The authors report on the feasibility of obtaining diagnostic-quality bilateral decubitus CT myelography in a single session, avoiding the need to schedule separate examinations for the left and right sides on different days.
SUMMARY:
Lateral decubitus CT myelography is a sensitive technique for detecting CSF-venous fistulas in patients with spontaneous intracranial hypotension. It might be necessary to perform bilateral studies to locate a fistula. We report on the feasibility of obtaining diagnostic-quality bilateral decubitus CT myelography in a single session, avoiding the need to schedule separate examinations for the left and right sides on different days.
Spontaneous intracranial hypotension (SIH) is a debilitating condition that results from leakage of CSF from the spine. In up to one-quarter of patients with SIH, the underlying cause is a CSF-venous fistula (CVF), an abnormal connection between the subarachnoid space of a nerve root sheath and adjacent veins.1 Targeted treatment of CVFs, by injection of blood and/or fibrin sealant,2 endovascular occlusion, or neurosurgery requires accurate localization of the fistula. Performing CT myelography (CTM) or digital subtraction myelography (DSM) with the patient in the lateral decubitus position increases the sensitivity for CVFs by exposing the nerve root sleeves on the dependent side to a higher concentration of contrast.1,3,4 Although most CVFs occur in the thoracic spine on the right side,5 on which side a CVF will be found cannot be known beforehand. Moreover, CVFs can rarely occur bilaterally.6,7 A complete examination, therefore, requires bilateral decubitus myelograms. Currently most centers undertaking lateral decubitus CTM or DSM examine the right and left sides on separate days, primarily because of manufacturer-set dose constraints on the maximum intrathecal iodine dose8,9 but also because the contrast is diluted by CSF when turning the patient to the opposite side, which leads to decreased opacification of meningeal diverticula.8 This practice is inconvenient for patients and radiology departments alike, even if studies are scheduled across 2 consecutive days. We report our experience in performing bilateral decubitus CTM in the same session.
MATERIALS AND METHODS
Subjects
Institutional review board approval was not required for this retrospective service evaluation. We evaluated our service by reviewing consecutive patients with SIH who were investigated by same-day bilateral decubitus CTM for a suspected CVF between December 2020 and May 2021, all of whom met the International Classification of Headache Disorders, 3rd edition, criteria for SIH.10 All patients had undergone brain MR imaging with contrast and spine MR imaging; one of the patients had previously undergone prone CTM, which had negative findings for a cause of the CSF leak.
Procedural Technique
The technique is a modification of that described previously.8 Patients were placed in the lateral decubitus position on the CT scanner table with the pelvis raised above their shoulders by a foam wedge and with their arms raised by their faces or above their heads. CT fluoroscopically-guided lumbar puncture was performed at L3–L4 or L2–L3 with a 22-ga spinal needle and the subarachnoid position of the needle tip confirmed by a test injection of 0.5 mL of iohexol containing 300 mg I/mL (Omnipaque, GE Healthcare). The theca was then prepressurized by a slow injection of 10 mL of sterile normal saline in an attempt to encourage leaking and also to dilute the test dose of contrast before 6–8 mL of contrast was injected intrathecally, after which the spine was scanned immediately from the lumbar puncture needle to the craniocervical junction during breath-holding in maximum inspiration. Acquisition parameters were pitch = 0.984, rotation time = 800 ms, reconstruction thickness = 0.625 mm, automated exposure control, tube current = 300–800 mA, tube voltage = 120 kV(peak). Images were reviewed at the console for signs of a CVF, including a hyperdense paraspinal vein and opacification of radicular veins in the neural foramen adjacent to a meningeal diverticulum. With the needle still in place, the patient was then assisted to turn carefully from the first decubitus position to lie initially prone and then to the opposite lateral decubitus position. The position of the needle was monitored at all times by the neuroradiologist performing the procedure, while additional staff assisted the patient with turning, avoiding bending or twisting of the lumbar spine. A new scan projection radiograph was acquired, and a limited scan was acquired through the needle to check that it had not become displaced from the vertebral canal during patient repositioning. A further 6–8 mL of iodinated contrast was then injected, and scanning of the spine was repeated as on the first side.
RESULTS
Three patients, 54, 64, and 67 years of age, underwent same-day bilateral decubitus CTM during the study period. Two subjects were women. All subjects had orthostatic headache, and 1 also had cognitive impairment in the form of a frontotemporal dementia-like presentation of SIH. Brain MR imaging showed features of SIH in all cases: All subjects showed diffuse dural enhancement, venous sinus distension, pituitary enlargement, and brain sag; none had subdural fluid collections. The Bern score for brain MR imaging was 8 for all subjects, indicating a high probability of a spinal CSF leak.11 No subjects had spinal epidural fluid collections; thus, a CVF was considered the most likely cause of the SIH, with a distal nerve root sleeve dural tear beyond the epidural compartment being possible but less likely.12 In 2 cases, the right side was examined first. In all 3 cases, decubitus CTM showed a single CVF, located in the right T11, right T5, and left T5 neural foramina, respectively. In 2 cases, the CVF was on the second side to be examined. The left T5 CVF drained to a right paravertebral vein via an intraosseous vein (Fig 1), the right T11 and right T5 CVFs drained into an ipsilateral paravertebral vein at the same level (Fig 2). The procedures were well-tolerated by the subjects with no adverse events during repositioning aside from minor bending of the spinal needle; in no case was the needle tip displaced from the spinal subarachnoid space, and no cases of subdural or epidural contrast injection occurred. Total contrast volumes injected ranged from 12 to 16 mL, split equally between sides. Each subject subsequently underwent a CT-guided percutaneous injection of fibrin sealant (TISSEEL; Baxter) directed at the CVF, targeting the junction of the diverticulum and the draining vein to place fibrin sealant within and around the diverticulum. This resulted in symptom improvement and improvement of imaging findings of SIH in all cases (Fig 3).
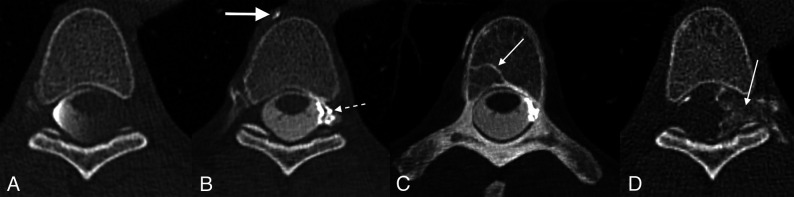
FIG 1.
Left T5 CVF with contralateral venous drainage and subsequent treatment. A, Axial right lateral decubitus CTM image shows dependent layering of contrast on the right side of the subarachnoid space but no leak. B, Axial left lateral decubitus CTM image shows more uniform distribution of contrast within the subarachnoid space following turning of the patient but also abnormal left radicular veins opacified by dense contrast (dashed arrow) and a hyperdense right paravertebral vein (arrow). C, Axial left lateral decubitus CTM MIP image shows transvertebral intraosseous drainage of the CVF to the right side (arrow). D, Axial posttreatment CT shows contrast-opacified fibrin sealant filling the foramen (arrow) and extending into the epidural space of vertebral canal.
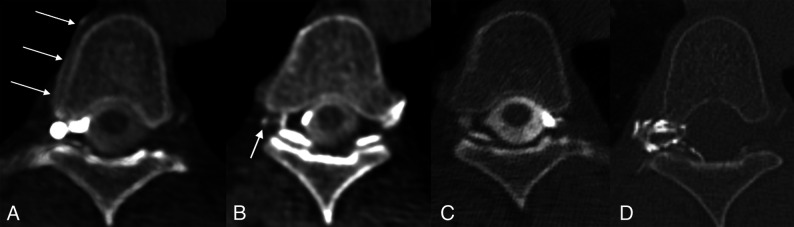
FIG 2.
Right T5 CVF and subsequent treatment. A, Axial right lateral decubitus CTM image shows a right foraminal meningeal diverticulum and a hyperdense right paraspinal vein (arrows). B, Axial right lateral decubitus CTM image obtained immediately caudal to (A) shows opacification of a small radicular vein (arrow). C, Axial left lateral decubitus CTM image shows no abnormality. D, Axial posttreatment CT shows contrast-opacified fibrin sealant filling the foramen and extending into the epidural space of vertebral canal.
FIG 3.
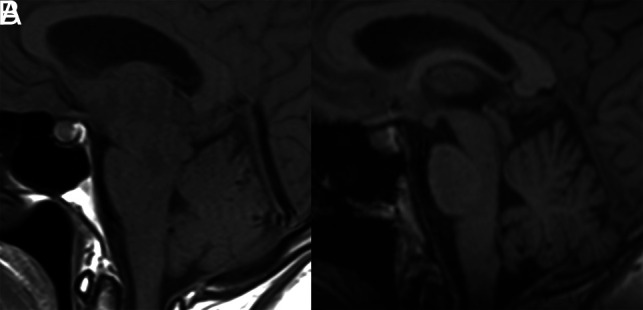
Pre- and posttreatment MRIs of the patient in Fig 2. A, Sagittal T1-weighted MR imaging shows brain sag with narrowing of the mamillopontine distance, narrowing of the prepontine cistern, and inferior sloping of the floor of the third ventricle. There is also pituitary enlargement and distension of the straight sinus. B, Resolution of these changes following CT-guided injection of fibrin sealant.
DISCUSSION
This report presents the feasibility of obtaining diagnostic-quality bilateral decubitus CTM during a single session. Hitherto, it has been the usual practice to schedule decubitus CTM or DSM examinations of the right and left sides on separate days to comply with dose constraints for iodinated contrast in view of the possible risks of neurotoxicity, including seizures and arachnoiditis. The manufacturer-set intrathecal dose limits of 3 g of iodine for the nonionic contrast media iohexol and iopamidol were based on earlier toxicity studies of the ionic agent metrizamide, despite evidence that the newer agents were less neurotoxic.13 Subsequently, dose-finding studies comparing iomeprol, iopamidol, and iohexol for myelography have found that doses up to 4.5 g of iodine were well-tolerated,14 and intrathecal doses of up to 6 g of iodine have been used in dynamic fluoroscopic myelography for localizing spinal CSF leaks without adverse effects.15 We determined, therefore, that the manufacturers’ 3 g dose limit should not necessarily be a barrier to performing bilateral decubitus CTM on the same day, and we were able to obtain diagnostic-quality bilateral studies with total doses between 3.6 and 4.8 g. Because this was an off-label use of the contrast medium, patients gave consent regarding this use. Because our prior practice for single-side decubitus CTM had been to use 10 mL of iohexol, 300 mg I/mL, in accordance with a previously reported technique,8 we initially used 8 mL of contrast per side for the same-day technique to decrease the risk of achieving suboptimal intrathecal opacification and a nondiagnostic study. When a nondiagnostic study did not occur, we subsequently decreased the dose further to 6 mL per side without any deleterious effect on intrathecal opacification or the ability to identify a CVF. It may be possible to obtain diagnostic-quality bilateral decubitus CTM with as little as 5 mL of contrast per side, thus not exceeding the recommended dose limit for intrathecal iodine; this dose is the subject of current investigation.
In 2 cases, the CVF was on the second side to be examined. Given the right-sided preponderance of CVFs,5 an argument could be made for starting with a right lateral decubitus CTM to maximize the chance of detecting a CVF at the earliest stage, potentially obviating the need to examine the opposite side. However, in view of the reported occurrence, albeit rare, of bilateral CVFs,6,7 we recommend always examining both sides even if a CVF is detected on the first side studied.
Benefits of our approach include greater convenience for the patient, including avoidance of a second lumbar puncture for opposite side decubitus CTM on a subsequent day and more efficient use of the CT scanner and neuroradiologist’s time. Avoiding a second CT fluoroscopy–guided lumbar puncture also decreases the radiation dose of same-day decubitus CTM compared with 2 procedures on separate days. Potential drawbacks include the risk of needle displacement during repositioning and subsequent injury to neural or vascular structures, as well as the potential for subdural or epidural contrast injection if the needle tip moves, though this did not occur in any of our cases. Although we observed that the spinal needle became bent during repositioning, this result did not have any adverse effect on the subjects or the performance or outcome of the procedure. Although we did not experience any difficulty repositioning patients on the foam wedge, alternative means of elevating the patient’s pelvis without requiring movement on the CT table, such as placing an inflatable mattress (HoverMatt; HoverTech International) under the patient’s hips and inflating it transiently after the intrathecal injection of contrast, might represent further refinements to the technique.16
CONCLUSIONS
Same-day bilateral decubitus CT myelography can be performed successfully and can result in a diagnostic-quality examination without adverse effects on the patient. This is more convenient for patients and radiology departments, obviating the need for a second procedure, which may carry potential additional risk.
ABBREVIATIONS:
- CTM
CT myelography
- CVF
CSF-venous fistula
- DSM
digital subtraction myelography
- SIH
spontaneous intracranial hypotension
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine 2019. Sep 13 [Epub ahead of print] 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 2.Mamlouk MD, Shen PY, Sedrak MF, et al. CT-guided fibrin glue occlusion of cerebrospinal fluid–venous fistulas. Radiology 2021;299:409–18 10.1148/radiol.2021204231 [DOI] [PubMed] [Google Scholar]
- 3.Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019;40:754–56 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020;41:21–28 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlobin NA, Shah VN, Chin CT, et al. Cerebrospinal fluid-venous fistulas: a systematic review and examination of individual patient data. Neurosurgery 2021;88:931–41 10.1093/neuros/nyaa558 [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Savastano LE, Atkinson JLD, et al. A novel endovascular therapy for CSF hypotension secondary to CSF-venous fistulas. AJNR Am J Neuroradiol 2021;42:882–87 10.3174/ajnr.A7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schievink WI, Maya MM, Moser F, et al. Multiple spinal CSF leaks in spontaneous intracranial hypotension: do they exist? Neurol Clin Pract 2021;11:e691–97 10.1212/CPJ.0000000000001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamlouk MD, Ochi RP, Jun P, et al. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol 2021;42:32–36 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope MC, Carr CM, Brinjikji W, et al. Safety of consecutive bilateral decubitus digital subtraction myelography in patients with spontaneous intracranial hypotension and occult CSF leak. AJNR Am J Neuroradiol 2020;41:1953–57 10.3174/ajnr.A6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 11.Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol 2019;76:580–87 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: A systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 2019;40:745–53 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpin SFS, Guest PJ, Byrne J. V. Theory and practice: how much contrast for myelography? Neuroradiology 1991;33:411–13 10.1007/BF00598614 [DOI] [PubMed] [Google Scholar]
- 14.Katayama H, Heneine N, Van Gessel R, et al. Clinical experience with iomeprol in myelography and myelo-CT: clinical pharmacology and double-blind comparisons with iopamidol, iohexol, and iotrolan. Invest Radiol 2001;36:22–32 10.1097/00004424-200101000-00004 [DOI] [PubMed] [Google Scholar]
- 15.Piechowiak EI, Pospieszny K, Haeni L, et al. Role of conventional dynamic myelography for detection of high-flow cerebrospinal fluid leaks: optimizing the technique. Clin Neuroradiol 2021;31:633–41 10.1007/s00062-020-00943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soderlund KA, Mamlouk MD, Shah VN, et al. Cerebrospinal fluid-lymphatic fistula causing spontaneous intracranial hypotension in a child with kaposiform lymphangiomatosis. Pediatr Radiol 2021;51:2093–97 10.1007/s00247-021-05132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]