Abstract
While in vitro results at clinically relevant concentrations do not predict abacavir (1592U89) interactions with drugs highly metabolized by cytochrome P450, the potential does exist for a pharmacokinetic interaction between abacavir and ethanol, as both are metabolized by alcohol dehydrogenase. Twenty-five subjects were enrolled in an open-label, randomized, three-way-crossover, phase I study of human immunodeficiency virus-infected male subjects. The three treatments were administration of (i) 600 mg of abacavir, (ii) 0.7 g of ethanol per kg of body weight, and (iii) 600 mg of abacavir and 0.7 g of ethanol per kg. Twenty-four subjects completed the study with no unexpected adverse events reported. Ethanol pharmacokinetic parameters were unchanged with abacavir coadministration. The geometric least squares mean area under the concentration curve extrapolated to infinite time for abacavir increased 41% (from 11.07 to 15.62 μg · h/ml), and the half-life increased 26% (from 1.42 to 1.79 h) in the presence of ethanol (mean ethanol maximum concentration in plasma of 498 μg/ml). The percentages of abacavir dose recovered in urine as abacavir and its two major metabolites were each altered in the presence of ethanol, but there was no change in the total percentage (≈50%) of administered dose recovered in the 12-h collection interval. In conclusion, while a single 600-mg dose of abacavir does not alter blood ethanol concentration, ethanol does increase plasma abacavir concentrations.
Abacavir (1592U89) is a nucleoside analogue recently approved as an antiretroviral drug for treatment of human immunodeficiency virus type 1 infection. Studies with human cell lines have shown that abacavir is phosphorylated by a unique metabolic pathway to produce the bioactive form, carbocyclic GTP (1144U88 triphosphate), which is a potent reverse transcriptase inhibitor (5). Metabolic interactions between clinically relevant concentrations of abacavir and other drugs that undergo metabolism mainly by cytochrome P450 isozymes are not predicted since the major metabolic pathways are through cytosolic alcohol dehydrogenase (ADH) and UDP glucuronyl transferase (UDP-GT) (Glaxo Wellcome, Inc., data on file; J. R. Ravitch, B. J. Bryant, M. J. Reese, C. C. Boehlert, J. S. Walsh, J. P. McDowell, and B. M. Sadler, Abstr. 5th Conf. Retroviruses Opportunistic Infect., abstr. 634, 1998). Abacavir has a carboxylate and a glucuronide as its two major metabolites: cytosolic ADH catalyzes the formation of the carboxylate 2269W93, and UDP-GT catalyzes the formation of the glucuronide 361W94 (8). Ethanol metabolism also requires ADH, and there is evidence that ethanol interacts with other compounds through the glucuronidation pathway (11). Hence, it is reasonable to assume that there could be a pharmacokinetic interaction between abacavir and ethanol in either of the two major metabolic pathways for abacavir. The potential for ethanol to alter abacavir metabolism was studied in vitro with human liver slices (n = 2) (J. S. Walsh, M. J. Reese, J. Ravitch, J. A. McDowell, and K. Edwards, Abstr. 5th Int. ISSX Meet., abstr. 193, 1998). Ethanol markedly inhibited formation of abacavir carboxylate with a trend toward both increased abacavir glucuronide formation and reduced abacavir metabolism.
A drug interaction which alters the pharmacokinetic parameters of ethanol may have important social, medical, and legal implications. Interactions between abacavir and ethanol at the acetaldehyde stage of ethanol metabolism might produce a disulfiram-type adverse effect (9). Interactions between abacavir and ethanol interaction could alter abacavir pharmacokinetics, changing plasma abacavir concentration and creating potential effects on safety and efficacy. Thus, the objective of this study (Glaxo Wellcome protocol CNAA1010) was to determine the extent of pharmacokinetic interactions in vivo between abacavir and ethanol.
MATERIALS AND METHODS
Study population.
Subjects were eligible for the study if they met the following criteria: male; aged 18 to 50 years; weight, 55 to 95 kg inclusive; human immunodeficiency virus type 1 seropositive; CD4+ cell count of ≥200 cells/μl within 14 days prior to initial treatment; regular consumption of alcohol with no recent changes in pattern of alcohol consumption; and agreement to use a barrier method of contraception while enrolled in the study and for a minimum of 1 month after the last dose of study drug. In addition, subjects were required to have adequate hematology and chemistry profiles at screening (hemoglobin, ≥10.0 g/dl; neutrophil count, ≥1,000 cells/μl; platelet count, ≥75,000/μl; aspartate aminotransferase and alanine aminotransferase levels, ≤2.5 times the upper limit of normal; serum amylase, ≤1.5 times the upper limit of normal; estimated creatinine clearance, >50 ml/min). Exclusion criteria included the following: regular weekly consumption of greater than 21 U of alcohol (1 U of alcohol is equivalent to 1/2 pint of beer, 1 glass of wine, or 1 oz of liquor); documented history of alcoholism; positive breath alcohol test upon arrival at the study center prior to treatment; history of clinically relevant hepatitis or pancreatitis within the previous 6 months; a malabsorption syndrome or other gastrointestinal dysfunction which may have interfered with drug absorption; an active diagnosis of AIDS (other than nonvisceral Kaposi's sarcoma); abnormal laboratory values considered clinically significant by the investigator; treatment with immunomodulating agents, cytotoxic chemotherapeutic agents, or radiation therapy within the previous 6 weeks; participation in a research study within the previous month; history of hypersensitivity or idiosyncratic reaction to nucleoside analogues; and use of concurrent medications which could not be withheld for a time from 48 h (24 h for antiretrovirals) prior to study drug administration until 12 h after study drug administration on each dosing day.
Based on published results from a similar pharmacokinetic ethanol interaction study (14), a sample size of 24 would provide 80% power to detect a difference of 20% in Cmax and >80% power to detect a 20% difference in area under the concentration curve (AUC). Data from another study (17) evaluating the interaction among zidovudine, lamivudine, and abacavir indicated that a sample size of 24 would provide at least 80% power to detect a 20% difference in Cmax and AUC. Only males were eligible to participate in this study; women were excluded because they may have lower gastric ADH activity and a smaller ethanol volume of distribution than men (7, 13). These differences would result in the same ethanol dose causing higher blood ethanol concentrations for female subjects, potentially increasing intersubject variability.
Study design.
This phase I study was conducted at a single center from January through May 1997. The study was approved by the Institutional Review Board affiliated with the study center prior to study initiation. All subjects provided written informed consent prior to enrolling in the study.
The study employed an open-label, balanced, three-way-crossover design in which each subject received abacavir (treatment 1), ethanol (treatment 2), and abacavir plus ethanol (treatment 3). Following initial screening evaluations, eligible subjects were randomized to receive the first treatment within 14 days of screening. The sequence of treatments for each subject was determined using a three-by-three Williams' square design, with four patients randomly assigned to each of the following treatment regimens: 123, 132, 213, 231, 312, and 321. Subjects were administered a single treatment on each of three dosing days, with a washout period of at least 7 days between treatments. A follow-up evaluation was performed 7 to 10 days after the final treatment.
Subjects arrived at the treatment facility on the evening before the treatment day and were given a breath alcohol test; a positive test result would disqualify the subject from further participation in the study. Subjects fasted after midnight and the following morning received a standardized high-fat breakfast, consisting of 2 slices of toasted white bread with butter, 2 eggs fried in butter, 2 slices of bacon, 2 ounces of hash-browned potatoes, and 8 oz of whole milk. This standard breakfast provided approximately 970 kcal, with 67 g of fat, 33 g of protein, and 58 g of carbohydrate. Subjects were asked to consume the entire breakfast within 30 min. Five minutes prior to dosing, predose urine samples were collected (if possible) and predose blood samples were drawn, as described below. Dosing occurred 30 min after breakfast was served, and subjects were allowed 5 min to ingest the abacavir and/or ethanol. Water was withheld for a period from 4 h prior to dosing until 4 h postdosing, although subjects were given 200 ml of water 2 h after ethanol ingestion to minimize dehydration. On treatment days, foods and beverages containing methyl xanthine, such as coffee, tea, and chocolate, were prohibited until 6 h postdosing, and tobacco was prohibited from 4 h predosing until 4 h postdosing. Standard lunch and dinner were provided 4 and 10 h postdosing, respectively. Subjects remained in the facility overnight and were discharged the following morning once postdosing assessments were completed, provided that the subjects were well and had a negative breath alcohol test.
Study medication.
Abacavir was administered as six 100-mg caplets provided by Glaxo Wellcome, Research Triangle Park, N.C. Ethanol was administered as 95% grain alcohol (Everclear brand) diluted in orange juice to 20% (vol/vol). The dose of ethanol was 0.7 g/kg of body weight, which is equivalent to approximately 4.7 U of alcohol. When subjects received only abacavir (treatment 1), they also consumed a quantity of orange juice equivalent to the quantity provided with the ethanol treatments.
Clinical and laboratory assessments.
Screening evaluation included physical examination, comprehensive medical history, electrocardiogram (ECG), review of concurrent medical conditions and use of concomitant medications, routine hematology and clinical chemistry, dipstick urinalysis for blood and protein, and assessment of total lymphocytes including percentage and absolute CD4 and CD8 counts. On arrival at the study center on the evening prior to each dosing day, subjects were given an alcohol breath test and a urine screen for illicit drug use. Assessments performed on each dosing day and at the follow-up evaluation included routine hematology and clinical chemistry, dipstick urinalysis for blood and protein, and adverse event monitoring. Vital signs were monitored at the screening and follow-up visits and on each dosing day at 15 min prior to dosing and thereafter at 1, 2, 3, 4, 6, 8, 12, and 24 h postdosing. Physical examination and ECG were performed during the follow-up evaluation scheduled to occur 7 to 10 days after the final dosing day.
Sample collections.
Serial blood samples were drawn by venipuncture or peripheral venous catheter over a 12-h period following administration of each treatment. To establish plasma concentration-time profiles for abacavir in treatments 1 (abacavir) and 3 (abacavir and ethanol), 3-ml blood samples were collected 5 min prior to dosing and thereafter at 10, 20, 30, 40, 50, 60, 75, 90, 105, 120, 150, 180, 240, 300, 360, 420, 480, 600, and 720 min postdosing. For analysis of ethanol concentrations, 5-ml blood samples were collected at the same time intervals during treatments 2 (ethanol) and 3 (abacavir and ethanol). The 3-ml blood samples for analysis of abacavir concentrations were drawn into a lavender-stoppered Vacutainer tube (containing dipotassium EDTA), gently inverted 8 to 10 times, stored on ice or refrigerated, and centrifuged within 30 min of collection to separate plasma. Plasma was transferred into a polypropylene tube, and the samples were stored at −20°C or lower until shipment on dry ice to Glaxo Wellcome for analysis. After collection, the 5-ml blood samples for analysis of ethanol concentrations were transferred to 15-ml polypropylene tubes containing sodium fluoride, sodium nitrite, and potassium oxalate. The tubes were gently inverted 8 to 10 times to ensure thorough mixing, and the samples were frozen immediately at −20°C or lower until shipment on dry ice to Cedra Corporation (Austin, Tex.) for analysis.
Urine samples were collected predosing (if possible) and at 0 to 6 h and 6 to 12 h postdosing for analysis of abacavir concentrations. Urine samples comprising each collection interval were mixed thoroughly, and a 10-ml aliquot was transferred to a 13-ml polypropylene tube and stored at −20°C or lower until shipment on dry ice to Glaxo Wellcome for analysis.
Analytical methods.
Ethanol concentrations in blood were determined using headspace gas chromatography with flame ionization detection (12) by Cedra Corporation, Pflugerville, Tex. The internal standard was n-propanol. Using sodium nitrite, ethanol and n-propanol were partitioned from blood into the headspace. The headspace sample was analyzed, with quality control (QC) samples and standards included in the analytical run. The assay procedure was linear over the 1- to 400-μg/ml range. Standards were assayed in duplicate, and study samples were assayed singly. Three or four concentrations of QC samples were assayed in duplicate, interspersed among study samples. For each QC concentration, the mean coefficient of variation never exceeded 6.1%, with the absolute deviation from the nominal concentration never exceeding 3.2%.
Plasma abacavir concentrations were determined using a validated reversed high-pressure liquid chromatography (HPLC) assay with UV detection at 284 nm over a quantifiable range of 25 to 5,000 ng/ml (10). Briefly, 0.1 ml of 10% trichloroacetic acid was added to 0.2 ml of plasma samples, which was then mixed by vortexing and centrifuged at 8,800 × g for 10 min. Supernatant (0.1 ml) was then injected onto a Rainin (4.6- by 250-mm) C18 Microsorb MV column (Varian, Walnut Creek, Calif.). The mobile phase consisted of 40% methanol in phosphate-triethylamine at a flow rate of 1.0 ml/min. The approximate retention time for abacavir was 9 min under these conditions. Bias ranged from −5% at low abacavir concentrations (50 ng/ml) to −2% at high abacavir concentrations (4,000 ng/ml), with coefficients of variation at these concentrations ranging from 8 to 2%, respectively.
Analysis of abacavir and its major metabolites in urine was conducted by HPLC using an octodecyl reversed-phase HPLC column with UV detection. Samples were centrifuged to remove particulates, and aliquots of the supernatants were diluted 1:10 with HPLC mobile phase and injected onto the HPLC system, along with calibration standards and QC samples. Approximately 20% of the diluted urine samples had analyte concentrations exceeding the upper limit of the calibration curve; aliquots of these diluted samples were further diluted 1:5 and injected onto the HPLC system with appropriate calibration samples and QC samples. For abacavir, 2269W93, and 361W94, the QC samples were accurate within 5.1, 7.0, and 3.0%, respectively. The corresponding coefficients of variation were within 5.13, 4.12, and 4.88% for the QC samples for abacavir, 2269W93, and 361W94, respectively.
Pharmacokinetic analysis.
WinNonlin version 1.2 (Scientific Consulting, Inc., Cary, N.C.) was used for calculation of the noncompartmental pharmacokinetic parameters. Parameters calculated were AUC from zero to the last quantifiable concentration in plasma (AUClast), AUC extrapolated to infinite time (AUC∞), percentage of AUC∞ that is extrapolated, Cmax, sample time associated with Cmax, terminal elimination rate constant in plasma (λz), plasma abacavir terminal half-life (t1/2), apparent total clearance from plasma, and apparent volume of distribution. The linear trapezoidal rule was used to calculate AUC estimates for both abacavir and ethanol. The abacavir t1/2 was calculated as ln(2)/λz. Urine samples were assayed for the concentrations of abacavir and its two major metabolites, 2269W93 and 361W94. Excreted amounts of these three analytes were calculated for each urine sample from the volume of urine and the concentration of each analyte. Amounts of the two metabolites were converted to abacavir equivalents for data analysis.
Statistical analysis.
Statistical analysis of data was performed using SAS software, version 6.12 (SAS Institute, Inc., Cary, N.C.). Descriptive statistics (geometric least squares mean and 95% confidence interval [CI]) were used to summarize pharmacokinetic parameters. For comparisons of treatments of abacavir alone or ethanol alone with abacavir-ethanol treatment, analysis of variance (ANOVA) was calculated using log-transformed parameters. In the mixed model, fixed effects included sequence, period, and treatment, while the random effect was subject within sequence. The Wilcoxon signed-rank test was used to compare plasma sample time associated with Cmax parameters, and the estimated median difference and 90% CI were calculated.
The Wilcoxon signed-rank test was used to compare urinary excretion data from the abacavir treatment (treatment 1) with urinary excretion data from the abacavir-ethanol treatment (treatment 3). The estimated median difference and 90% CI were calculated.
RESULTS
Baseline characteristics.
Twenty-five subjects enrolled in this study, and 24 subjects completed the study. One subject chose to withdraw after the first treatment period and was replaced by another subject who completed all three treatments. The mean demographic characteristics of the 25 subjects were 34.2 years in age (range, 25 to 46 years), 75.3 kg in weight (range, 54.5 to 96.8 kg), and 463 CD4+ cells/mm3 (range, 265 to 964 cells/mm3). Nineteen of the subjects were white, five were black, and one subject was categorized as being of other ethnic origin. Twenty-two of the subjects (including the subject who withdrew) were classified as class A (asymptomatic), and the remaining three subjects were classified as class B (symptomatic and non-AIDS) (3).
Pharmacokinetics.
While three subjects experienced vomiting on this trial (one from each treatment), this had no effect on the pharmacokinetic calculations since the vomiting occurred 4, 6, and 7 days after dose administration, well after the last pharmacokinetic sample was taken.
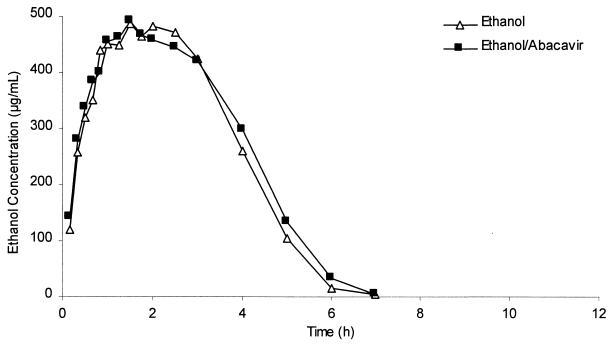
No statistically significant differences in blood ethanol pharmacokinetic parameters were seen in the comparison of the presence with the absence of abacavir (Table 1). Analysis was based on AUClast rather than AUC∞: blood levels of ethanol were undetectable in this study by 10 h postdosing. It was inappropriate to extrapolate AUC since ethanol follows zero-order elimination kinetics. Blood ethanol median profiles from the two treatments were essentially superimposable, as shown in Fig. 1, indicating that a single 600-mg abacavir dose has no effect on blood ethanol concentrations.
TABLE 1.
Ethanol pharmacokinetic parameters for ethanol alone and ethanol coadministered with abacavir
| Pharmacokinetic parameter | Ethanola (n = 24) | Ethanol-abacavira (n = 24) | Ratio of parametersb |
|---|---|---|---|
| AUClast (μg · h/ml) | 1,736 (1,494, 2,016) | 1,776 (1,529, 2,063) | 1.02 (0.93, 1.13) |
| Cmax (μg/ml) | 502 (441, 571) | 498 (437, 567) | 0.99 (0.90, 1.10) |
Geometric least squares mean and 95% CI values (in parentheses) for each treatment group.
Geometric least squares mean ratio and 90% CI values (in parentheses) of treatment comparison based on ANOVA.
FIG. 1.
Comparative linear plots of the median concentration-time profiles of ethanol in blood following administration of ethanol with and without abacavir.
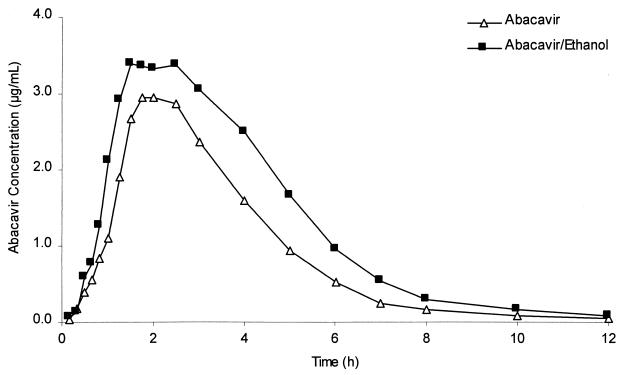
Abacavir pharmacokinetic parameters showed statistically significant differences in the presence of ethanol, as shown in Table 2. Based on geometric least squares mean ratios, concurrent administration of ethanol produced a 15% increase in Cmax (from 3.60 to 4.13 μg/ml), a 41% increase in AUC∞ (from 11.07 to 15.62 μg · h/ml), a 21% reduction in λz (from 0.49 to 0.39 liter/h), and a 26% increase in t1/2 (from 1.42 to 1.79 h). Plasma abacavir profiles from the two treatments are shown in Fig. 2.
TABLE 2.
Abacavir pharmacokinetic parameters for abacavir alone and abacavir coadministered with ethanol
| Pharmacokinetic parameter | Abacavira (n = 25) | Abacavir-ethanola (n = 24) | Ratio of parametersb |
|---|---|---|---|
| AUC∞ (μg · h/ml) | 11.07 (10.05, 12.20) | 15.62 (14.17, 17.22) | 1.41 (1.35, 1.48) |
| Cmax (μg/ml) | 3.60 (3.16, 4.09) | 4.13 (3.63, 4.69) | 1.15 (1.03, 1.28) |
| λz (liter/h) | 0.49 (0.45, 0.53) | 0.39 (0.36, 0.42) | 0.79 (0.74, 0.85) |
| t1/2 (h) | 1.42 (1.32, 1.53) | 1.79 (1.67, 1.93) | 1.26 (1.17, 1.36) |
Geometric least squares mean and 95% CI values (in parentheses) for each treatment group.
Geometric least squares mean ratio and 90% CI values (in parentheses) of treatment comparison based on ANOVA.
FIG. 2.
Comparative linear plots of the median concentration-time profiles of abacavir in plasma following administration of abacavir with and without ethanol.
Urinary excretion data from the two abacavir treatments indicated statistically significant differences in the amounts of abacavir and its metabolites excreted but not in the total percentages of abacavir equivalents excreted (Table 3). Comparing treatment with abacavir alone to treatment with abacavir and ethanol, the median difference in urinary excretion recovery increased by 1.38% for abacavir, decreased by 17% for 2269W93, and increased by 11% for 361W94 (P < 0.05 for all comparisons). Urinary excretion ratios of metabolite to parent drug decreased for 2269W93 from 10.54 for abacavir alone to 2.59 for abacavir in the presence of ethanol. The ratios for 361W94 were essentially unchanged: 9.66 in the absence of ethanol and 9.14 in the presence of ethanol.
TABLE 3.
Treatment effect on urinary excretion profile of abacavir, 2269W93, and 361W94, as percentage of administered dose
| Drug and parameter | Abacavir treatment (n = 25) | Abacavir-ethanol treatment (n = 24) | Median difference in % excreteda |
|---|---|---|---|
| Abacavirb | |||
| GLSM (95% CI)c | 2.54 (2.15, 2.94) | 3.91 (3.37, 4.44) | |
| Median (range) | 2.51 (0.98, 5.60) | 3.80 (1.69, 7.24) | 1.38* (0.73, 1.91) |
| 2269W93b | |||
| GLSM (95% CI) | 26.88 (24.39, 29.36) | 10.14 (8.34, 11.95) | |
| Median (range) | 26.78 (16.04, 40.55) | 9.00 (5.11, 22.56) | −17* (−17.70, −13.04) |
| 361W94b | |||
| GLSM (95% CI) | 24.54 (22.50, 26.57) | 35.72 (31.00, 40.43) | |
| Median (range) | 23.51 (17.01, 36.40) | 36.60 (7.34, 63.39) | 11.0* (7.28, 14.11) |
| Totalb | |||
| GLSM (95% CI) | 53.96 (50.10, 57.82) | 49.77 (44.25, 55.29) | |
| Median (range) | 51.08 (37.44, 71.58) | 53.81 (18.80, 71.35) | −4.0 (−8.11, 1.58) |
Data for 2269W93 and 361W94 are expressed as abacavir equivalents. ∗, P ≤ 0.05.
Treatment difference represents median percentage change from treatment 1 (abacavir) to treatment 2 (abacavir plus ethanol) with 90% CI.
Geometric least squares means (GLSMs) with 95% CIs are presented as percentages of administered doses excreted within the 12-h postdosing collection interval.
Safety.
A total of 161 adverse events were reported by 25 subjects. The most common adverse events were headache (68% of subjects), dizziness (44%), nausea (32%), drowsiness (24%), dyspepsia (16%), and fatigue (16%). Unsteady gait, abrasion, and ecchymosis of arm(s) were each reported by three subjects (12%). The most common abacavir treatment adverse events were headache (28% of subjects), nausea (16%), dyspepsia (12%), and fatigue (12%). The most common adverse events from the ethanol treatment were headache (42%), dizziness (25%), and nausea (13%). The combination ethanol-abacavir treatment had headache (58%), nausea (25%), dizziness (21%), drowsiness (17%), and unsteady gait (13%) as the most common adverse events.
The majority of adverse events experienced with ethanol treatment or the ethanol-abacavir treatment were consistent with the known side effects of ethanol consumption: neurological (e.g., headache, dizziness, drowsiness, and unsteady gait) and gastrointestinal (e.g., nausea, dyspepsia, and vomiting). Neurological events were reported for both ethanol-containing groups for over twice the number of subjects for which they were reported for the abacavir-alone group.
With three exceptions, all adverse events were assessed as mild or moderate in intensity or as grade 1 or 2 in severity. Following coadministration of abacavir and ethanol, two subjects reported unsteady gait (categorized as grade 3 in severity), and one subject reported diarrhea (categorized as grade 3 in severity). The three subjects reporting unsteady gait had similar ethanol concentrations in both ethanol-containing treatments. The ethanol AUC ratios (ethanol-abacavir treatment AUC divided by ethanol treatment AUC) were 89.2, 93.0, and 104.5% while the Cmax ratios were 89.0, 97.3, and 99.1%. Of the 161 total adverse events, there were 100 events which were considered to be possibly related to study treatment. All adverse events related to the study drug had resolved at the time of the follow-up evaluation.
There were no clinically significant changes in median hematology, clinical chemistry, or urinalysis parameters for any of the subjects during the course of the study. The physical examinations at screening did not reveal any clinically significant abnormalities, and there were no clinically significant changes from baseline in any subjects. The ECG abnormalities noted at screening were not considered clinically significant, and there were no clinically significant changes in ECG in any subjects during the course of the study. No clinically significant changes in mean vital signs were attributable to abacavir. Changes in vital signs in the ethanol treatment groups were similar and consistent with known vasodilatory effects of ethanol.
DISCUSSION
This study did not demonstrate any alteration in the pharmacokinetic parameters of ethanol by abacavir coadministration; blood ethanol median profiles following ethanol administration in the presence and absence of abacavir were essentially superimposable. There was no evidence that coadministration of abacavir interferes with ethanol metabolism. There were no disulfiram-type reactions in any subject who received coadministration of abacavir and ethanol.
No new adverse events were reported with abacavir, and adverse events reported following ethanol administration were consistent with known side effects of ethanol. Three subjects reporting unsteady gait while taking ethanol had similar ethanol concentrations with and without abacavir cotherapy. None of the adverse events reported raised new safety concerns, either for administration of abacavir alone or for coadministration of abacavir and ethanol.
Ethanol altered the pharmacokinetic parameters of abacavir. The 41% increase in AUC∞, the 15% increase in Cmax of abacavir, and the 26% increase in abacavir half-life are consistent with competition between abacavir and ethanol for metabolism. While these changes in abacavir pharmacokinetics were statistically significant, they are not considered clinically significant. The safety of abacavir has been evaluated in clinical trials with doses up to 1,800 mg per day given for periods up to 12 weeks (15) and with 1,200 mg per day given for over a year (S. Staszewski, C. Katlama, T. Harrer, P. Massip, P. Yeni, A. Cutrell, and H. M. Steel, Abstr. 12th Int. Conf. AIDS, abstr. 12212, 1998). The increased plasma abacavir concentrations observed in this study remained well within the range seen in previous studies where no additional safety concerns were demonstrated.
The 600-mg abacavir single dose used in this study is twice the single dose for the clinically approved 300-mg twice-daily abacavir therapy. The clinically approved abacavir dose had not been determined at the time of this study, and the highest abacavir dose being considered was the 600-mg twice-daily therapy. The choice of the 600-mg abacavir single dose for this study was chosen to test the abacavir-ethanol interaction at the highest abacavir dose being considered to ensure that an interaction was not missed.
Drug-ethanol interaction study designs have varied in food intake, ethanol dose, and subject number, reflecting either social drinking situations or the testing of a scientific hypothesis. The design of this study, where the ethanol dose was administered following a standard high-fat breakfast, was thought to provide a maximal drug-ethanol effect, if one existed (6, 7, 16). The high-fat meal administered in this study also served to reduce the incidence of gastritis following ethanol consumption at a mean exposure of ∼500 μg/ml.
Our results indicate that coadministration of abacavir and ethanol resulted in a clear decrease in the urinary excretion ratio of 2269W93 (formed via ADH) to parent drug but essentially no change in the urinary excretion ratio of 361W94 (formed via UDP-GT) to parent drug and no change in the total percentage recovered as abacavir and the two metabolites. These results indicate competition between ethanol and abacavir for metabolism by ADH. The decreased formation of 2269W93 reflects less carboxylation due to the presence of ethanol and appears to be partially compensated for by an increase in glucuronidation. The switch to the glucuronidation pathway is consistent with minimal inhibition of net abacavir clearance observed in this study. These observations are also consistent with the results of an in vitro study examining the relationship of abacavir and ethanol (Walsh et al., 5th Int. ISSX Meet.).
In conclusion, this study demonstrated that abacavir does not affect blood ethanol concentrations but that ethanol does statistically increase exposure to abacavir. The increase in abacavir exposure due to the presence of ethanol is not considered clinically significant because the increased exposure remained well within the range seen in previous studies.
ACKNOWLEDGMENTS
We thank Bill Mahony for assaying concentrations of abacavir in plasma, Joshua Ravitch for assaying concentrations of abacavir and its major metabolites in urine, Andrew Kurtz at Cedra for assaying ethanol concentrations in plasma, Edward A. Kelly (PPD-Pharmaco Inc.) for the clinical conduct of the study, and Barbara J. Rutledge and Belinda Ha for manuscript preparation.
This work was supported by Glaxo Wellcome, Inc.
REFERENCES
- 1.Bagasra O, Whittle P, Kajdacsy-Ball A, Lischner H W. Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV-1 and on CD4 and CD8 lymphocytes. Prog Clin Biol Res. 1990;325:351–358. [PubMed] [Google Scholar]
- 2.Bagasra O, Bachman S E, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz R J. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep. 1992;41:RR-17. [PubMed] [Google Scholar]
- 4.Cook R T, Stapleton J T, Ballas Z K, Klinzman D. Effect of a single ethanol exposure on HIV replication in human lymphocytes. J Investig Med. 1997;45:265–271. [PubMed] [Google Scholar]
- 5.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser A G, Hudson M, Sawyer A M, Rosalki S B, Pounder R E. Short report: the effect of ranitidine on the postprandial absorption of a low dose of alcohol. Aliment Pharmacol Ther. 1992;6:267–271. doi: 10.1111/j.1365-2036.1992.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 7.Frezza M, DiPadova C, Pozzato G, Terpin M, Baraona E, Lieber C S. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 8.Good S S, Daluge S M, Ching S V, Ayers M M, Mahony W B, Falletto M B, Domin B A, Owens B S, Dornsife R E, McDowell J A, LaFon S W, Symonds W T. 1592U89 succinate—preclinical toxicological and disposition studies and preliminary clinical pharmacokinetics. Antivir Res. 1995;26:A229. [Google Scholar]
- 9.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar P N, Sweet D E, McDowell J A, Symonds W, Lou Y, Hetherington S, LaFon S. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber C S. Mechanism of ethanol-drug-nutrition interactions. J Toxicol Clin Toxicol. 1994;32:631–681. doi: 10.3109/15563659409017974. [DOI] [PubMed] [Google Scholar]
- 12.Manno B R, Manno J E. A simple approach to gas chromatographic microanalysis of alcohol in blood and urine by a direct-injection technique. J Anal Toxicol. 1978;2:257–261. [Google Scholar]
- 13.Marshall A W, Kingstone D, Boss M, Morgan M Y. Ethanol elimination in males and females: relationship to menstrual cycle and body composition. Hepatology. 1983;3:701–706. doi: 10.1002/hep.1840030513. [DOI] [PubMed] [Google Scholar]
- 14.Melander O, Linden A, Melander A. Pharmacokinetic interactions of alcohol and acetylsalicylic acid. Eur J Clin Pharmacol. 1995;48:151–153. doi: 10.1007/BF00192741. [DOI] [PubMed] [Google Scholar]
- 15.Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell S M, Harrigan P R, Steel H, Lanier E R, Pearce G. A dose-ranging study to evaluate the safety and efficacy of three doses of abacavir (1592U89) alone or in combination with zidovudine and lamivudine in antiretroviral treatment naive subjects. AIDS. 1998;12:F197–F202. doi: 10.1097/00002030-199816000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Toon S, Khan A Z, Holt B I, Mullins F G P, Langley S J, Rowland M M. Absence of effect of ranitidine on blood alcohol concentrations when taken morning, midday, or evening with or without food. Clin Pharmacol Ther. 1994;55:385–391. doi: 10.1038/clpt.1994.46. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chittick G E, McDowell J A. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43:1708–1715. doi: 10.1128/aac.43.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]