function of the stereocilia bundle (37). In contrast, SLC7A14 is an intracellular transporter localized to the lysosomal membrane solely in IHCs. SLC7A14 mutation led to degeneration of IHCs, but not OHCs, producing a hearing loss phenotype characteristic of auditory neuropathy. Second, unlike some of the Usher type I and type II mutations, which cause moderate to severe congenital deafness, mutation of SLC7A14 does not affect differentiation and development of hair cells and photoreceptor cells. The onset of hearing and vision loss is progressive, similar to Usher type III. However, unlike Usher types I and III, which display a vestibular phenotype, SLC7A14 mutation does not lead to vestibular disorder, likely due to the lack of SLC7A14 expression in mature vestibular hair cells. Last, we should add that although we did not examine cognitive function in mice and humans, the specific, high-level expression of SLC7A14/SLC7A14 in hippocampal neurons requires further investigation to implicate a functional role of SLC7A14 in the central nervous system, which would also distinguish SLC7A14 from other genes in syndromic hearing loss.
One limitation of this study was the small sample size of human participants. Audiometric testing of additional patients with the same mutation or other variants in SLC7A14 would strengthen our conclusion. While the p.Gly330Arg variant has a minor allelic frequency of 0.17% globally and 2.3% in the east Asian population (gnomAD v2.1.1), it should be noted that homozygous mutations are rare and, so far, have been reported only in east Asian populations (15, 52). Hearing loss due to homozygous SLC7A14 p.Gly330Arg mutation is indicative of a loss-of-function phenotype, as heterozygous mice and human patients showed no obvious phenotype, suggesting that one copy of the SLC7A14 gene appears to be sufficient for function and survival of IHCs. However, it needs to be determined whether haploinsufficiency is a risk factor for accelerated age-related hearing and vision loss or increased vulnerability to noise, intense light exposure, and ototoxicity. Similarly, investigation of transgenic mouse models with other causative autosomal recessive or compound heterozygous mutations of SLC7A14 identified in patients with RP would solidify association of the gene with syndromic hearing loss. While our study suggests that SLC7A14 may contribute to lysosomal homeostasis in a neuronal cell line, further investigation in IHCs and photoreceptors is necessary to fully depict the mechanism of SLC7A14-associated dysfunction inducing pathogenic cascades that cause sensory cell degeneration and death.
In summary, we showed that SLC7A14 is a novel marker for vertebrate hair cells and is highly specific to IHCs in mammals. As a transmembrane transporter, SLC7A14 is trafficked via a direct intracellular route to the lysosomal membrane. Loss-of-function mutation causes auditory neuropathy and RP in mice and humans, indicative of similarities in a key homeostatic mechanism in these cell types. Our study suggests that loss of SLC7A14 function up-regulates basal autophagy, perturbing cellular homeostasis and leading to degeneration of IHCs and photoreceptors. This is the first study to show that lysosomal-autophagy dysfunction causes syndromic hearing loss in mice and humans. SLC7A14 joins the long list of other SLC transporters linked to human disease (53). Recognition of SLC7A14 as an additional candidate gene in patients with syndromic vision and hearing loss is important for proper prognosis and treatment. Since the degeneration of IHCs and photoreceptors progresses after birth, this is an ideal syndromic disease model for targeted gene therapy to correct pathogenic variants before the onset of sensory deficits, thereby preventing permanent hearing and vision impairment.
METHODS
Experimental design
To determine whether mutation of SLC7A14 causes syndromic hearing loss, we used the use of transgenic KO and KI mouse models, as well as human patients. The mechanisms of pathogenesis were investigated both in vitro, using cell culture, and in vivo using animal models. Advanced imaging, immunocytochemistry, molecular biology, and electrophysiology techniques used throughout the study are described in detail below.
Animal models
Wild-type male and female C57BL/6J mice were obtained from The Jackson Laboratory and bred for gene and protein expression analyses. KO and KI mice aged 1 month to a year, both male and female, were used for expression assays and electrophysiology experiments. All mouse experiments were approved by the Creighton Institutional Animal Care and Use Committee or Wenzhou Medical University and Capital Medical University in China. Other species used in vertebrate expression experiments included Tg(Pou4f3:GFP) zebrafish (Creighton University), brown leghorn chicken, red-eared slider turtle (Stanford University), and Sprague-Dawley rat [National Institutes of Health (NIH)/National Institute on Deafness and Other Communication Disorders (NIDCD)]. All animal procedures were approved by the respective institution animal care and use committees.
The KO mice were generated previously (15). Heterozygous mice were bred to obtain Slc7a14+/+ (wild type), Slc7a14+/− (heterozygous), and Slc7a14−/− (KO) mice. Genotyping was conducted using genomic DNA extracted from tissue samples. PCR reactions were performed using a Taq PCR master mix kit (Qiagen) with the following primers: 5′-TCTATCAGCAAACCTTCACTGCAAC-3′ (forward) and 5′-CTGTCAATCATACTGTCAACATGGGTTC-3′ (reverse). Because the distinct KO phenotype is a result of only a 10–base pair (bp) deletion, Sanger sequencing of the ~550-bp product followed by genomic alignment confirmed the genotype of experimental mice (fig. S3). Loss of SLC7A14 expression was confirmed by antibody-mediated immunofluorescence.
The KI mice were generated using CRISPR-Cas9–mediated genomic recombination. DNA donor sequences, encoding mouse exon 6 with the human mutant codon c.AGG (p.Gly330Arg), together with Cas9 nickase and single-guide RNA were injected into C57BL/6 mouse zygotes. Founder mice (F0) were screened for the substitution and then outcrossed with wild-type mice to generate a stable F1 generation. DNA sequencing was performed to confirm that the target gene had the correct missense mutation encoding SLC7A14 p.Gly330Arg (fig. S3). Heterozygous mice (Slc7a14+/c.AGG) were mated to generate the experimental genotypes including wild-type (Slc7a14+/+) and homozygous KI mice (Slc7a14c.AGG/c.AGG). Mice were genotyped with forward (5′-CGTATGTGTCTGTGAGCATGA-3′) and reverse (5′-CAAGGACGGCAGGTTTTTGG-3′) primers, and the missense mutation was verified by Sanger sequencing.
Human participants
Patient participants with RP were identified and clinically diagnosed in a previous study. For this study, further ophthalmic examination and auditory tests were conducted with informed consent of each participant. The protocol was approved by the ethics committees of Wenzhou Medical University and Capital Medical University.
Sample processing and histology
Mice were anesthetized with a ketamine/xylazine mixture and then decapitated. Tissues of interest, including the brain, eyes, and otic capsules, were removed and placed in 4% paraformaldehyde (PFA) in 1× phosphate-buffered saline (PBS) for 24 hours at 4°C. Adult cochlea were decalcified in 120 mM EDTA for 3 to 5 days at 4°C until the bony tissue was soft and flexible. Fixed tissues were washed in 1× PBS three times, then dehydrated using a standard ethanol series, embedded in paraffin, and sectioned (8 to 15 μm thick). The brain, retina, and cochlear sections were used for subsequent gene and protein expression assays.
RNAscope
The RNAscope-based smFISH assay from Advanced Cell Diagnostics (ACD) was used to examine Slc7a14 mRNA expression in the developing mouse cochlea. Samples were pretreated according to the ACD protocol for formalin-fixed paraffin-embedded tissue. However, because of the fragility of the tissues, the pretreatment target retrieval and protease steps were modified. Tissues were covered with freshly prepared 0.5% pepsin + 5 mM HCl in deionized water and incubated in the humidifying chamber at 37°C for 10 min. The remaining protocol was conducted according to the RNAscope 2.5 HD Detection Reagent RED user manual and published protocols (54). Following the final wash, samples were immunolabeled using the protocol described below to label hair cells using anti-MYO7A primary antibodies. Probes were detected by red channel fluorescence, with no probe treatment included as a negative control.
Immunofluorescence
Mouse tissues prepared as described above were used for immunofluorescence experiments. Formalin-fixed paraffin-embedded tissues were sectioned, then deparaffinized, and rehydrated. Additional otic capsules were dissected for whole-mount preparations. Samples were washed in 1× PBS, then permeabilized, and blocked in 1× PBS with 0.2% Triton X-100 and 5% normal goat serum (NGS), followed by incubation with primary antibodies (table S1) overnight at 4°C. Samples were rinsed in 1× PBS three times and then incubated with Alexa Fluor (AF)–conjugated secondary antibodies (table S1) for 1 hour at room temperature followed by subsequent washes. For some samples, additional staining with 4′,6-diamidino-2-phenylindole (DAPI) or AF488-phalloidin (anti–F-actin) was used to label nuclei and stereocilia, respectively. Last, samples were rinsed three times in 1× PBS and mounted with SlowFade (Invitrogen) and then imaged.
Tissues from vertebrate species were collected following euthanasia according to respective institutional animal care and use protocols. Zebrafish Tg(Pou4f3:,GFP) larvae (72 hours postfertilization) were euthanized and fixed in 4% PFA in PBS overnight at 4°C. Immunostaining was conducted as previously described (55). Red-eared sliders were decapitated, the lower jaw removed, head bisected, brain removed, and bone of the ear capsule dissected away to allow penetration of fixative. The bisected heads were stored in 4% PFA in PBS at room temperature overnight. The papilla was then dissected out of the bony capsule in PBS. Fertilized chicken eggs were purchased from AA Laboratory Eggs Inc. (Westminster, CA). Following euthanasia and decapitation, posthatch day 7 chicken heads were bisected, brain and lower jaw were removed, and the bony ear capsule (containing all auditory and vestibular organs) was excised with surgical scissors. Following dissection to expose the basal region, the tissue was placed in 4% PFA at room temperature for 1 hour. Immunostaining of chicken and turtle tissues was conducted as previously described (56), modifying the protocol to accommodate free-floating vibratome sections, which were stained in mesh-bottom baskets (57). Rat cochleae were prepared similarly to the mouse cochlea as described above. Human cochlear sections were provided by the NIDCD National Temporal Bone Laboratory at UCLA. Celloidin-embedded human inner ear sections were immunostained using a published protocol (58). DAPI was used to label the nuclei of inner ear cells, and anti-SLC7A14 detected cell-specific protein expression.
Confocal microscopy
Visualization of smFISH and immunofluorescence was conducted using Zeiss laser scanning confocal microscopes LSM700, LSM710, or LSM880. Sections were imaged at 1.0 zoom at ×40 or ×63 magnification using Zen Black acquisition software. In addition, samples for colocalization analyses, including cultured cells, were imaged on a Nikon Ti-E with a Yokogawa Spinning Disc Confocal microscope with a Flash 4.0 Hamamatsu Monochrome camera.
Scanning electron microscopy
The protocol described by Jia et al. (59) was referenced for sample preparation and imaging protocol. Briefly, cochleae from wild-type and KO mice were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) containing 2 mM CaCl2 for 24 hours at 4°C. The cochleae were postfixed for 1 hour in 1% OsO4 in 0.1 M sodium cacodylate buffer. Samples were then dehydrated in ethanol, critical point–dried from CO2, and sputter-coated with gold. SEM was conducted on a FEI Quanta 200 at the University of Nebraska Medical Center imaging core, and images were acquired digitally.
Auditory electrophysiology and retinography
To characterize the auditory phenotype of the mice, we measured both the ABR and DPOAE using our established protocols (7, 60). For all experiments, both male and female mice were included, with a minimum sample size based on a priori power analysis. ABR measurements were elicited with standard tone bursts from 4 to 50 kHz. The response signals were amplified (100,000×), filtered, and acquired by a TDT RZ6 (Tucker-Davis Technologies). Each averaged response was based on 500 stimulus repetitions. DPOAE thresholds were elicited by input of two primary tones of different frequencies (f1 and f2) from two electrostatic speakers, with the f2 level of 10 dB lower than the f1 level (Tucker-Davis Technologies, EC1 phones). The sound pressure obtained from the microphone in the ear canal was amplified, and fast Fourier transforms were computed from averaged waveforms of ear canal sound pressure. The DPOAE response was measured in response to f1 and f2, with f2/f1 = 1.2. Recorded ABR and DPOAE thresholds were graphed, and statistical analysis was conducted using GraphPad Prism.
To test the auditory function in diagnosed patients with autosomal recessive RP, otoscopy and tympanometry were performed to examine the ear canal and middle ear, and pure-tone audiometry was conducted according to a standard protocol (61). Normal air conduction threshold search screening was conducted by presenting pure tones from 250 Hz to 8 kHz at the upper limits of human hearing. Decreasing intensities, at 10-dB intervals, were presented until the patient no longer responded. If no deficit was detected up to 8 kHz, extended-range testing up to 16 kHz was conducted. Hearing thresholds were recorded for both the left and right ears and compared to age-matched average hearing thresholds (62). DPOAE measurements of both left and right ears from human patients were conducted on the basis of similar principles described above. The DP-gram was generated with standard clinical level separation with f1 level set to 65 dB and f2 at 55 dB, with the frequency ratio of 1.2. The mean noise floor was measured from the amplitudes in the frequency bins above and below the 2f1-f2 component. Details for ophthalmic examinations, including fundoscopy, optical coherence tomography (OCT), and electroretinography (ERG), were described in a previous publication (15).
In vitro experiments
Cell culture
The SH-SY5Y human bone marrow neuroblast cell line [American Type Culture Collection (ATCC) CRL-2266] was selected for in vitro assays based on endogenous expression of SLC7A14. Cells were cultured according to the product data sheet from ATCC and grown in Dulbecco’s minimum essential medium:F12 medium supplemented with 10% fetal bovine serum. Cells were maintained in a humidified 37°C incubator with 5% CO2, and the culture medium was refreshed every 3 to 4 days. Adherent differentiated cells resembling neurons were used for all experiments described below.
Cell transfection
The pcDNA3.1+C-eGFP plasmids (Genscript) containing the wild-type SLC7A14 (WT pcDNA3.1) or mutant SLC7A14 c.988G>A (MT pcDNA3.1) were amplified using the high-efficiency transformation protocol (New England Biolabs). Transformed cells were selected by ampicillin resistance, amplified in liquid LB + ampicillin media, and then incubated at 37°C for 18 hours in a shaker. Plasmids were isolated using the QIAprep Spin Miniprep Kit (Qiagen), and concentration was measured by Nanodrop. Plasmids were sequenced by Sanger sequencing using the mammalian cytomegalovirus promoter and aligned to the reference sequence to confirm the correct open reading frame sequences for both the wild-type and mutant plasmids.
For transfection experiments, SH-SY5Y cells were seeded into six-well plates and incubated until they were 75% confluent. One hour before transfection, the cell medium was replaced with Opti-MEM medium. Cells were transfected using Lipofectamine 3000 (Thermo Fisher Scientific) reagent with approximately 2.0 μg/μl of WT or MT plasmid per well. The transfection media was added to the well plates and incubated for 6 hours. After the incubation period, cells were supplemented with normal medium and incubated under normal conditions for 48 hours and then screened for transfection efficiency.
Immunofluorescence
For in vitro immunofluorescence experiments, SH-SY5Y cells were seeded at 0.5 × 106 in glass-bottomed 35-mm dishes and incubated overnight under normal culture conditions. Media was replaced with 4% PFA, and cells were incubated for 2 hours at 4°C. After fixation, the cells were rinsed three times with 1× PBS and then incubated with 0.2% Triton X-100 + 5% NGS for 20 min. Primary antibodies targeting proteins of interest (table S1) were incubated overnight at 4°C. Following repeat 1× PBS washes, samples were incubated with conjugated secondary antibodies (table S1) for 2 hours at room temperature. Cells were incubated with DAPI for 10 min, washed, then covered with a 50% glycerol/50% 1× PBS solution, and then stored at 4°C before imaging. Cells were imaged on a Nikon Ti-E with a Yokogawa Spinning Disc Confocal microscope with a Flash 4.0 Hamamatsu Monochrome camera.
Colocalization analysis
Live cells were examined for localization of SLC7A14-eGFP to the lysosome. Following transfection, live cells were labeled with LysoTracker Red DND-99 (Thermo Fisher Scientific) at a working concentration of 75 nM. The red fluorophore is acidotropic, which labels the acidic lumen of the lysosomes. Addition of Hoechst 33342 (5 μM; Thermo Fisher Scientific) stained the nuclei of the live cells. Transfected cells were incubated with the labeling mixture for 1 hour at 37°C, washed with 1× PBS, and then supplemented with normal medium.
Colocalization assays were conducted both in vitro in cultured SH-SY5Y cells and in vivo using cochleae from P30 wild-type C57BL/6 mice. In addition, cochleae from the KI mice were used to examine the localization of the mutant SLC7A14 p.Gly330Arg protein. Protocols for immunofluorescence of these cells and tissues are described above. It is important to note that different LAMP1 and LAMP2 antibodies were used to target human and mouse proteins in SH-SY5Y cells and cochlear tissue. In addition, the calreticulin primary antibody was conjugated; therefore, no secondary antibody incubation was required.
Samples were imaged on a Nikon Ti-E confocal microscope, and the colocalization function in the NIS Element software (Nikon) was used to quantify the colocalization of the green (SLC7A14) and red (protein label of target organelle). Thresholding of each fluorophore, as well as selecting a region of interest in the image, allowed for subtraction of background signal to maximize the signal for each channel. PCC, a statistical measure of the co-occurrence of the respective fluorophores in each pixel, was used to determine colocalization (19). The PCC measure for each layer in the z-stack image was calculated separately, exported to Excel, and then averaged for each sample. As a result, each colabeled sample (n = 3 per genotype and per target) was imaged in at least three cochlear (apical to low apical) locations, and each image consisted of 10 to 15 layers. Therefore, the average represented at least 100 calculated PCC values. Post hoc analysis of CTCF of the LysoTracker label was quantified in ImageJ (CTCF = integrated density − [area of selected cell × mean fluorescence of background readings]).
Gene knockdown
SLC7A14 gene knockdown was achieved using siRNA treatment. One day before treatment, SH-SY5Y cells were seeded in 24-well plates at 0.05 × 106 cells. The target SLC7A14 siRNA or negative control siRNA was diluted in the Accell delivery media (Dharmacon). Normal medium was replaced with 500 μl of the siRNA mixture for a final concentration of 1 μM per well. Following incubation for 96 hours, the cells were collected, and total RNA or protein was isolated and analyzed as described below.
Reverse transcription polymerase chain reaction
Total RNA from cultured cells was isolated using the RNeasy mini kit (Qiagen), and concentrations were measured using a Nanodrop 2000. Replicate cDNA libraries were constructed from 1 μg of total RNA using the QuantiTect reverse transcription kit (Qiagen). RT-PCR was performed to detect the expression of SLC7A14 [5′-CACGGCACATGGAACTAAGC-3′ (forward primer) and 5′-TGATTCTTCACCTTTCCCCAGG-3′ (reverse primer)] and GAPDH [5′-AAGACGGGCGGAGAGAAACC-3′ (forward primer) and 5′-GGGGCAGAGATGATGACCCT-3′ (reverse primer)]. The reaction proceeded as follows: 94°C for 4 min; 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min × 35 cycles; and 72°C for 10 min. The PCR products were separated by electrophoresis on a 2% agarose gel with SYBR Safe DNA gel stain (Invitrogen), followed by ultraviolet imaging to detect PCR products. Band intensities were quantified using Bio-Rad Image Lab 6.1 software and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot
Protein expression in cultured cells was quantified by Western blot. Samples were washed with cold PBS and then isolated in radioimmunoprecipitation assay cell lysis buffer and 1× Halt protease inhibitor cocktail (Thermo Fisher Scientific). Protein concentration (in micrograms per milliliter) was measured using the Pierce rapid gold BCA protein assay kit (Thermo Fisher Scientific) and a Varioskan LUX plate reader (3020-80010) to quantify absorbance at 480 nm. Samples were run on a mini-PROTEAN TGX-stain free gel (Bio-Rad) with a 4 to 20% gradient to separate proteins, along with molecular weight markers. The gel was run at 200 V for 30 min then imaged. Transfer was completed using the Trans blot turbo system (Bio-Rad), followed by activation and imaging to ensure transfer. The membrane was washed in TBS-T (1× tris-buffered saline + 1% Tween 20) and then blocked in EveryBlot blocking buffer (Bio-Rad). The membrane was incubated with primary antibodies (table S1) in blocking buffer overnight at 4°C. Following four washes in TBS-T, the blot was incubated with horseradish peroxidase–conjugated secondary antibodies (table S1) for 1 hour at room temperature. Following additional wash steps, the membrane was incubated with the chemiluminescence substrate (Bio-Rad) for 5 min and then imaged. Quantification of the bands of interest was conducted using Bio-Rad Image Lab 6.1 software, and expression of proteins of interest was normalized to α-tubulin or β-actin intensity.
Autophagy activity
After confirming successful gene knockdown, we repeated the experiment to measure changes in autophagy activity after decrease in SLC7A14 expression. The starvation treatment was simply a 2-hour incubation in Hank’s balanced salt solution (HBSS), which successfully increased autophagy in wild-type SH-SY5Y cells. Immediately following the 96-hour incubation in the knockdown media, the cells were supplemented with normal medium for 2 hours to ensure that autophagy activity was not up-regulated due to the reduced serum Accell media used for knockdown. The cells were then washed twice with PBS, followed by incubation in HBSS (starvation treatment) or HBSS plus 2 μM BafA1 (Cell Signaling Technology, #54645) (starvation treatment plus inhibitor) for 2 hours. Cells were harvested for total RNA or total protein (described above), and autophagy activity was analyzed by Western blot (see protocol above; antibodies listed in table S1). Autophagy activity was measured on the basis of the LC3-II–to–LC3-I expression ratio, which indicates active formation of autolysosomes (25). Additional samples were incubated in normal medium or HBSS for 2 hours, with LysoTracker Red and Hoechst 33342 added to label lysosomes and nuclei, respectively (described above). After washing cells with PBS, the live cells were imaged with a Nikon Ti-E confocal microscope. CTCF of the pH-sensitive LysoTracker label in live cells was quantified in ImageJ (CTCF = integrated density − [area of selected cell × mean fluorescence of background readings]).
Autophagy in vivo was examined using immunofluorescence of the LC3B protein (63). To specifically examine basal autophagy, mice were not subjected to noise exposure (i.e., ABR testing) or stress-inducing starvation immediately before tissue harvesting. The wild-type, KO, and KI mice cochleae (aged 1.5 to 3 months, two per genotype) were immunostained with anti-LC3B antibody, phalloidin-AF488, and DAPI and then imaged with a Zeiss 710 confocal microscope. The number of puncta per IHC was quantified in three locations in apical to low apical turn regions per cochlea using ImageJ.
Statistical analysis
All statistical analyses were conducted in GraphPad Prism 6. Details of statistical analysis of experiments are described in the figure legends. Statistical comparisons between groups were performed by one-way analysis of variance (ANOVA), two-way ANOVA, or Student’s t test, with post hoc analysis conducted as needed. Differences were indicated as statistically significant when P < 0.05, with error bars indicating ± SE.
Acknowledgments
We would like to thank H. Stessman for the gift of the SH-SY5Y cells. We also want to thank I. Rebustini at NIH-NIDCD for imaging rat tissues and M. Schnee at Stanford University for isolating turtle papilla. Last, we would like to acknowledge M. Zallocchi and T. Kaur for technical assistance with Western blots and preparation of cochlear slices.
Funding: This work was supported by the following: NIH grant R01 DC016807 (D.Z.H.); Béllucci Depaoli Family Foundation (D.Z.H.); Beijing Natural Science Foundation Z20J00122 (Z.-B.J.); National Natural Science Foundation of China 81970838 (Z.-B.J.); National Natural Science Foundation of China 81870718 and 81770996 (Y.L.); NIH grant 1P20GM139762-01, NIGMS, and Béllucci Depaoli Family Foundation (The Imaging and Molecular Biology Cores at the Translational Hearing Research Center of Creighton University School of Medicine); and NIH/NIDCD Grant U24: 1U24DC015910-01 (human cochlear samples provided by the NIDCD National Temporal Bone Laboratory at UCLA).
Author contributions: Conceptualization and design of experiments: K.P.G., Z.-B.J., and D.Z.H. Data collection and analysis for imaging experiments: K.P.G., H.L., A.J., B.K., and D.Z.H. Data collection for mouse auditory measurements: Y.L., X.-C.Z., T.W., S.S.G., and D.Z.H. Analysis of electrophysiological data: K.P.G., Y.L., H.L., and D.Z.H. Auditory and visual function tests of human participants: C.-J.Z., R.-J. S., B-B.C., and Z.-B.J. In vitro data collection and analysis: K.P.G., H.L., and D.Z.H. Authored original draft of manuscript: K.P.G. and D.Z.H. The final version of the manuscript was produced with input from all authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S4
Table S1
REFERENCES AND NOTES
- 1.Luzio J. P., Pryor P. R., Bright N. A., Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Settembre C., Fraldi A., Medina D. L., Ballabio A., Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P., Gu M., Xu H., Lysosomal ion channels as decoders of cellular signals. Trends Biochem. Sci. 44, 110–124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt F. M., d’Azzo A., Davidson B. L., Neufeld E. F., Tifft C. J., Lysosomal storage diseases. Nat. Rev. Dis. Primers. 4, 1–25 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Dallos P., The active cochlea. J. Neurosci. 12, 4575–4585 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corwin J. T., Warchol M. E., Auditory hair cells: Structure, function, development, and regeneration. Annu. Rev. Neurosci. 14, 301–333 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Dallos P., Wu X., Cheatham M. A., Gao J., Zheng J., Anderson C. T., Jia S., Wang X., Cheng W. H. Y., Sengupta S., He D. Z. Z., Zuo J., Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58, 333–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y., Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196 (1985). [DOI] [PubMed] [Google Scholar]
- 9.Liberman M. C., Dodds L. W., Pierce S., Afferent and efferent innervation of the cat cochlea: Quantitative analysis with light and electron microscopy. J. Comp. Neurol. 301, 443–460 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Liu H., Giffen K. P., Chen L., Beisel K. W., He D. Z. Z., Transcriptomes of cochlear inner and outer hair cells from adult mice. Sci. Data 5, 180199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Pecka J. L., Zhang Q., Soukup G. A., Beisel K. W., He D. Z. Z., Characterization of transcriptomes of cochlear inner and outer hair cells. J. Neurosci. 34, 11085–11095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H., Chen L., Giffen K. P., Stringham S. T., Li Y., Judge P. D., Beisel K. W., He D. Z. Z., Cell-specific transcriptome analysis shows that adult pillar and Deiters’ cells express genes encoding machinery for specializations of cochlear hair cells. Front Mol Neurosci. 11, 356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fotiadis D., Kanai Y., Palacín M., The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 34, 139–158 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Jaenecke I., Boissel J.-P., Lemke M., Rupp J., Gasnier B., Closs E. I., A chimera carrying the functional domain of the orphan protein Slc7a14 in the backbone of Slc7a2 mediates trans-stimulated arginine transport. J. Biol. Chem. 287, 30853–30860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Z.-B., Huang X.-F., Lv J.-N., Xiang L., Li D.-Q., Chen J., Huang C., Wu J., Lu F., Qu J., SLC7A14 linked to autosomal recessive retinitis pigmentosa. Nat. Commun. 5, 3517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T.-M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H.-W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K.-R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., Jones A. R., Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Zhuang Y.-Y., Xiang L., Wen X.-R., Shen R.-J., Zhao N., Zheng S.-S., Han R.-Y., Qu J., Lu F., Jin Z.-B., Slc7a14 is indispensable in zebrafish retinas. Front. Cell Dev. Biol. 7, 333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barta C. L., Liu H., Chen L., Giffen K. P., Li Y., Kramer K. L., Beisel K. W., He D. Z., RNA-seq transcriptomic analysis of adult zebrafish inner ear hair cells. Sci. Data. 5, 180005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow A. L., Macleod A., Noppen S., Sanderson J., Guérin C. J., Colocalization analysis in fluorescence micrographs: Verification of a more accurate calculation of pearson’s correlation coefficient. Microsc. Microanal. 16, 710–724 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Saftig P., Klumperman J., Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Bissa B., Beedle A., Govindarajan R., Lysosomal solute carrier transporters gain momentum in research. Clin. Pharmacol. Ther. 100, 431–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt F. M., Emptying the stores: Lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 17, 133–150 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Closs E. I., Boissel J.-P., Habermeier A., Rotmann A., Structure and function of cationic amino acid transporters (CATs). J. Membr. Biol. 213, 67–77 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Levine B., Kroemer G., Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orhon I., Reggiori F., Assays to monitor autophagy progression in cell cultures. Cells 6, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvezin C., Neufeld T. P., Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11, 1437–1438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noben-Trauth K., Zheng Q. Y., Johnson K. R., Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 35, 21–23 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubisch C., Schroeder B. C., Friedrich T., Lütjohann B., El-Amraoui A., Marlin S., Petit C., Jentsch T. J., Kcnq4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96, 437–446 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Furness D. N., Johnson S. L., Manor U., Rüttiger L., Tocchetti A., Offenhauser N., Olt J., Goodyear R. J., Vijayakumar S., Dai Y., Hackney C. M., Franz C., Fiore P. P. D., Masetto S., Jones S. M., Knipper M., Holley M. C., Richardson G. P., Kachar B., Marcotti W., Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc. Natl. Acad. Sci. U.S.A. 110, 13898–13903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rance G., Starr A., Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain 138, 3141–3158 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Ruel J., Emery S., Nouvian R., Bersot T., Amilhon B., Van Rybroek J. M., Rebillard G., Lenoir M., Eybalin M., Delprat B., Sivakumaran T. A., Giros B., El Mestikawy S., Moser T., Smith R. J. H., Lesperance M. M., Puel J.-L., Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am. J. Hum. Genet. 83, 278–292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starr A., Sininger Y., Winter M., Derebery M. J., Oba S., Michalewski H. J., Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 19, 169–179 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Yasunaga S., Grati M., Cohen-Salmon M., El-Amraoui A., Mustapha M., Salem N., El-Zir E., Loiselet J., Petit C., A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 21, 363–369 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Baig S. M., Koschak A., Lieb A., Gebhart M., Dafinger C., Nürnberg G., Ali A., Ahmad I., Sinnegger-Brauns M. J., Brandt N., Engel J., Mangoni M. E., Farooq M., Khan H. U., Nürnberg P., Striessnig J., Bolz H. J., Loss of Cav1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 14, 77–84 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Mencía Á., Modamio-Høybjør S., Redshaw N., Morín M., Mayo-Merino F., Olavarrieta L., Aguirre L. A., del Castillo I., Steel K. P., Dalmay T., Moreno F., Moreno-Pelayo M. Á., Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat. Genet. 41, 609–613 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Kawashima Y., Géléoc G. S. G., Kurima K., Labay V., Lelli A., Asai Y., Makishima T., Wu D. K., Santina C. C. D., Holt J. R., Griffith A. J., Mechanotransduction in mouse inner ear hair cells requires transmembrane channel–like genes. J. Clin. Invest. 121, 4796–4809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosgrove D., Zallocchi M., Usher protein functions in hair cells and photoreceptors. Int. J. Biochem. Cell Biol. 46, 80–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnett J., Emery S. B., Kim T. B., Boerst A. K., Lee K., Leal S. M., Lesperance M. M., Autosomal dominant progressive sensorineural hearing loss due to a novel mutation in the KCNQ4 gene. Arch. Otolaryngol. Head Neck Surg. 137, 54–59 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews G., Fuchs P., The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 11, 812–822 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser T., Grabner C. P., Schmitz F., Sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 100, 103–144 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Rebsamen M., Superti-Furga G., SLC38A9: A lysosomal amino acid transporter at the core of the amino acid-sensing machinery that controls MTORC1. Autophagy 12, 1061–1062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebsamen M., Pochini L., Stasyk T., de Araújo M. E. G., Galluccio M., Kandasamy R. K., Snijder B., Fauster A., Rudashevskaya E. L., Bruckner M., Scorzoni S., Filipek P. A., Huber K. V. M., Bigenzahn J. W., Heinz L. X., Kraft C., Bennett K. L., Indiveri C., Huber L. A., Superti-Furga G., SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Tsun Z.-Y., Wolfson R. L., Shen K., Wyant G. A., Plovanich M. E., Yuan E. D., Jones T. D., Chantranupong L., Comb W., Wang T., Bar-Peled L., Zoncu R., Straub C., Kim C., Park J., Sabatini B. L., Sabatini D. M., Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nixon R. A., The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Muela N., Koga H., García-Ledo L., de la Villa P., de la Rosa E. J., Cuervo A. M., Boya P., Balance between autophagic pathways preserves retinal homeostasis. Aging Cell 12, 478–488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto C., Iwasaki S., Urata S., Morishita H., Sakamaki Y., Fujioka M., Kondo K., Mizushima N., Yamasoba T., Autophagy is essential for hearing in mice. Cell Death Dis. 8, e2780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohlemiller K. K., Hennig A. K., Lett J. M., Heidbreder A. F., Sands M. S., Inner ear pathology in the mucopolysaccharidosis VII mouse. Hear. Res. 169, 69–84 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Schachern P. A., Cureoglu S., Tsuprun V., Paparella M. M., Whitley C. B., Age-related functional and histopathological changes of the ear in the MPS I mouse. Int. J. Pediatr. Otorhinolaryngol. 71, 197–203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiwatpanit T., Remis N. N., Ahmad A., Zhou Y., Clancy J. C., Cheatham M. A., García-Añoveros J., Codeficiency of lysosomal mucolipins 3 and 1 in cochlear hair cells diminishes outer hair cell longevity and accelerates age-related hearing loss. J. Neurosci. 38, 3177–3189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koffler T., Ushakov K., Avraham K. B., Genetics of hearing loss. Otolaryngol. Clin. North Am. 48, 1041–1061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolz H. J., Hereditary hearing loss and its syndromes third edition. Eur. J. Hum. Genet. 24, 1650 (2016). [Google Scholar]
- 52.Sugahara M., Oishi M., Oishi A., Ogino K., Morooka S., Gotoh N., Kang I., Yoshimura N., Screening for SLC7A14 gene mutations in patients with autosomal recessive or sporadic retinitis pigmentosa. Ophthalmic Genet. 38, 70–73 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Kandasamy P., Gyimesi G., Kanai Y., Hediger M. A., Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 43, 752–789 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Salehi P., Nelson C. N., Chen Y., Lei D., Crish S. D., Nelson J., Zuo H., Bao J., Detection of single mRNAs in individual cells of the auditory system. Hear. Res. 367, 88–96 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Giffen K. P., Liu H., Kramer K. L., He D. Z., Expression of protein-coding gene orthologs in zebrafish and mouse inner ear non-sensory supporting cells. Front. Neurosci. 13, 1117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheibinger M., Ellwanger D. C., Corrales C. E., Stone J. S., Heller S., Aminoglycoside damage and hair cell regeneration in the chicken utricle. J. Assoc. Res. Otolaryngol. 19, 17–29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sive H. L., Grainger R. M., Harland R. M., Baskets for in situ hybridization and immunohistochemistry. Cold Spring Harb. Protoc. 8, pdb.prot4777 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Lopez I. A., Ishiyama G., Hosokawa S., Hosokawa K., Acuna D., Linthicum F. H., Ishiyama A., Immunohistochemical techniques for the human inner ear. Histochem. Cell Biol. 146, 367–387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia S., Yang S., Guo W., He D. Z. Z., Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J. Neurosci. 29, 15277–15285 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q., Liu H., McGee J., Walsh E. J., Soukup G. A., He D. Z. Z., Identifying microRNAs involved in degeneration of the organ of Corti during age-related hearing loss. PLOS ONE 8, e62786 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker J., Cleveland L., Davis J., Seales J., Audiometry screening and interpretation. Am. Fam. Physician 87, 41–47 (2013). [PubMed] [Google Scholar]
- 62.Rodríguez Valiente A., Trinidad A., García Berrocal J. R., Górriz C., Ramírez Camacho R., Extended high-frequency (9-20 kHz) audiometry reference thresholds in 645 healthy subjects. Int. J. Audiol. 53, 531–545 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Yuan H., Wang X., Hill K., Chen J., Lemasters J., Yang S.-M., Sha S.-H., Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 22, 1308–1324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S4
Table S1