ABSTRACT
Since the reintroduction of dengue viruses in 1987, Sao Paulo State (SP), Brazil, has experienced recurrent epidemics in a growing number of municipalities, each time with more cases and deaths. In the present study, we investigated the spatio-temporal dynamics of dengue-related deaths and associated factors in SP. This was an ecological study with spatial and temporal components, based on notified dengue-related deaths in the municipalities of SP between 2007 and 2017. A latent Gaussian Bayesian model with Poisson probability distribution was used to estimate the standardized mortality ratios (SMR) for dengue and relative risks (RR) for the socioeconomic, demographic, healthcare-related, and epidemiological factors considered. Epidemiological factors included the annual information on the number of circulating serotypes. A total of 1,019 dengue-related deaths (0.22 per 100,000 inhabitant-years) between 2007 and 2017 were confirmed in SP by laboratory testing. Mortality increased with age, peaking at 70 years or older (1.41 deaths per 100,000 inhabitant-years). Mortality was highest in 2015, and the highest SMR values were found in the North, Northwest, West, and coastal regions of SP. An increase of one circulating serotype, one standard deviation in the number of years with cases, and one standard deviation in the degree of urbanization were associated with increases of 75, 35, and 45% in the risk of death from dengue, respectively. The risk of death from dengue increased with age, and the distribution of deaths was heterogeneous in space and time. The positive relationship found between the number of dengue serotypes circulating and years with cases at the municipality/micro-region level indicates that this information can be used to identify risk areas, intensify surveillance and control measures, and organize healthcare to better respond to this disease.
KEYWORDS: Dengue mortality, Risk factor, Surveillance, Spatiotemporal
INTRODUCTION
The dengue virus (DENV; genus Flavivirus , family Flaviviridae) is transmitted by females of Aedes aegypti mosquitoes, and has at least four different serotypes (DENV1, DENV2, DENV3, and DENV4). The disease has a wide clinical spectrum, varying from asymptomatic forms to symptomatic ones, ranging from mild to severe, and even fatal. Dengue is primarily transmitted in urban areas, particularly in tropical and subtropical regions, with a strong seasonal influence and epidemic cycles occurring every 3-to-5 years 1 .
Approximately 4 billion people are estimated to be at risk of infection with the dengue virus, and an average of 100-400 million new infections occur each year 2 . There were an estimated 57 million cases of dengue (uncertainty interval: 37,083,098.01-101,350,556.42) in 2019, and more than 36,000 deaths worldwide. These numbers represent the highest global records of the disease since 1990 3 .
Since its re-emergence in Brazil in the 1980s, the number of cases of dengue and the number of regions in Brazil affected by this disease have increased every year, with endemic and epidemic cycles. The four viral serotypes of dengue have been cocirculating in Brazil since at least 2010, and the scenario of the disease in the country is becoming increasingly complex with hyper endemicity and an increasing number of deaths 4 - 8 .
Between 2008 and 2019, approximately 10.6 million dengue cases were reported in Brazil, with the years 2015 and 2019 ranking first and second in number of cases in the historical series, respectively. In the same period, 6,429 dengue-related deaths were confirmed in the country, 986 deaths in 2015, and 840 in 2019 9 . In subsequent years, there was a reduction in the number of dengue cases in Brazil, and between epidemiological weeks 1 and 51 of 2021, there were 526,032 cases (incidence of 246.6 cases per 100,000 inhabitants), of which 364 were severe and 235 were confirmed deaths 10 .
The determinants of disease expansion and increased mortality are not entirely clear, and the intensity of dengue transmission varies significantly according to spatial and temporal parameters. Intrinsic (host-related) factors can contribute to these variations, since individuals develop homotypic immunity after subsequent infections with different serotypes 4 , 5 . They are also related to the clinical manifestation of the disease, as secondary infections by any of the four serotypes tend to be more severe, with a higher risk of death due to the presence of heterotypic antibodies 11 .
When severe cases of dengue emerge, the organization of health services and the adequate provision of treatment may alter the mortality rate from this disease. Extrinsic factors, such as the biological characteristics of the vector and socio-environmental aspects (climate, demographic and economic factors, urbanization etc.) also influence the spatio-temporal distribution of dengue, as well as its heterogeneity 12 - 14 .
Although there is an abundance of information available regarding dengue, few studies have addressed the spatial and spatio-temporal dynamics of dengue-related deaths and the role of socioeconomic, demographic, and epidemiological determinants in this process. The lack of such data can be considered a knowledge gap, since studies that consider geographical space are important in the identification of risk areas and contextual factors that determine the spatio-temporal heterogeneity of this disease. The results of such studies can also be extremely useful in developing surveillance, planning and control activities 15 .
In a study developed in Latin America and the Caribbean (LAC), which considered as the unit of analysis the countries in that particular region, dengue mortality was evaluated by taking into account contextual factors and a partial approach on the circulation of viral serotypes by including data on DENV2 in the overall analysis 16 . Other studies have sought to relate mortality with contextual factors specific to geographic areas of Brazil as units of analysis, but without considering the history of circulating serotypes, which was pointed out as a limitation by the authors 17 , 18 . It is also noteworthy that the studies cited, even using data from specific geographic areas, did not take into account the possible presence of a spatial autocorrelation of the studied phenomenon, as established by the first law of geography 19 .
For this reason, the aim of the present study was to investigate the spatio-temporal dynamics of dengue-related deaths, from 2007 to 2017, in Sao Paulo State (SP) municipalities, and to identify potentially associated factors, including socioeconomic, demographic, healthcare, and epidemiological aspects. It should also be noted that the epidemiologic factors evaluated included information about the annual circulation of the four DENV serotypes, which we considered from both, spatial and temporal points of view.
MATERIALS AND METHODS
Ethical aspects
The present study was approved by the University of Sao Paulo School of Public Health’s institutional review board (Nº 1.687.650), and was conducted in accordance with the Brazilian Guidelines and Standards for Research Involving Humans (CNS Resolution 466 of December 12, 2012).
Study area, design, and study period
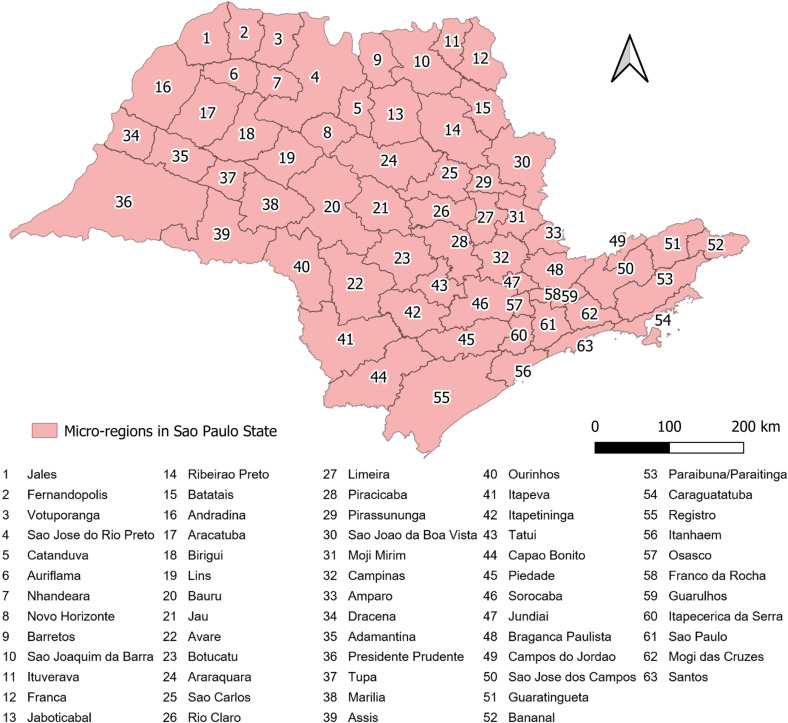
The present study focused on SP State, located in Southeastern Brazil (19–25° S, 44–53° W) 20 . Prior to 2017, when intermediate/immediate regions were implemented, SP was geographically divided into 63 micro-regions, which are spatial aggregates that express the organization at the micro or local level based on specific characteristics resulting from the presence of environmental elements or social and economic relationships 20 . Currently, SP has 645 municipalities ( Figure 1 ), and according to the 2010 demographic census, 41,262,199 inhabitants live in 248,219,63 km2 of territory , with a population density of 166.23 inhabitants/km2, and approximately 95.96% of the population living in urban areas 20 . This was an ecological study with spatial and temporal components, based on notified dengue-related deaths from 2007 to 2017, which utilized the municipalities of SP as units for analysis.
Figure 1. Geographical characteristics of the study area: Micro-regions in Sao Paulo State.
Conceptual model
Hyper endemic areas, characterized as areas in which different viral serotypes cocirculate, and longer viral transmission times, are associated with an increased risk of epidemics and dengue mortality, likely due to effects of previous circulation of dengue virus serotypes and previous infections in the population. Furthermore, rapid and unplanned processes of urbanization in areas with high demographic densities often act favoring the occurrence of vector-transmitted diseases. The local ability to respond and prevent severe cases can also vary, based on the availability of hospital beds and budget resources. For this reason, we selected variables that could provide an approximation of this scenario, considering the spatio-temporal structure of data over a period that would allow the observation of the epidemic cycles, and reflect their variations in order to estimate the risk of death from dengue in SP.
Data sources, inclusion and exclusion criteria and study variables
Data on dengue-related deaths were obtained from the Brazilian Notifiable Disease Information System (SINAN). Notification data for dengue-related deaths, along with the city of residence in SP State were provided by the SP State Department of Health’s Epidemiological Surveillance Center (CVE/SES-SP) as dBase files (SINAN database export format), one for each year in the period of 2007 to 2017. Confirmed cases of dengue in residents of SP, documented in SINAN, with symptoms that began during the study period and progressed to death were included. Deaths confirmed only by clinical-epidemiological criteria were excluded. The following variables were evaluated for deaths due to dengue: age, gender and municipality of residence.
The Brazilian Institute of Geography and Statistics (IBGE) databases were used to obtain census population data, population estimates and digital municipal networks related to the municipalities of SP. They also provided data on socioeconomic variables, demographic density, degree of urbanization and per capita gross domestic product (GDP), which were used in the data modeling process 20 .
Dengue in SP was characterized in epidemiological terms using the following variables for each year of study: number of years with confirmed cases that were based on the introduction of the virus to the calendar year, time interval between introduction of the virus and the calendar year for each municipality of residence, and number of serotypes identified by micro-region per year. The serotype data were obtained from the SP State Department of Health, provided by the Adolfo Lutz Institute (IAL). These analyzes were performed by micro-regions, as samples are sent to IAL for regional viral monitoring.
The hospital bed ratio (ratio between the number of beds per micro-region and its population, multiplied by 1,000) was used to estimate the health care capacity. Since not all municipalities in SP have inpatient beds in public or private hospitals, and the micro-region system groups neighboring municipalities according to their specific characteristics, we chose this unit of analysis for this variable instead of the municipal data 20 . Information on hospital beds was obtained from DATASUS, the Health Informatics Department of the Brazilian Ministry of Health 21 .
Data analyzes
The number of observed dengue-related deaths from 2007 to 2017, according to the 645 municipalities of SP, was modeled using latent Gaussian Bayesian models, considering Poisson and zero-inflated Poisson probability distributions and a spatio-temporal structure. Initially, only models with intercept, spatial and temporal random effects relative to the interaction of space and time were considered. Next, covariates were included in the model. The best models were selected and those with the lowest deviance information criterion (DIC) values were chosen 22 .
The temporal autocorrelation of data was represented by a structured random effect obtained from an autoregressive model of the random walk-1 (RW1) type, and by an unstructured random effect 22 . A Besag-York-Mollié (BYM) type model, composed of spatially structured and unstructured random effects, was considered to represent the spatial dependence of data 23 . The BYM model was used with the parameterization proposed by Riebler et al . 24 . The interaction between space and time was represented by unstructured random effects in both, space and time 22 .
The expected dengue-related deaths, according to municipality and year, were estimated by indirect standardization, taking into account the respective age and gender structures of each municipality and year. To do so, the deaths that occurred during the study period (2007-2017) and the mortality rates for the entire SP State were calculated by age group and gender, based on the population in 2012 (midpoint). To consider the expected deaths in the models allowed us to interpret the coefficients generated after exponentiation as relative risks (RR), and to obtain the standardized mortality ratios (SMR) for dengue in the units of analysis and years evaluated.
Before including the covariates in the modeling, an exploratory analysis was performed to identify outliers and collinearity. Covariates with 2-to-2 correlation coefficients ≥ 0.60 were considered collinear. Since the covariates “number of serotypes present” and “hospital bed ratio” were expressed by micro-regions, an unstructured independent and identified distributed (iid) random effect was considered to represent the grouping of deaths according to micro-region. All covariates included in the modeling, except for the number of serotypes present in the micro-regions, were standardized by subtracting the respective means and dividing by the respective standard deviations. In this way, the RR obtained from this modeling, for each of the standardized covariates, could be interpreted as an increase or decrease in risk, corresponding to its variation of one standard deviation.
The modeling was performed in a Bayesian context, using the integrated nested Laplace approximation (INLA), which is a more efficient computational option than the Markov chain Monte Carlo (MCMC) model 25 . The INLA approach is especially advantageous over MCMC for spatially- and temporally-structured data 22 . Minimally informative priors were considered for the fixed effects. For the random effects related to grouping the data by micro-regions, temporal random effects and spatial random effects, penalized complexity priors were considered, as proposed by Simpson et al . 26 .
Software used
The R software environment v. 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) with the Tidyverse v.1.2.1, Epitools v. 0.5-10, R-INLA v. INLA_18.05.28, and INLA Outputs v. 1.4.0 packages were used for database construction and statistical analyses, and QGis v. 2.18.9 (QGIS Development Team) was used to build the maps for each topic.
RESULTS
From 2007 to 2017, 1,019 (505 males and 514 females) deaths due to dengue were confirmed by laboratory testing in SP State. The overall mortality rate for this period was 0.22 deaths per 100,000 inhabitant-years, with the same rates for both genders. The ≥ 70 age group was the most affected, with 1.41 deaths per 100,000 inhabitant-years, and the rate in this group was higher in males than in females (1.95 vs. 1.05, respectively). These data can be seen in Table 1 .
Table 1. Dengue mortality rates (per 100,000 inhabitant-years) by gender and age in Sao Paulo State, 2007-2017.
| Age group (years) | 0-14 | 15-29 | 30-49 | 50-69 | 70+ | Total |
|---|---|---|---|---|---|---|
| Female | ||||||
| Deaths | 27 | 54 | 140 | 140 | 153 | 514 |
| Population | 4,264,364 | 5,293,253 | 6,600,292 | 4,042,128 | 1,330,837 | 21,530,874 |
| Rate | 0.06 | 0.09 | 0.19 | 0.31 | 1.05 | 0.22 |
| Male | ||||||
| Deaths | 31 | 38 | 86 | 157 | 193 | 505 |
| Population | 4,423,307 | 5,365,067 | 6,225,351 | 3,496,412 | 898,986 | 20,409,123 |
| Rate | 0.06 | 0.06 | 0.13 | 0.41 | 1.95 | 0.22 |
| Total | ||||||
| Deaths | 58 | 92 | 226 | 297 | 346 | 1019 |
| Population | 8,687,671 | 10,658,320 | 12,825,643 | 7,538,540 | 2,229,823 | 41,939,997 |
| Rate | 0.06 | 0.08 | 0.16 | 0.36 | 1.41 | 0.22 |
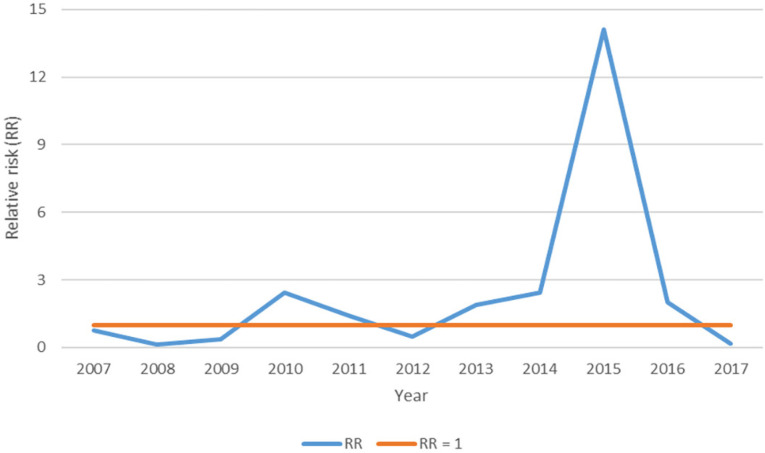
The models considering the Poisson probability distribution were better adjusted to the data than those that considered a zero-inflated distribution. Figure 2 shows the posteriors means of the temporal random effects, interpreted after exponentiation as RR, and illustrates that these values were above the unit in 2010 and 2011 and between 2013 and 2016; 2015 was the most notable year, at 14.1. Figure 3 shows the posterior means of the predicted SMR for the 645 municipalities in SP between 2007 and 2017. Between 2007 and 2009, the SMR was below the unit in almost all municipalities in SP, although higher values were observed in the Western portion of the State, and lower values in the Eastern. In 2010, many municipalities in Western SP exhibited values above the unit, along with municipalities in the center of the State and in the coastal Caraguatatuba, Itanhaem and Santos micro-regions (represented on Figure 1 as micro-regions 54, 56, and 63, respectively). This was again seen in 2013 (at a lower intensity) and 2014. In 2015, the SMR was above the unit for all municipalities, except for a few located in Sao Paulo (61) and Itapeva (41) micro-regions. Meanwhile, 2016 resembled 2010, 2013, and 2014, and 2017 was similar to 2008, with municipalities presenting the lowest RR during the study period.
Figure 2. Posterior means of temporal random effects (exponentiated and presented as relative risk) in the period 2007-2017, in Sao Paulo State.
Figure 3. Posterior means of standardized mortality ratios (SMR) of dengue in the period 2007–2017 and municipalities in Sao Paulo State.
The years with temporal RR values above the unit were those in which most municipalities had SMR values above the unit, culminating in 2015, when these values were high in nearly all municipalities. In comparison (with the partial exception of 2015), in the remaining years, the municipalities in the Western portion of the State generally exhibited higher SMR values than those in the Eastern.
The exploratory analysis of covariates revealed collinearity between the covariates “number of years with dengue cases” and “time interval since dengue introduction,” and of these, we opted to use the former in the modeling. Table 2 presents the posterior means and 95% credibility intervals for the fixed effects, which were interpreted as RRs. The covariates considered important in explaining the risk of death from dengue were “degree of urbanization” (one standard deviation increase in the degree of urbanization was associated with an increased in the risk of death of 45%), “number of years with dengue cases” (one standard deviation increase in this covariate was associated with a 35% increase in the risk of death) and “number of serotypes in the micro-region” (one unit increase in the number of circulating serotypes was associated with a 75% increase in the risk of death due to dengue). At the same time, the covariates “population density,” “GDP per capita” and “hospital bed ratio in the micro-region” were not found to be associated with the risk of dengue-related deaths in SP, between 2007 and 2017.
Table 2. Posterior means of fixed effects presented as relative risks and their respective 95% credibility intervals for municipalities in the Sao Paulo State, 2007–2017.
| Covariates | Mean | Quantile 0.0250 | Quantile 0.0975 |
|---|---|---|---|
| Intercept | 0.02 | 0.01 | 0.05 |
| Demographic density | 0.95 | 0.85 | 1.06 |
| Degree of urbanization | 1.45 | 1.06 | 1.98 |
| Per capita GDP | 0.97 | 0.87 | 1.08 |
| Number of years with dengue cases | 1.35 | 1.12 | 1.63 |
| Number of serotypes in the micro-region | 1.75 | 1.32 | 2.29 |
| Hospital bed ratio in the micro-region | 1.09 | 0.88 | 1.32 |
GDP = gross domestic product (per capita).
Figure 4 shows the number of circulating serotypes per year in the State’s micro-regions and their evolution over time. Between 2007 and 2010, the viral circulation pattern remained stable, with three serotypes circulating annually in approximately 60% of the regions. In 2011, the pattern changed, with circulation of an additional serotype, initially in limited areas but, increasing to one-third of the micro-regions in 2012, and rapidly extending to nearly 60% as early as 2013, and from 2013 onwards it remained stable until 2017.
Figure 4. A) Percentage of micro-regions and number of dengue virus serotypes circulating per year in Sao Paulo State, 2007-2017; B) Dengue serotypes circulating in the micro-regions of Sao Paulo State in 2010-2013. Numbers in the maps represent the micro-regions shown in Figure 1 .
A comparison between the dengue-related death occurrence patterns ( Figure 3 ) and the distribution patterns of circulating dengue virus serotypes ( Figure 4 ) confirmed the positive association found between these two variables in the model. A partial coincidence between micro-regions in which three serotypes circulated, and municipalities with SMR above the unit in 2010 was found in the Western part of SP, as well as in some central and coastal micro-regions. The death patterns followed the same pattern in 2013 and 2014, despite the addition of the fourth serotype in 2011. However, in 2015, the pattern of deaths followed the distribution of circulating serotypes from 2013 onwards, and the micro-regions in the Central/Southern portion of the State in which one or two serotypes circulated, corresponded to the areas with the lowest SMRs, as seen in Figure 3 . In most other areas of the State, four serotypes circulated in 2015, and the SMR values were also above the unit.
A comparison of the number of years between 2007 and 2017 in which dengue cases occurred in SP municipalities (Supplementary Figure S1) and the SMR values ( Figure 3 ) confirmed the positive association between these two variables. The behavior of this covariate in specific years (2007, 2010 and 2015; Supplementary Figure S2) in relation to the SMRs values ( Figure 3 ) has also confirmed this association. The number of years in which dengue cases occurred increased, and there was a correlation between areas with higher values for this variable and higher SMRs. As the degree of urbanization in the municipalities of SP did not change significantly during the study period, for purposes of comparison with the SMRs, Supplementary Figure S3 maps this variable for 2012 (the midpoint of the study period). A correlation in the regions in the Central/Southern portions of the State between lower SMR and lower degrees of urbanization can be seen. This phenomenon has also occurred in 2015, when the highest SMR values were found throughout the entire study period.
DISCUSSION
The year 2015 was the one with the highest dengue-related mortality in SP State, coinciding with the year in which most deaths from this disease occurred throughout the entire country, approximately 1,000, with those in SP accounting for half of that number 5 . The number of dengue-related deaths in SP between 2007 and 2017 was approximately ten times that found between 1998 and 2006. This increase occurred in parallel with the development of the disease in Brazil: mortality rates, which had already increased between 2001 and 2011 17 , increased even more in the period from 2011 to 2017 in relation to the period of 2001 to 2010 5 .
Some studies in Brazil 8 , 14 and Panama 27 observed higher dengue-related mortality rates among older people, similar to the results of the present study, which have been associated with a greater presence of comorbidities 8 , 13 , 27 . Although we did not identify any differences in mortality rates between genders, when the entire age range was considered, a higher risk of death was observed in males over the age of 70. As such, the results of the present study partially agree with those of previous studies in which differences between genders 8 were either not identified or a higher risk of death in males was found 14 .
The heterogeneous pattern of the spatial distribution of dengue-related mortality in the municipalities of SP was not associated with per capita GDP in the model obtained in the present study. Other studies, however, showed a relationship between higher dengue-related mortality and poor local socioeconomic conditions, indicating the need to obtain variables that can more comprehensively characterize the determinants of this relationship in future models 16 , 18 , 28 . The hospital bed ratio in each micro-region was also considered not important to explain the risk of death from dengue, which was also observed in a study that sought to associate mortality with contextual factors using Brazilian municipalities as units of analysis 17 . It is possible that the measure of health care capacity in relation to severe cases of dengue provided by this variable does not reflect the quality and punctuality of care offered to patients, factors that have been indicated as relevant for the reduction of dengue-related mortality.
The relationship between demographic changes and the spatial heterogeneity of dengue-related mortality in the municipalities of SP was assessed via the degree of urbanization and population density, and it was found that only the degree of urbanization was associated with a higher risk of death due to the disease. The association between urbanization and dengue-related deaths was also verified in another study involving Brazilian municipalities 17 . Lack of infrastructure and sanitation, in addition to significant social inequality and environmental degradation, are causes of accelerated and unplanned urbanization processes that are related to vector-borne diseases 29 . For this reason, the risks of dengue may be higher in municipalities in which urbanization is disordered and the health care network may be less effective, which would increase the risk of death due to this disease, as has already been hypothesized in a study that considered contextual factors to assess dengue-related mortality in LAC 16 . Urbanization, among other factors, has also been associated with an increase in vector-borne diseases that have been occurring around the world in recent decades, dengue in particular 29 .
It is logical to assume that the more experience a municipality is with subsequent dengue epidemics, the more prepared will be its health teams to recognize the first cases, identify severity and reduce deaths, while in municipalities in which the disease is a more recent development, an increased number of deaths would be more likely. However, based on the findings of the present study (which are in accordance with the results of a relevant study involving LAC countries), an increased number of years with dengue cases was associated with a higher risk of dengue-related mortality 16 . Considering the entire population as being previously susceptible, and the number of years with dengue cases as an approximation of the virus transmission time in the municipality, its increase is accompanied by accumulated immunity among those exposed, and therefore, a progressively greater chance of sequential infections over the years. According to the results of the model obtained in the present study, there was an increase in the risk of dengue-related death. For this reason, municipalities are possibly able to improve their capacity to organize and manage services in the face of serial dengue epidemics, but the mechanisms that determine the severity of the disease will continue to be present, and the training of professionals in each seasonal period should be encouraged as a strategy to reduce mortality. The association between the number of years with dengue cases and a higher risk of dengue-related mortality points to an increase in the severity of cases and an increased rate of mortality in an inadequate health care condition. Therefore, in addition to the generalized improvement in healthcare throughout SP, the time of dengue transmission could be a criterion for prioritizing the allocation of resources to improve care in the municipalities.
Previous reports have pointed to an association between viral serotype replacement and increased severity or mortality due to dengue, based on descriptive temporal or spatio-temporal relationships 8 , 30 , 31 . An ecological study investigating the effects of environmental, demographic, socioeconomic and biological factors on dengue-related mortality observed an increased risk of death due to the disease when DENV2 was circulating, but the emergence and re-emergence of different serotypes were not analyzed 16 . For this reason, the finding of the present study of the existence of a relationship between the number of circulating serotypes and an increased risk of death from dengue is an unprecedented demonstration of the effect of hyper endemicity on dengue-related mortality in the literature, in ecological terms.
Given the potential use of serotyping in defining risk areas for dengue-related deaths, viral circulation monitoring should be more widely used, with representative sample form geographical regions that permit targeted and timely actions. However, the number of DENV serotyping that can be performed by the Brazilian laboratory infrastructure is still insufficient to develop an appropriate regional viral monitoring according to the needs of the country 4 , so that investments in this area are essential.
Molecular epidemiology studies have associated the presence or absence of symptoms in infected people with DENV and their frequencies, as well as the increase in the number of dengue cases and their severity, with circulation of different serotypes and diversity of DENV genotypes 27 , 32 - 34 , indicating the importance of a genotypic surveillance alongside other epidemiological surveillance activities. In a case reported in Brazil, in 2019, the occurrence of death from dengue was associated with a viral lineage that had not been detected in the country before 35 . This type of information, when available in space and time, could be useful in modifying the ecological modeling used in the present study and improve the process of identifying areas of risk for deaths.
The limitations of the present study include the use of data from secondary sources based on passive epidemiological surveillance, which is consequently subject to underreporting and underestimation of the magnitude of both, morbidity and dengue-related mortality 36 . As an ecological study, we were careful to interpret our findings at the level of the analysis units used, rather than extrapolating our results to the individuals.
Modeling by regression of variables with spatial and temporal distribution (such as dengue-related mortality) could violate the basic assumption of independence between the observed values 37 . To avoid this, we considered the existence of spatial and temporal dependence in the phenomenon studied, which has not been the rule in spatio-temporal studies on dengue 38 . To this end, spatially and temporally structured random effects were included in our models, in addition to the interactions between them, which allowed the self-correlation found in the data to be incorporated into the structure of the models 22 , 39 . Additionally, the hierarchical Bayesian models used in the present study are recognized as suitable for investigating spatiotemporal disease patterns 40 .
The growing number of dengue cases and deaths worldwide has shown the poor effectiveness of control measures, a situation requiring urgent attention 5 . Using tools to identify risk areas for the occurrence of severe cases and increased deaths could make dengue surveillance and control systems more effective. Despite its limitations, the strengths of the present study ensure its validity, so its findings can contribute to the improvement of this system.
CONCLUSION
The overall mortality rate due to dengue in SP during the study period was 0.22 deaths per 100,000 inhabitant-years, increasing with age, with higher values observed in people aged 70 years and over (1.41 per 100,000 inhabitant-years). Mortality rates did not differ between genders when considering all age groups; however, among people aged 70 years or more, the risk of death was higher in males. The distribution of dengue-related deaths was found to be heterogeneous in space and time. The highest SMR values were observed in the Northern, Northwestern, Western, and coastal regions of the State. The mortality rate was highest in 2015, and the SMR was above the unit in nearly all municipalities in SP during that year. The number of circulating serotypes, number of years with dengue cases, and degree of urbanization were associated with a higher relative risk of dengue-related mortality.
Footnotes
Supplementary Material available from:
REFERENCES
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Hay SI. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic Arbovirus. Annu Rev Entomol. 2020;65:191–208. doi: 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- 3.University of Washington. Institute for Health Metrics and Evaluation Global health data exchange: GBD results tool. [[cited 2022 Feb 18]]. Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/fda228226164e24692c3c9c54e4a2bf5 .
- 4.Salles TS, Encarnação Sá-Guimarães T, Alvarenga ES, Guimarães-Ribeiro V, Meneses MD, Castro-Salles PF, et al. History, epidemiology and diagnostics of dengue in the American and Brazilian contexts: a review. Parasit Vectors. 2018;11:264–264. doi: 10.1186/s13071-018-2830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrioli DC, Busato MA, Lutinski JA. Spatial and temporal distribution of dengue in Brazil, 1990-2017. PLoS One. 2020;15:e0228346. doi: 10.1371/journal.pone.0228346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito AF, Machado LC, Oidtman RJ, Siconelli MJ, Tran QM, Fauver JR, et al. Lying in wait: the resurgence of dengue virus after the Zika epidemic in Brazil. Nat Commun. 2021;12:2619–2619. doi: 10.1038/s41467-021-22921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezerra JM, Sousa SC, Tauil PL, Carneiro M, Barbosa DS. Entry of dengue virus serotypes and their geographic distribution in Brazilian federative units: a systematic review. Rev Bras Epidemiol. 2021;24:e210020. doi: 10.1590/1980-549720210020. [DOI] [PubMed] [Google Scholar]
- 8.Nunes PC, Daumas RP, Sánchez-Arcila JC, Nogueira RM, Horta MA, Santos FB. 30 years of fatal dengue cases in Brazil: a review. BMC Public Health. 2019;19:329–329. doi: 10.1186/s12889-019-6641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil. Ministério da Saúde Secretaria de Vigilância em Saúde. Óbito por arboviroses no Brasil, 2008 a 2019. [[cited 2022 Feb 18]];Bol Epidemiol. 2020 51:1–28. Availabe from: http://plataforma.saude.gov.br/anomalias-congenitas/boletim-epidemiologico-SVS-33-2020.pdf . [Google Scholar]
- 10.Brasil. Ministério da Saúde Secretaria de Vigilância em Saúde. Monitoramento dos casos de arboviroses urbanas causados por vírus transmitidos pelo mosquito Aedes (dengue, chikungunya e zika), semanas epidemiológicas 1 a 51, 2021. [[cited 2022 Feb 18]];Bol Epidemiol. 2021 52:17–17. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos-1/boletins-epidemiologicos/2021/boletim-epidemiologico-vol-52-no-48.pdf/view . [Google Scholar]
- 11.Katzelnick LC, Coloma J, Harris E. Dengue: knowledge gaps, unmet needs, and research priorities. Lancet Infect Dis. 2017;17:e88–e100. doi: 10.1016/S1473-3099(16)30473-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci U S A. 2006;103:11802–11807. doi: 10.1073/pnas.0602960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carabali M, Hernandez LM, Arauz MJ, Villar LA, Ridde V. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect Dis. 2015;15:301–301. doi: 10.1186/s12879-015-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moraes GH, Duarte EF, Duarte EC. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. Am J Trop Med Hyg. 2013;88:670–676. doi: 10.4269/ajtmh.11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baquero OS, Machado G. Spatiotemporal dynamics and risk factors for human Leptospirosis in Brazil. Sci Rep. 2018;8:15170–15170. doi: 10.1038/s41598-018-33381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Quijano FA, Waldman EA. Factors associated with dengue mortality in Latin America and the Caribbean, 1995-2009: an ecological study. Am J Trop Med Hyg. 2012;86:328–334. doi: 10.4269/ajtmh.2012.11-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paixão ES, Costa MC, Rodrigues LC, Rasella D, Cardim LL, Brasileiro AC, et al. Trends and factors associated with dengue mortality and fatality in Brazil. Rev Soc Bras Med Trop. 2015;48:399–405. doi: 10.1590/0037-8682-0145-2015. [DOI] [PubMed] [Google Scholar]
- 18.Silva MD, Branco MD, Aquino J, Junior, Queiroz RC, Bani E, Moreira EP, et al. Spatial-temporal analysis of dengue deaths: identifying social vulnerabilities. Rev Soc Bras Med Trop. 2017;50:104–109. doi: 10.1590/0037-8682-0272-2016. [DOI] [PubMed] [Google Scholar]
- 19.Tobler WR. A computer movie simulating urban growth in the Detroit Region. Econ Geogr. 1970;46(Suppl):234–240. [Google Scholar]
- 20.Instituto Brasileiro de Geografia e Estatística [[cited 2022 Feb 18]];Downloads. Available from: https://www.ibge.gov.br/estatisticas/downloads-estatisticas.html . [Google Scholar]
- 21.Brasil. Ministério da Saúde . DATASUS. CNES: estabelecimentos com tipo de atendimento prestado: internação; São Paulo: [[cited 2022 Feb 18]]. Available from: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?cnes/cnv/atintsp.def . [Google Scholar]
- 22.Blangiardo M, Cameletti M. Spatial and spatio-temporal Bayesian models with R-INLA. Chichester: John Wiley and Sons; 2015. [Google Scholar]
- 23.Besag J, York J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–20. [Google Scholar]
- 24.Riebler A, Sørbye SH, Simpson D, Rue H. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Stat Methods Med Res. 2016;25:1145–1165. doi: 10.1177/0962280216660421. [DOI] [PubMed] [Google Scholar]
- 25.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc B. 2009;71:319–392. [Google Scholar]
- 26.Simpson D, Rue H, Riebler A, Martins TG, Sørbye SH. Penalising model component complexity: a principled, practical approach to constructing priors. Stat Sci. 2017;32:1–28. [Google Scholar]
- 27.Díaz Y, Chen-Germán M, Quiroz E, Carrera JP, Cisneros J, Moreno B, et al. Molecular epidemiology of dengue in Panama: 25 years of circulation. Viruses. 2019;11:764–764. doi: 10.3390/v11080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siqueira JB, Jr, Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tauil PL. Urbanização e ecologia do dengue. Cad Saude Publica. 2001;17(Suppl):S99–S102. [PubMed] [Google Scholar]
- 30.Shirin T, Muraduzzaman AK, Alam AN, Sultana S, Siddiqua M, Khan MH, et al. Largest dengue outbreak of the decade with high fatality may be due to reemergence of DEN-3 serotype in Dhaka, Bangladesh, necessitating immediate public health attention. New Microbes New Infect. 2019;29:100511–100511. doi: 10.1016/j.nmni.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatura SN, Denis D, Santoso MS, Hayati RF, Kepel BJ, Yohan B, et al. Outbreak of severe dengue associated with DENV-3 in the city of Manado, North Sulawesi, Indonesia. Int J Infect Dis. 2021;106:185–196. doi: 10.1016/j.ijid.2021.03.065. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-García E, Muñoz ML, David RE, Pérez-Ramírez G, Navarrete-Espinosa J, Díaz-Badillo A, et al. Epidemiological implications of the genetic diversification of dengue virus (DENV) serotypes and genotypes in Mexico. Infect Genet Evol. 2020;84:104391–104391. doi: 10.1016/j.meegid.2020.104391. [DOI] [PubMed] [Google Scholar]
- 33.Adams B, Holmes EC, Zhang C, Mammen MP, Jr, Nimmannitya S, Kalayanarooj S, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci U S A. 2006;103:14234–14239. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suppiah J, Ching SM, Amin-Nordin S, Mat-Nor LA, Ahmad-Najimudin NA, Low GK, et al. Clinical manifestations of dengue in relation to dengue serotype and genotype in Malaysia: a retrospective observational study. PLoS Negl Trop Dis. 2018;12:e0006817. doi: 10.1371/journal.pntd.0006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha MP, Duarte AN, Neto, Pour SZ, Hajjar LA, Frassetto FP, Dolhnikoff M, et al. Systemic dengue infection associated with a new dengue virus type 2 introduction in Brazil: a case report. BMC Infect Dis. 2021;21:311–311. doi: 10.1186/s12879-021-05959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gómez-Dantés H, Willoquet JR. Dengue in the Americas: challenges for prevention and control. Cad Saude Publica. 2009;25(Suppl 1):S19–S31. doi: 10.1590/s0102-311x2009001300003. [DOI] [PubMed] [Google Scholar]
- 37.Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol. 2005;20:328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Aswi A, Cramb SM, Moraga P, Mengersen K. Bayesian spatial and spatio-temporal approaches to modelling dengue fever: a systematic review. Epidemiol Infect. 2018;147:e33. doi: 10.1017/S0950268818002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SA, Jarvis CI, Edmunds WJ, Economou T, Lowe R. Spatial connectivity in mosquito-borne disease models: a systematic review of methods and assumptions. J R Soc Interface. 2021;18:20210096–20210096. doi: 10.1098/rsif.2021.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres-Signes A, Dip JA. A Bayesian functional methodology for dengue risk mapping in Latin America and the Caribbean. Acta Trop. 2021;215:105788–105788. doi: 10.1016/j.actatropica.2020.105788. [DOI] [PubMed] [Google Scholar]