Abstract
The therapeutic responses to the eight most widely used antimalarial drugs were assessed in 207 adult patients with Plasmodium vivax malaria. This parasite does not cause marked sequestration, so parasite clearance can be used as a direct measure of antimalarial activity. The activities of these drugs in descending order were artesunate, artemether, chloroquine, mefloquine, quinine, halofantrine, primaquine, and pyrimethamine-sulfadoxine (PS). Therapeutic responses to PS were poor; parasitemias did not clear in 5 of the 12 PS-treated patients, whereas all the other patients made an initial recovery. Of 166 patients monitored for ≥28 days, 35% had reappearance of vivax malaria 11 to 65 days later and 7% developed falciparum malaria 5 to 21 days after the start of treatment. There were no significant differences in the times taken for vivax malaria reappearance among the different groups except for those given mefloquine and chloroquine, in which all vivax malaria reappearances developed >28 days after treatment, suggesting suppression of the first relapse by these slowly eliminated drugs. There was no evidence of chloroquine resistance. The antimalarial drugs vary considerably in their intrinsic activities and stage specificities of action.
Plasmodium vivax affects millions of people living in tropical areas and is an important cause of morbidity in Central and South Americas and Asia. Until recently, P. vivax has remained uniformly sensitive to chloroquine, and this cheap and widely available antimalarial agent has been the treatment of choice for the past 50 years. Although P. vivax developed resistance to the dihydrofolate reductase inhibitors within a few years of their initial deployment as single drugs (7), chloroquine resistance has developed only in the last decade. High-level chloroquine resistance has been well documented on the northern part of the island of New Guinea and in Sumatra, Indonesia, and there have been sporadic reports from other geographic locations (1). The evaluation of alternative antimalarial drugs for the treatment of vivax malaria is therefore needed.
Most research on the efficacies of antimalarial drug treatments concerns Plasmodium falciparum, the most dangerous and the most drug resistant of the four human malaria parasites. P. vivax is less virulent than P. falciparum because it does not reach high parasite densities and it does not sequester in the capillaries and venules. As all stages of asexual development are present in the peripheral blood, the initial decline in the level of parasitemia following drug treatment of P. vivax malaria reflects antimalarial activity and not a combination of accelerated parasite clearance and sequestration (13). This allows a direct comparison of the antimalarial activities of different antimalarial agents, including drugs which act predominantly in the second half of the asexual life cycle. In contrast, in falciparum malaria comparison of the pharmacodynamic properties of antimalarial agents in vivo is confounded by the sequestration of parasitized red blood cells in the microvasculature. The present study compared the intrinsic antimalarial activities of different drugs in the treatment of acute vivax malaria.
MATERIALS AND METHODS
Patients.
The study was conducted with adult male patients with acute symptomatic P. vivax malaria admitted to the Bangkok Hospital for Tropical Diseases, Bangkok, Thailand, between 1992 and 1998. Fully informed consent was obtained from each subject. Patients with mixed infections, those who gave a history of drug hypersensitivity or who had taken any antimalarial drugs within the previous 48 h, or those whose urine was positive by screening tests for sulfonamides (lignin test) or 4-aminoquinolines (Wilson-Edeson test) were excluded. Patients with glucose-6-phosphate dehydrogenase deficiency were not recruited for studies involving primaquine or sulfadoxine. The study was approved by the ethics committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Management.
After clinical assessment and confirmation of the diagnosis from thick and thin blood smears, baseline blood samples were taken for routine hematology and biochemistry analyses. Patients were allocated at random to one of the following nine treatment regimens: (i) chloroquine (Thai Government Pharmaceutical Organization; 150 mg of base/tablet) at 10 mg of base/kg of body weight, followed 6 h later by 5 mg of base/kg repeated at 24 and 36 h (total dose, 25 mg of base/kg), followed by primaquine (Thai Government Pharmaceutical Organization; 75 mg of base/tablet) at 15 mg of base/day for 14 days; (ii) chloroquine at 10 mg of base/kg, followed 6 h later by 5 mg of base/kg repeated at 24 and 36 h (total dose, 25 mg of base/kg); (iii) primaquine (Thai Government Pharmaceutical Organization) at 0.25 mg of base/kg daily (adult dose, 15 mg of base/day) for 14 days; (iv) quinine sulfate (Thai Government Pharmaceutical Organization; 300 mg of salt/tablet) at 10 mg of salt/kg three times a day for 7 days; (v) mefloquine (Lariam; Hoffmann-La Roche, Basel, Switzerland; 250 mg of base/tablet) at 15 mg of base/kg as a single dose; (vi) halofantrine (SmithKline & Beecham Laboratories, Paris, France; 233 mg of base/tablet) at 8 mg base/kg three times a day for 1 day; (vii) artesunate (Guilin No. 1 Factory, Guangxi, People's Republic of China; 50 mg of salt/tablet) at 3.3 mg/kg (adult dose, 200 mg) on the first day and then at 1.65 mg/kg (adult dose, 100 mg/day) for a further 4 days; (viii) artemether (Kunming Pharmaceutical Factory, Kunming, People's Republic of China; 40 mg of salt/capsule) at 2.7 mg/kg (adult dose, 160 mg) on the first day and then at 1.3 mg/kg daily (adult dose, 80 mg/day) for a further 4 days; and (ix) Fansidar (Roche, Basel, Switzerland; 500 mg of sulfadoxine plus 25 mg of pyrimethamine [PS] per tablet) at 25/1.25 mg/kg (adult dose, three tablets) as a single dose.
Oral acetaminophen (0.5 to 1 g every 4 h) was given for a temperature of ≥38°C. Vital signs were recorded every 4 h until resolution of fever and thereafter every 6 to 12 h. Fever clearance times (FCTs) were expressed as FCTA, the time taken for the body temperature first to fall below 37.5°C, and FCTB, the time taken for the body temperature to fall below 37.5°C and to remain below this value for >48 h. Patients who were subsequently unable to stay in the hospital until clearance of both fever and parasites were excluded from the study. Early treatment failure was defined as persistence of fever and parasitemia for more than 7 days or persistence of parasitemia in the absence of fever for more than 2 weeks. For any regimen with a ≥40% treatment failure rate, further recruitment of patients was terminated. Reappearance of infection was assessed in patients who remained in Bangkok either in the hospital or at home (i.e., outside the malaria transmission area) for at least 28 days. Patients who failed to respond to the studied therapies or those who had recurrent vivax malaria were treated subsequently with the standard dose of chloroquine and primaquine (regimen 1). Patients with delayed appearance of P. falciparum were treated with a 7-day course of quinine (10 mg of the salt/kg every 8 h) combined with tetracycline (250 mg every 6 h).
Laboratory investigations.
Parasite counts were measured every 6 h in thin films until the parasitemia became detectable only in thick films and then every 12 h until clearance and thereafter daily for 28 days. Parasitemia was expressed as the number of parasites per microliter of blood, derived from the numbers of parasites per 1,000 red blood cells in a thin film stained with Giemsa or Field stain or calculated from the white cell count and the numbers of parasites per 200 white blood cells in a thick film. The following variables were chosen prospectively to describe parasite clearance: time taken from the start of antimalarial treatment until the asexual malaria parasite count fell by 50% (PC50) or by 90% (PC90) of the admission value and the time for the parasite count to fall below detectable levels in a peripheral blood smear (parasite clearance time [PCT]). Variables to define the rates of parasite reduction were the ratio of the parasite count before treatment to the counts at 24 h (PRR24) or at 48 h (PRR48) and the ratio of the parasite count at 48 h to the count at 96 h (PRR96). Routine biochemical and hematological tests were performed on admission and were repeated weekly thereafter.
Statistical analysis.
The data from each treatment group were compared by one-way analysis of variance with post-hoc multiple comparisons by using the Bonferroni correction. Nonparametric data were compared by the Kruskal-Wallis test. The cumulative cure rates were calculated by Kaplan-Meier survival analysis, and rates were compared by the Logrank test. Associations between fever clearance, parasite clearance, and rates of parasite reduction were measured by using Spearman's rank correlation coefficient. All statistical analyses were performed with the statistical computing package SPSS (version 8 for Windows; SPSS, Gorinchem, The Netherlands).
RESULTS
Patients.
The study included 207 male patients (ages, between 15 and 64 years; mean age, 25 ± 9 years) with P. vivax malaria. The patients were from all parts of Thailand, but most came from the west (69%) and the east (14.5%), where P. falciparum multidrug resistance is an increasing problem. The majority of these patients (69%) had history of a previous malaria parasite infection. There were no significant differences in age distributions, admission laboratory data (Table 1), numbers of previous malaria attacks, or the proportion of patients among the various treatment groups who returned for follow-up (P ≥ 0.061).
TABLE 1.
Laboratory findings on admission for patients with P. vivax infection in various treatment groupsa
| Treatment group | Total no. of patients | Hematocrit (%)b | WBC (no. [103]/μl)b | Creatinine concn (mg/dl)b | Total bilirubin concn (mg/dl)c | SGOT level (units/liter)c |
|---|---|---|---|---|---|---|
| CP | 30 | 40 (4) | 9.8 (6.3) | 1.1 (0.2) | 1.2 (0.8–2.0) | 37 (20–48) |
| Chloroquine | 30 | 34 (9) | 6.3 (1.7) | 1.0 (0.2) | 1.0 (0.4–3.3) | 25 (10–197) |
| Primaquine | 30 | 36 (6) | 6.4 (1.7) | 1.1 (0.2) | 1.1 (0.3–3.7) | 21 (8–70) |
| Quinine | 22 | 38 (8) | 7.2 (2.2) | 1.1 (0.2) | 1.5 (0.7–7.6) | 19 (8–60) |
| Mefloquine | 20 | 36 (9) | 8.6 (2.6) | 1.2 (0.3) | 1.9 (0.7–4.7) | 23 (10–173) |
| Halofantrine | 23 | 36 (5) | 7.2 (3.2) | 1.0 (0.1) | 1.6 (0.4–3.9) | 20 (10–94) |
| Artesunate | 20 | 35 (8) | 7.0 (2.7) | 1.0 (0.2) | 1.7 (0.4–4.3) | 18 (10–219) |
| Artemether | 20 | 40 (5) | 7.1 (2.5) | 1.2 (.2) | 1.6 (0.3–4.9) | 21 (10–114) |
| PS | 12 | 32 (9) | 6.8 (2.3) | 1.1 (0.3) | 1.9 (0.9–5.5) | 17 (10–198) |
| Total | 207 | 37 (7) | 7.4 (3.9) | 1.1 (0.2) | 1.4 (0.3–7.6) | 20 (8–219) |
Abbreviations: WBC, white blood cell; SGOT, serum glutamic oxalacetic transaminase; CP, chloroquine-primaquine.
Data are means (standard deviations).
Data are means (ranges).
Clinical response.
Clinical recovery following treatment occurred in all except five PS-treated patients (Table 2). Of the five patients who failed PS treatment, one had persistence of fever and parasitemia for more than 168 h, and the other four patients became afebrile in 52 to 155 h (mean, 107 ± 43 h) but the parasitemia persisted. Following treatment, FCTA ranged from 3 to 30 h (median, 8 h). There were no significant differences in FCTA between the treatment groups studied (P > 0.01). The overall median (range) FCTB was 27 h (range, 3 to >168 h). The FCT was fastest in patients treated with artesunate or artemether (P < 0.001) and was slowest in patients treated with PS (P = 0.001). Patients treated with mefloquine had significantly shorter FCTBs compared to the FCTBs for those treated with halofantrine (P = 0.002). There were no significant differences in FCTB when the FCTBs for other pairs of related treatment compounds were compared: artesunate and artemether, chloroquine and quinine, as well as quinine and primaquine (P ≥ 0.49). None of the patients developed serious adverse effects, as determined by monitoring of clinical symptoms and from laboratory data.
TABLE 2.
Parasite and fever clearance times for patients with P. vivax infection in various treatment groups
| Treatment group | Total no. of patients | Parasite clearance times (h)a
|
FCT (h)b
|
|||
|---|---|---|---|---|---|---|
| PC50 | PC90 | PCT | FCTA | FCTB | ||
| CPc | 30 | 15 (11) | 25 (12) | 64 (22) | 9 (2–24) | 30 (4–72) |
| Chloroquine | 30 | 9 (6) | 20 (7) | 65 (18) | 10 (2–30) | 31 (4–60) |
| Primaquine | 30 | 23 (16) | 48 (19) | 93 (34) | 4 (4–44) | 28 (4–96) |
| Quinine | 22 | 15 (8) | 33 (8) | 98 (23) | 8 (4–24) | 31 (6–169) |
| Mefloquine | 20 | 10 (5) | 24 (9) | 76 (22) | 4 (2–19) | 21 (4–39) |
| Halofantrine | 23 | 13 (7) | 37 (12) | 85 (24) | 8 (4–23) | 35 (8–104) |
| Artesunate | 20 | 6 (2) | 9 (3) | 38 (13) | 9 (3–24) | 14 (6–72) |
| Artemether | 20 | 6 (3) | 10 (4) | 50 (11) | 4 (3–25) | 17 (3–114) |
| PS | 12 | 13 (7) | 46 (15) | 114d (44) | 7 (4–16) | 58 (15–>168) |
| Total | 207 | 12 (10) | 28 (17) | 74 (31) | 8 (2–30) | 27 (3–>168) |
Data are means (standard deviations).
Data are means (ranges).
CP, Chloroquine-primaquine.
Excluding five patients with treatment failure.
PS responses.
Five patients in the PS group had early treatment failures (5 of 12; 42%). Recruitment to this treatment group was therefore terminated. A further three of the five patients who could be monitored for ≥28 days had recurrent vivax malaria. Fever and PCTs were slow in these patients (Tables 2 and 3).
TABLE 3.
PRRs for patients with P. vivax infection described for the various treatment groups
| Group | PRR24a | No. of patients for PRR24 | PRR48a | No. of patients for PRR48 | PRR96a | No. of patients for PRR96 |
|---|---|---|---|---|---|---|
| CPb | 12 (2–509) | 27 | 90 (2–2,747) | 21 | 882 (457–1,306) | 2 |
| Chloroquine | 36 (1–1,184) | 30 | 438 (33–2,575) | 23 | 972 | 1 |
| Primaquine | 2 (<1–692) | 28 | 9 (<1–692) | 27 | 315 (9–2,579) | 13 |
| Quinine | 4 (1–54) | 22 | 97 (3.5–561) | 22 | 1,498 (178–3,266) | 8 |
| Mefloquine | 12 (2–332) | 20 | 449 (18–3,182) | 19 | 1,572 (754–1,968) | 4 |
| Halofantrine | 7 (1–276) | 23 | 201 (<1–2,079) | 20 | 1,427 (521–2,020) | 4 |
| Artesunate | 844 (110–2,613) | 17 | 1,507 (146–2,531) | 5 | 0 | |
| Artemether | 508 (57–3,869) | 20 | 1,720 (339–2,311) | 8 | 0 | |
| PS | 2 (<1–381) | 12 | 15 (2–1,330) | 11 | 139 (13–2,160) | 10 |
| Total | 12 (<1–3,869) | 199 | 112 (<1–3,182) | 156 | 641 (9–3,266) | 42 |
Data are medians (ranges).
CP, chloroquine-primaquine.
PCTs.
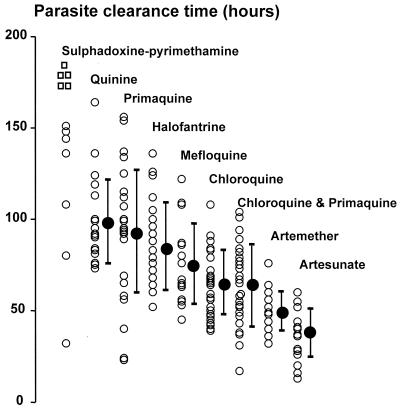
Following treatment, the PCTs for the different treatment groups varied from 13 to more than 168 h with a hierarchy of drug activities (Fig. 1; Table 2). Excluding the five PS-treated patients who failed early treatment, the overall mean (standard deviation) PCT for the remaining 202 patients was 73.5 h (30.5 h). PCT was correlated positively with FCTB (r = 0.39; P < 0.001). Most of the patients (91%; n = 189) cleared the parasites after fever clearance (median interval, 45 h; range, 1 to 130 h). The median ratio of PCT − FCTB/PCT was 0.63 (range, −0.3 to +0.97), and this ratio was not significantly different among the treatment groups (P = 0.8). Parasitological responses (PC50, PC90, and PCT) for all groups were fastest in patients who were treated with artesunate and were slowest in patients who received PS (Fig. 1).
FIG. 1.
PCTs for various groups of patients treated for P. vivax infection. Data (closed circles) are shown as means (standard deviations).
The PCT following the standard treatment, chloroquine-primaquine, was significantly longer compared to that following treatment with artesunate (P = 0.005) and was significantly shorter compared to those following treatment with quinine, primaquine, and PS (P < 0.001). PCTs for chloroquine-primaquine were not significantly different from those for chloroquine, artemether, mefloquine, and halofantrine (P ≥ 0.07). In a comparison of the responses to treatment with different antimalarial drugs, the PC90s mirrored the PCTs, whereas PC50s were not significantly different between the groups (P ≥ 0.02). In a comparison of the two qinghaosu derivatives, the PCTs for artesunate were shorter than the PCTs for artemether (P = 0.002), although their PC50s and PC90s were similar (P > 0.12). Patients treated with chloroquine alone also had significantly faster parasitological responses (PC50, PC90, and PCT) compared to those for patients treated with quinine (P ≤ 0.01). There were no significant differences in the parasitological responses between the mefloquine and halofantrine groups (P = 0.21).
PRRs.
Following treatment, the calculated PRRs (PRR24, PRR48, and PRR96) for all patients varied widely, ranging from <1 to >3,000 (P ≤ 0.018; Table 3). The median PRR24s and PRR48s for artesunate and artemether were highest and were at least 14-fold greater than those for the other treatments. There were no significant differences in PRR24 and PRR48 between artesunate and artemether (P ≥ 0.14) or between halofantrine and mefloquine (P ≥ 0.14). Chloroquine had significantly higher PRR24s and PRR48s compared to the reduction ratios observed following quinine treatment (P ≤ 0.002) (Table 3).
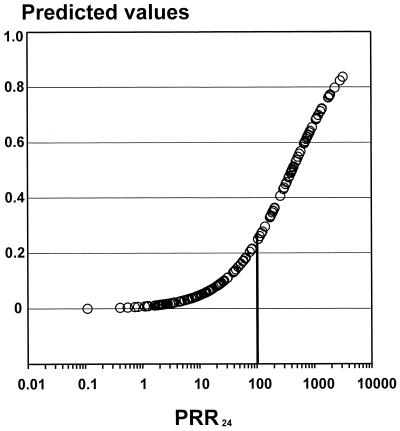
As expected from the first-order decline in parasitemia following treatment, the overall PCT for all 202 patients correlated with the PRRs and the PC50s and PC90s (r = 0.32 to 0.72; P ≤ 0.001). A PRR24 of ≥100 increased the probability of parasite clearance within 48 h 12.4-fold (95% confidence interval, 5.5 to 27.9; P < 0.001) (Fig. 2), giving positive and negative predictive values of 0.56 and 0.95, respectively.
FIG. 2.
PRR24-dependent predictive value for PCT of ≤48 h in patients with P. vivax infection.
Clinical course.
Overall, 166 (80%) of the recruited patients completed 28 days of follow-up or remained in the hospital until the appearance of vivax or falciparum malaria (Table 4). Of these, 32 (19.3%) patients returned for subsequent monthly follow-up (outside the protocol) for 1 to 2 months. Reappearance of the vivax malaria, which was observed in all treatment groups, occurred in 58 patients (35%), and delayed appearance of falciparum malaria occurred in 11 patients (7%).
TABLE 4.
Clinical outcomes for monitored patients
| Treatment group | No. of patients
|
||||
|---|---|---|---|---|---|
| Subsequent appearance of:
|
Complete cure | Total | |||
|
P. vivax
|
P. falciparum | ||||
| ≤28 days | >28 days | ||||
| CPa | 1 | 21 | 22 | ||
| Chloroquine | 1 | 6 | 14 | 21 | |
| Primaquine | 3 | 2 | 3 | 18 | 26 |
| Quinine | 11 | 2 | 4 | 17 | |
| Mefloquine | 2 | 15 | 17 | ||
| Halofantrine | 9 | 1 | 2 | 5 | 17 |
| Artesunate | 12 | 7 | 19 | ||
| Artemether | 9 | 2 | 6 | 17 | |
| PS | 3 + 5 TFsb | 2 | 10 | ||
| Total | 47 + 5 TFs | 11 | 11 | 92 | 166 |
CP, chloroquine-primaquine.
TFs, treatment failures.
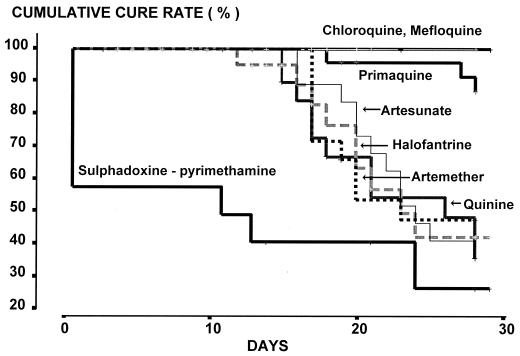
The cumulative cure rates of vivax malaria with 28 days of follow-up for the standard treatment of chloroquine plus primaquine and for mefloquine were 100%. Assessed at 28 days, both drugs were significantly more efficacious than the remaining drugs (P ≤ 0.017). Among the antimalarial drug monotherapies, the 28-day cumulative cure rates for quinine, halofantrine, artemether, and artesunate were not significantly different, but for all drugs they were lower than those for primaquine, chloroquine, and mefloquine (Fig. 3). Within the 28-day follow-up period the median time to the reappearance of P. vivax was 20 days (range, 11 to 28 days). Among those patients given mefloquine or chloroquine, reappearance of P. vivax occurred in 3 of 32 patients monitored after 28 days of treatment. There were no significant differences in the time for the reappearance of vivax malaria among the remaining treatment groups (P = 0.42).
FIG. 3.
Cumulative cure rates for various groups of patients treated for P. vivax infection.
After treatment and clearance of the first P. vivax infection, delayed appearance of falciparum malaria was observed in 11 patients from only three treatment groups: the chloroquine, primaquine, and halofantrine treatment groups (Table 4). The overall mean (standard deviation) time to detection of falciparum malaria in these patients was 13.1 days (5.7 days) and ranged from 5 to 21 days after the initiation of treatment for vivax malaria. There was no significant difference in the time to the appearance of falciparum malaria between these groups (P = 0.14). All of these patients were treated successfully with a 7-day course of quinine and tetracycline.
The apparent success rates of the different treatments (no subsequent appearances of either vivax or falciparum malaria) were significantly higher for the standard treatment, chloroquine and primaquine (95.5%), and mefloquine (88.2%) than for the remaining treatments (P ≤ 0.01). The success rate for chloroquine treatment was significantly higher than that for quinine treatment (67 versus 24%; P = 0.021). There was no significant difference in the success rates following artesunate and artemether treatments (37 versus 35%; P = 0.83).
Parasite clearance rates in patients with and without reappearance of P. vivax infection.
There were no significant differences in either FCTs (FCTA and FCTB) or parasitological responses (PCTs or PRRs) between patients with and without reappearance of P. vivax infection during the 28-day follow-up (P ≥ 0.14). However, after the exclusion of data for the rapidly acting drugs (artemether and artesunate) from the analysis, patients with reappearance of P. vivax infection had significantly longer PCTs compared to those for patients without P. vivax infection reappearance (mean, 97 ± 31 versus 78 ± 28 h; P = 0.003), although their PRRs and FCTs were not significantly different (P ≥ 0.21). There were significant correlations between the time to onset of reappearance of P. vivax infection and PCT (r = −0.52; P = 0.001), PRR24 (r = 0.45; P = 0.007), or PRR48 (r = 0.47; P = 0.006). Patients with PCTs of more than 96 h had a 2.1 (95% confidence interval, 1.2 to 3.8) relative risk for reappearance of P. vivax infection compared to that for patients who had cleared the parasites within 96 h (P = 0.021). These associations did not exist for patients given either artesunate or artemether.
DISCUSSION
In Asia and South and Central Americas, P. vivax is a major cause of morbidity. Vivax malaria differs from falciparum malaria in several important respects; it seldom causes death (9), until recently it has been uniformly sensitive to chloroquine, and it causes relapses which derive from persistent liver stages (hypnozoites) of the parasite. The majority of antimalarial drug trials in recent years have concerned falciparum malaria. The recent emergence of resistance to chloroquine in vivax malaria deserves attention and has reawakened interest in P. vivax drug sensitivity. Alternative antimalarial treatments for P. vivax are needed. As this parasite is not sequestered markedly in the microcirculation, the clearance of parasites from the blood can be used as a direct measure of antimalarial activity. This allows comparison of the different drugs in terms of both intrinsic activity and also stage specificity. In falciparum malaria such comparisons are confounded by almost complete sequestration; the drugs which act on the second half of the 2-day asexual cycle cannot be compared easily, as these stages are not visible to the microscopist.
The artemisinin derivatives are the most rapidly acting and potent of the antimalarial drugs. In this series the times to reduction of parasitemia by 50% were less than 6 h for both derivatives; in contrast, values generally exceed 9 h for the other antimalarial drugs. This reflects the early stage specificity of action of the artemisinin derivatives on young ring-form parasites. Chloroquine, with or without primaquine, produced more rapid parasite clearance than quinine, which confirms the results of previous studies (6, 11, 14) and which also reflects the early stage specificity of drug action (12). In contrast, drugs with weaker intrinsic activities and/or later stage specificities, such as primaquine (10) and, in particular, PS, gave slow rates of parasite clearance. The ratio of the baseline parasite count to that 48 h later (i.e., after one asexual life cycle) allows the antimalarial activities of different drugs to be compared without the confounder of stage specificity (13). Large differences in intrinsic antimalarial activity between the drugs were evident, with the artemisinin derivatives having the greatest activities and primaquine and PS having the weakest activities. Indeed, 5 of the 12 patients treated with PS had high-grade failures that required early treatment with chloroquine plus primaquine. This indicates a very high level of resistance to the antifolate-sulfonamide combination. This resistance results from mutations in the gene that encodes dihydrofolate reductase, as in P. falciparum (3). Although these antimalarial drugs have never been compared in a single study as treatments for falciparum malaria, this hierarchy of activity is similar to that which would be expected in P. falciparum parasites which were chloroquine sensitive but antifolate resistant.
Assessment of the therapeutic response to vivax malaria is complicated by the appearance of relapses. These result from persistent liver stages (hypnozoites) of the parasite which are insensitive to all antimalarial drugs except the 8-aminoquinolines. For this reason, radical treatment of vivax malaria requires treatment with primaquine. The relapse interval in Southeast Asian vivax malaria has traditionally been reported to be 6 weeks (2). However, it is evident that when primaquine is not given and vivax malaria is treated with effective short-half-life antimalarial drugs, then the infection reappears on average 3 weeks after the start of treatment (1). By comparison with the more drug-resistant falciparum malaria, for which failure rates would have been expected to be less than 20% following treatments with quinine or artemisinin derivatives, vivax malaria reappeared in over 50% of patients, despite rapid parasite clearance. The similarities in the cumulative cure rate profiles (Fig. 3) for artesunate, artemether, halofantrine, and quinine, despite their excellent activities against the blood stage of the parasite, suggest that these reappearances result from relapses of the infection and not recrudescence. This is consistent with other work conducted on the western border of Thailand, where approximately 50% of patients treated with chloroquine alone, but not with chloroquine-primaquine, suffer a relapse of vivax malaria (although this occurs at 6 weeks, not at 3 weeks) (5). When slowly eliminated antimalarial drugs such as chloroquine and mefloquine are given, the first relapse is presumably suppressed by residual antimalarial activity in the blood. The second relapse occurs 3 weeks after the first, giving a total relapse interval of 6 weeks. Thus, with 4 weeks of follow-up, large apparent differences in the antimalarial efficacies of different antimalarial drugs are found if cure rate is assessed, but this is an artifact of their elimination profiles. The second and subsequent relapses are not prevented by slowly eliminated drugs. Among the patients treated with primaquine, which is known to prevent relapses effectively, there were occurrences of vivax malaria within 28 days in 5 of the 26 patients who could be monitored. Taken together with very slow asexual-stage responses, it is likely that some or all of these represented true recrudescences. This confirms our previous report indicating the weak asexual-stage activity of primaquine against P. vivax (10). For PS the high early failure rates without parasite clearance confirm the very low intrinsic activities of these drugs against vivax malaria in Thailand.
Of the 166 patients with ≥28 days of follow-up and without the possibility of reinfection, 11 (7%) developed falciparum malaria. This high rate of mixed infection is consistent with our previous experience and compares with an approximate 30% rate of cryptic mixed infection in patients presenting with falciparum malaria. Interestingly, primaquine and chloroquine together appeared to have some activity in suppressing the multidrug-resistant P. falciparum strains prevalent in this area, as none of the 21 patients treated with chloroquine-primaquine had subsequent falciparum malaria, whereas 3 of 26 primaquine recipients and 6 of 21 recipients of only chloroquine had subsequent falciparum malaria. The efficacies of the other drugs in suppressing P. falciparum is anticipated from their known efficacy in this area of the world and would have been augmented by coexistent vivax malaria (8).
This study raises several interesting questions concerning the assessment of antimalarial drugs in vivax malaria. As the true relapse interval of Southeast Asian strains of P. vivax is 3 weeks and this is also the mean time to recrudescence following treatment with rapidly eliminated antimalarial agents, it is not possible to distinguish with confidence a recrudescence from a relapse. Presumably, the genotypes of strains that cause relapses and recrudescences are the same, as they derive from the same initial infection (although this remains to be confirmed) (4). Comparison of parasite clearance rates allows some assessment of the intrinsic antimalarial activity, but it is heavily influenced by stage specificity. On the other hand, prevention of relapse with primaquine does allow comparison of different drugs, but it is confounded by the intrinsic antimalarial activity of primaquine itself against the asexual stages. For accurate comparison of relapse and recrudescence rates in vivax malaria, at least 2 months of follow-up is required.
ACKNOWLEDGMENTS
We are grateful to the staff of the Hospital for Tropical Diseases Bangkok.
This study was part of the Wellcome-Mahidol University, Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Baird J K, Leksana B, Masbar S, Fryauff D J, Sutanihardja A M, Suradi, Wingnall F S, Hoffman S L. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 2.Collins W, Jeffery G M. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 3.de Pecoulas P E, Tahar R, Ouatas T, Mazabraud A, Basco L K. Sequence variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol Biochem Parasitol. 1998;92:265–273. doi: 10.1016/s0166-6851(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 4.Kirchgatter K, del Portillo H A. Molecular analysis of Plasmodium vivax relapses using the MSP1 molecule as a genetic marker. J Infect Dis. 1998;117:511–515. doi: 10.1086/517389. [DOI] [PubMed] [Google Scholar]
- 5.Luxemburger C, van Vugt M, Jonathan S, McGready R, Looareesuwan S, White N J, Nosten F. Treatment of vivax malaria in an endemic area on the western border of Thailand. Trans R Soc Trop Med Hyg. 1999;93:433–438. doi: 10.1016/s0035-9203(99)90149-9. [DOI] [PubMed] [Google Scholar]
- 6.Most H, London I M, Kane C A, Lavietes P H, Schroeder E F. Chloroquine for treatment of acute attacks of vivax malaria. JAMA. 1946;131:963–967. doi: 10.1001/jama.1946.02870290013005. [DOI] [PubMed] [Google Scholar]
- 7.Peters W. Experimental resistance. III. Sulphonamides, sulphones and related compounds. In: Peters W, editor. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. pp. 481–522. [Google Scholar]
- 8.Price R N, Nosten F, Luxemburger C, van Vugt M, Paiphun L, Chongsuphajaisiddhi T, White N J. Artesunate-mefloquine treatment of 1967 patients with multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–577. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 9.Pukrittayakamee S, Chantra A, Vanijanonta S, White N J. Pulmonary oedema in vivax malaria. Trans R Soc Trop Med Hyg. 1998;92:421–422. doi: 10.1016/s0035-9203(98)91075-6. [DOI] [PubMed] [Google Scholar]
- 10.Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White N J. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis. 1994;169:932–935. doi: 10.1093/infdis/169.4.932. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt L H. Comparative efficacies of quinine and chloroquine as companions to primaquine curative drug regimen. Am J Trop Med Hyg. 1981;30:20–25. doi: 10.4269/ajtmh.1981.30.20. [DOI] [PubMed] [Google Scholar]
- 12.ter Kuile F, White N J, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 13.White N J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White N J, Krishna S, Waller D, Craddock C, Kwiatkowski D, Brewster D. Open comparison of intramuscular chloroquine and quinine in children with severe chloroquine-sensitive falciparum malaria. Lancet. 1989;ii:1313–1316. doi: 10.1016/s0140-6736(89)91918-1. [DOI] [PubMed] [Google Scholar]