Abstract
Placental pathology can identify characteristic features of specific infectious pathogens. The histopathology of acute SARS-CoV-2 placental infection and exposure without infection has been well described. However, whether the characteristic placental pathology persists after the acute phase of the infection is less clear. We retrospectively identified 67 COVID-19–recovered pregnant patients who had placental pathology available. After reviewing the gross and histopathology, we categorized the findings and studied the placentas for evidence of chronic infection by immunohistochemistry for the spike protein of the virus. We found these placentas showed significantly increased prevalence of maternal and a trend towards significance of fetal vascular malperfusion when compared to a control group of placentas examined for the sole indication of maternal group B streptococcal colonization. None of the COVID-19–recovered placentas showed expression of the viral spike protein; therefore, we found no evidence of persistent infection of the placenta in women with a history of COVID-19 during their pregnancy. We conclude that recovery from a SARS-CoV-2 infection during pregnancy puts the pregnancy at risk for specific pathology.
Keywords: COVID-19, Placenta, Maternal vascular malperfusion, Fetal vascular malperfusion, SARSp-CoV-2
1. Introduction
The COVID-19 global pandemic led to concerns regarding pregnancy outcomes for infected mothers. There have been many studies describing the spectrum of placental involvement by SARS-CoV-2 and its impact on pregnancy. Placental pathology has identified histopathologic features associated with acute placental SARS-CoV-2 exposure: maternal and/or fetal vascular malperfusion [[1], [2], [3], [4]] and direct placental infection with the triad of histiocytic intervillositis, increased perivillous fibrin, and villous trophoblast necrosis (refer to the studies by Watkins et al [5] and Scwartz et al [6] for example). It is unclear if viral replication occurs in the placenta and therefore if persistent infection is possible or if a history of SARS-CoV-2 infection that occurred during pregnancy leads to similar or different placental pathologies in this nonacute setting. With these questions in mind, in this study, we wanted to identify if (1) viral proteins for SARS-CoV-2 persists in the placentas from patients who had tested positive for SARS-CoV-2 and then cleared (by time-based criteria) or tested negative before delivery (COVID-19–recovered, nonacute infection) using immunohistochemistry against viral proteins as a surrogate for viral persistence and (2) similar pathologies seen in acute infection are observed in these placentas.
2. Materials and methods
Sixty-seven SARS-CoV-2–recovered patients who gave birth between 01/01/2020 and 06/30/2021 (18 months) were identified from the case files of Massachusetts General Hospital, Boston, MA, with Institutional Review Board approval (2020P001116). Only patients with a history of SARS-CoV-2 infection (confirmed with a reverse transcriptase-polymerase chain reaction [RT-PCR] test) and a negative SARS-CoV-2 RT-PCR test before the time of delivery or those who were cleared by time-based criteria (no symptoms two weeks after a positive test) were included in the study. No neonates were tested within 24 h of delivery. Maternal age, time from positive SARS-CoV-2 test to delivery, gestational age at delivery, mode of delivery, vaccination status (and the number of vaccinations), infant birth weight, Apgar scores, and perinatal complications were obtained from the electronic medical record.
All placentas were weighed with the weight percentile for gestational age determined and underwent gross examination, and representative sections were examined with hematoxylin and eosin staining. Amsterdam Placental Workshop Group Consensus Statement recommendations were followed to sample and report pathologic findings. Diagnoses were reviewed and categorized into six major categories based on the primary diagnosis: gross and histologically normal, anatomic defects, maternal vascular malperfusion (MVM), fetal vascular malperfusion (FVM), inflammatory pathologic findings (e.g., villitis of unknown etiology, chronic histiocytic intervillositis), infectious pathologic findings (e.g., chorioamnionitis, infectious villitis), and other thrombotic findings (increased perivillous fibrin deposition, intervillous thrombus). The findings were compared to those of a previously published cohort of placentas pathologically examined for the sole indication of maternal group B streptococcal (GBS) colonization (n = 126). Categorical comparisons between COVID-19–recovered and GBS-colonized placental findings were conducted with Fisher's exact tests, with a p-value of 0.05 considered statistically significant. Statistics were conducted using StataIC, version 16.0.
One formalin-fixed and paraffin-embedded block of placental parenchyma was selected from each case. Immunohistochemistry was performed on 4-μm-thick sections from the selected blocks following antigen retrieval (pH 6.1 citrate buffer; Target Retrieval Solution, Dako, Carpinteria, CA) using a mouse monoclonal antibody directed against SARS-CoV-2 S (1:1000 dilution; clone 1A9, catalog number GTX632604; GeneTex, Irvine, CA), followed by detection using EnVision+ (Dako). One positive control was included in each run consisting of placental tissue with strong SARS-CoV-2 immunohistochemical staining [7].
3. Results
The patients’ age ranged from 16 to 42 (mean = 30.3) years, and the time from positive SARS-CoV-2 test to delivery ranged from 14 to 230 (mean = 87.9) days (see Table 1 ). Thirty-eight had a negative SARS-CoV-2 test before delivery, and 29 were cleared by time-based criteria. Only three patients received at least one dose of vaccine before delivery; for the remainder, none of the patients from 2020 were vaccinated and all except five from 2021 were vaccinated with at least one dose (all postpartum, except the three mentioned earlier). Gestational age ranged from 34.0 to 41.5 (mean = 39.0 weeks). Pregnancy complications were reported in 33 (49.2%) including gestational hypertension, gestational diabetes mellitus, GBS infection, obesity, breast cancer, acute kidney injury, anemia, Coxsackievirus infection, Zika virus exposure, and depression. Delivery complications were reported in 20 (29.8%) including tight/wrapped nuchal cord, shoulder dystocia, and failure to progress. The birth weights ranged from 993 to 4555 (mean = 3202.1) grams.
Table 1.
Basic demographic findings from COVID-19–recovered patients.
| Variable | COVID-19–recovered, nonacute SARS-CoV-2 (n = 67) |
|---|---|
| Age, range; mean ± SD (years) | 16-42; 30.3 ± 5.8 |
| Time from positive SARS-CoV-2 test to delivery, range; mean ± SD (days) | 14-230; 87.9 ± 56.5 |
| Gestational age, range; mean ± SD (weeks) | 34.0–41.5; 39.0 ± 1.1 |
| Pregnancy complications, n (%) | 33 (49.2) |
| Birth weight, mean ± SD (grams) | 993-4555; 3202.1 ± 538.7 |
| Delivery complications, n (%) | 20 (29.8) |
Abbreviations: n, number; SD, standard deviation.
The placentas weighed from 117 to 760 (mean = 427.1) grams (see Table 2 for details and percentile distribution); all underwent gross and microscopic examination. Four (6.0%) were normal and appropriate for gestational age, 14 (20.9%) showed maternal vascular malperfusion, 14 (20.9%) inflammatory pathologic findings, 11 (16.4%) fetal vascular malperfusion, 8 (11.9%) infectious pathologic findings, 7 (10.4%) other thrombotic findings, and 4 (6.0%) anatomic defects. The findings were compared to those observed in the cohort with maternal GBS colonization (see Table 3 ), and the statistically significant differences included higher rates of maternal vascular malperfusion (p: <0.001). A trend toward statistical significance was observed in fetal vascular malperfusion (p = 0.051), but no statistical significance was found for infectious, inflammatory, or other thrombotic findings between the two groups.
Table 2.
Placental pathology in COVID-recovered patients.
| Placental pathologic category | COVID-19–recovered, nonacute SARS-CoV-2 (n = 67) |
|---|---|
| Normal, n (%) | 4 (6.0) |
| Anatomic defects, n (%) | 4 (6.0) |
| MVM, n (%) | 14 (20.9) |
| FVM, n (%) | 11 (16.4) |
| Other thrombotic pathologic findings (increased fibrin, IVT etc.), n (%) | 7 (10.4) |
| Infectious pathologic findings (chorioamnionitis, infectious villitis), n (%) | 8 (11.9) |
| Inflammatory pathologic findings (VUE, CHI), n (%) | 14 (20.9) |
| Placental weight, range; mean ± SD (grams) | 177-760; 427.1 ± 113.1 |
| Placenta weight percentiles, n (%) [1] | <10%: 46 (65.7) 10–25%: 8 (11.9) 25–50%: 6 (8.9) 50–75%: 6 (8.9) 75–90%: 2 (3.0) >90%: 1 (1.5) |
Abbreviations: CHI, chronic histiocytic intervillositis; FVM, fetal vascular malperfusion; IVT, intervillous thrombus; MVM, maternal vascular malperfusion; n, number; SD, standard deviation; VUE, villitis of unknown etiology.
Reference: 1. Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med 1996; 16, 901–907.
Table 3.
Comparison of placenta findings from COVID-19–recovered patients with patients with GBS infection.
| COVID-19–recovered, nonacute SARS-CoV-2 (n = 67) | GBS-colonized (n = 126) | p-value | |
|---|---|---|---|
| MVM, n (%) | 14 (20.9) | 4 (3.2) | <0.001 |
| FVM, n (%) | 11 (16.4) | 9 (7.1) | 0.051 |
| Inflammatory pathologic findings, n (%) | 14 (20.9) | 24 (19.0) | 0.849 |
| Infectious pathologic findings, n (%) | 8 (11.9) | 19 (15.1) | 0.665 |
| Other thrombotic findings, n (%) | 7 (10.4) | 7 (5.6) | 0.248 |
Abbreviations: FVM, fetal vascular malperfusion; GBS, Group b streptococcal; MVM, maternal vascular malperfusion.
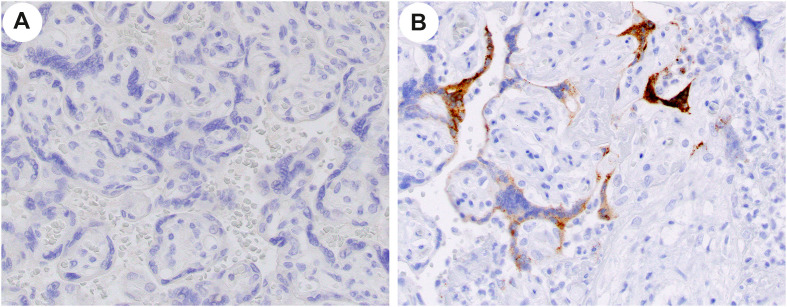
All placentas from patients with nonacute SARS-CoV-2 were examined immunohistochemically for SARS spike protein, and none showed expression of the protein (Fig. 1 ).
Fig. 1.
Example of immunohistochemical studies on a case (A) and control (B) placenta at x40 original. Note the absence of signal in the case (A) and positive signal in the control (B) villous trophoblast.
4. Discussion
Pregnancy complications have been reported in patients with acute SARS-CoV-2 infection, and histopathologic findings in these placentas have been relatively well described (refer to the studies by Watkins et al [5] and Scwartz et al [6] for example). However, it is unclear whether infection or pathology persists in placentas once the infection has cleared. In order to answer these questions, we identified a cohort of 67 patients who were COVID-19–recovered (>14 days after a positive test with a negative test after or cleared with time-based criteria), and herein, we report the pregnancy outcomes and detailed histopathologic findings from placental pathology and immunohistochemistry for viral protein.
Since the placenta is an organ known to be a site for acute SARS-CoV-2 infection, we studied the possibility of a placental reservoir of virus post acute infection. To answer this question, we performed immunohistochemistry against the SARS spike protein, which is regarded as a sign of probable placental infection [7]. Another large study investigated placental infection in the acute SARS-CoV-2 infection setting using immunohistochemistry in 64 placentas and did not identify any positives [8]. Similarly, none of the 67 placentas in our series showed any positivity. These two studies indicate that placental infection (at least as detected by immunohistochemical methods) is likely very low in the acute and non-acute setting.
Despite being negative for viral proteins with immunohistochemistry, the placentas in this study still showed pathologic findings, which we classified using Amsterdam Placental Workshop Group Consensus Statement recommendations [9]. Acute placental infection by SARS-CoV-2 results in a characteristic triad of histiocytic intervillositis, perivillous fibrin deposition, and trophoblast necrosis [5]. However, histopathologic findings in the nonacute setting have not been conclusive and the reported findings differ [10,11]. The few recent studies investigating placental findings in nonacute SARS-CoV-2 infection identified both fetal vascular malperfusion [10,12,13] and maternal vascular malperfusion [14] (significant only in one study [15]. One study reported fetal vascular malperfusion to be significantly less prevalent in nonacute infection than in acute infection, and the same study did not show significant maternal vascular malperfusion in the nonacute setting, suggesting the histopathologic findings may resolve over time [10].
In our cohort, we identified maternal vascular malperfusion to be significantly more common in the COVID-19–recovered placentas than in the controls with GBS infection, indicating that vascular pathologies persist regardless of the presence of the virus. The is also suggested by the trend toward significance with fetal vascular malperfusion. The other placental pathologies we compared (inflammatory, infections, anatomic) did not show significant differences. These findings suggest that patients with a history of COVID-19 during or pregnancy might be at risk for significant placental pathologies including maternal vascular malperfusion and fetal vascular malperfusion, both of which are associated with perinatal morbidity (refer to the studies by Ernst [16] and Redline and Ravishankar [17] and references therein for reviews).
This study covered cases from the first wave and early Delta wave of the pandemic, but not the Omicron wave. Specific variants might be relevant to the pathology [[18], [19], [20]]. SARS-CoV-2 placentitis is rare, and it is not possible to know if the placentas used in this study had it during their acute inffection. Additionally, in light of the prior study with 64 placentas and negative immunohistochemistry in the acute setting [8], perhaps 67 cases might be insufficient to capture enough that may have been infected during their acute phase. Given our prior study, it is likely that at least four placentas would have been infected in 67 cases [21]. It is also possible that if there had been SARS-CoV-2 placentitis, fetal death or delivery would have resulted and a pregnancy-recovered state would not follow. Without placental infection, of course, persistent infection would not occur; however, the current study may rule out placental viral replication and the placenta being a reservoir after acute infection.
We believe our study has unique strengths. Our study describes 67 cases all with antepartum and outcome data, all studied for viral protein expression, examined by experienced placental pathologists following Amsterdam criteria [8], and reviewed by an internationally respected placental pathologist (D. J. R.). Our study is the largest study of COVID-19–recovered placentas and the only one that examined for the presence of the virus in the placenta.
In summary, we studied 67 cases of COVID-19–recovered patients’ placentas and found no placentas with SARS-CoV-2 placentitis or evidence for viral infection by immunohistochemistry, suggesting that persistent infection does not occur post clearing of the acute infection. We describe, though, that exposure to SARS-CoV-2 during pregnancy is associated with increased maternal vascular malperfusion and a trend toward associated increased fetal vascular malperfusion pathologies, which suggests that SARS-CoV-2 viral infection during pregnancy, even cleared, can have lasting effects on the placenta.
Acknowledgements
Author contributions: D. J. R. – conceptualization, review and editing. B. B. – writing original draft, review and editing. K. J. – methodology. J. L. H. – investigation, methodology, reviewing and editing. The authors thank the histology and immunohistochemistry laboratories at our respective institutions, the residents and Pathologists' Assistants for their grossing, and the pregnant people who made up this study.
Footnotes
Disclosures: None.
References
- 1.Baergen R.N., Heller D.S. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhu M., Cagino K., Matthews K.C., Friedlander R.L., Glynn S.M., Kubiak J.M., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020 doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad M.F., Das S., Goldstein J.A., Shanes E.D., Mithal L.B., Miller E.S. Histopathologic findings in the placentas of pregnant women with COVID-19. Am J Clin Pathol. 2021;156:329–330. doi: 10.1093/ajcp/aqab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal N., Puri M., Agarwal K., Singh S., Yadav R., Tiwary N., et al. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur J Obstet Gynecol Reprod Biol. 2021;259:7–11. doi: 10.1016/j.ejogrb.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins J.C., Torous V.F., Roberts D.J. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis: a report of 7 cases with confirmatory in situ hybridization, distinct histomorphologic features, and evidence of complement deposition. Arch Pathol Lab Med. 2021 doi: 10.5858/arpa.2021-0246-SA. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz D.A., Baldewijns M., Benachi A., Bugatti M., Collins R.R.J., De Luca D., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor Associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 7.Roberts D.J., Edlow A.G., Romero R.J., Coyne C.B., Ting D.T., Hornick J.L., et al. National institutes of health/eunice kennedy shriver national institute of child H, human development S-C-piw. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the national institutes of health/eunice kennedy shriver national institute of child health and human development SARS-CoV-2 placental infection Workshop. Am J Obstet Gynecol. 2021;225:593 e591–593 e599. doi: 10.1016/j.ajog.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitan D., London V., McLaren R.A., Mann J.D., Cheng K., Silver M., et al. Histologic and immunohistochemical evaluation of 65 placentas from women with polymerase chain reaction-proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Arch Pathol Lab Med. 2021;145:648–656. doi: 10.5858/arpa.2020-0793-SA. [DOI] [PubMed] [Google Scholar]
- 9.Khong T.Y., Mooney E.E., Ariel I., Balmus N.C., Boyd T.K., Brundler M.A., et al. Sampling and definitions of placental lesions: Amsterdam placental Workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 10.Glynn S.M., Yang Y.J., Thomas C., Friedlander R.L., Cagino K.A., Matthews K.C., et al. SARS-CoV-2 and placental pathology: malperfusion patterns are dependent on timing of infection during pregnancy. Am J Surg Pathol. 2022;46:51–57. doi: 10.1097/PAS.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menter T., Mertz K.D., Jiang S., Chen H., Monod C., Tzankov A., et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patberg E.T., Adams T., Rekawek P., Vahanian S.A., Akerman M., Hernandez A., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382 e381. doi: 10.1016/j.ajog.2020.10.020. 382 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulersen M., Prasannan L., Tam Tam H., Metz C.N., Rochelson B., Meirowitz N., et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2:100211. doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharps M.C., Hayes D.J.L., Lee S., Zou Z., Brady C.A., Almoghrabi Y., et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst L.M. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–560. doi: 10.1111/apm.12833. [DOI] [PubMed] [Google Scholar]
- 17.Redline R.W., Ravishankar S. Fetal vascular malperfusion, an update. APMIS. 2018;126:561–569. doi: 10.1111/apm.12849. [DOI] [PubMed] [Google Scholar]
- 18.Shook L.L., Brigida S., Regan J., Flynn J.P., Mouhammadi A., Etemad B., et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. JID (J Infect Dis) 2021 doi: 10.1093/infdis/jiac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh A., Sehn J.K., Goldfarb I.T., Watkins J.C., Torous V.F., Heerema-McKenney A., et al. SARS-CoV-2 placentitis and intraparenchymal thrombohematomas among COVID-19 infections in pregnancy. JAMA Netw Open. 2022 doi: 10.1001/jamanetworkopen.2022.5345. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of Physicians of Ireland FoPatIoOaG . 2021. Covid placentitis: statement from the RCPI faculty of pathology and the institute of obstetricians and gynaecologists.https://www.rcpi.ie/news/releases/covid-placentitis-statement-from-the-rcpi-faculty-of-pathology-and-the-institute-of-obstetricians-and-gynaecologists/ Online. [Google Scholar]
- 21.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]