Abstract
Background
We explored the effect of discontinuing versus continuing angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) on clinical outcomes in patients with COVID-19 according to baseline disease severity.
Methods
We randomized 659 patients with a confirmed diagnosis of COVID-19 and classified them as having mild or moderate COVID-19 disease severity at hospital presentation using blood oxygen saturation and lung imaging. The primary outcome was the mean ratio of number of days alive and out of the hospital at 30 days according to disease severity.
Results
At presentation, 376 patients (57.1%) had mild and 283 (42.9%) had moderate COVID-19. In patients with mild disease, there was no significant difference in the number of days alive and out of the hospital between ACEI/ARB discontinuation (mean 23.5 [SD 6.3] days) and continuation (mean 23.8 [SD 6.5] days), with a mean ratio of 0.98 (95% CI 0.92-1.04). However, in patients with moderate disease, there were fewer days alive and out of the hospital with ACEI/ARB discontinuation (mean 19.6 [SD 9.5] days) than continuation (mean 21.6 [SD 7.6] days), with a mean ratio of 0.90 (95% CI 0.81-1.00; P-interaction = .01). The impact of discontinuing versus continuing ACEIs/ARBs on days alive and out of hospital through 30 days differed according to baseline COVID-19 disease severity.
Conclusions
Unlike patients with mild disease, patients with moderate disease who continued ACEIs/ARBs had more days alive and out of hospital through 30 days than those who discontinued ACEIs/ARBs. This suggests that ACEIs/ARBs should be continued for patients with moderate COVID-19 disease severity.
Clinical Trial Registration
ClinicalTrials.gov (NCT04364893).
Clinical experience and high-quality studies suggest that genetics, ethnicity, socioeconomic status, age, hypertension, cardiovascular disease, diabetes, and chronic respiratory disease are some of the important risk factors related to an increased risk of death in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19).1., 2., 3., 4. Early in the COVID-19 pandemic there were questions about the possibility that antihypertensive therapy with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) might facilitate SARS-CoV-2 infection.5 , 6 According to pre-clinical studies, the use of renin-angiotensin system (RAS) inhibitors could upregulate the angiotensin-converting enzyme 2 (ACE2) receptor, which is the way in which SARS-CoV-2 enters human cells.7 , 8 Interestingly, mechanistic considerations suggested that treatment with ACEIs and ARBs during the course of more extensive pulmonary inflammation might decrease lung damage and prevent angiotensin-II-mediated pulmonary permeability and inflammation.9 Thus, it can be inferred that the continuation of ACEIs and ARBs (mainly antagonists of AT1 receptor) in COVID‐19 might be a beneficial treatment in patients in a more intense state of inflammation to reduce the severity of the infection.10 , 11 The BRACE CORONA randomized clinical trial showed that in patients hospitalized with hypertension and COVID-19, there was no significant difference in the primary outcome of number of days alive and out of the hospital (DAOH) through 30 days between continuing or stopping ACEIs or ARBs. Although the results for the primary outcome were consistent across the majority of the predefined subgroups, there were a few significant interactions between treatment effect in some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission, favoring the group that continued ACEIs or ARBs.8
We hypothesize that the treatment effect of continuing versus discontinuing ACEIs/ARBs may be influenced by clinical severity at presentation. In the current study, we describe details of a prespecified analysis of the BRACE CORONA trial according to COVID-19 disease severity at presentation.
Methods
Study Design
The BRACE CORONA (NCT04364893) protocol and study results have been reported.12 , 13 The protocol was approved by local ethics committees, and all participants provided written informed consent. Briefly, BRACE CORONA was a prospective, multicenter, open-label, registry-based randomized trial in patients with hypertension who were hospitalized due to COVID-19 and were taking ACEIs or ARBs. The main objective was to determine whether discontinuation compared with continuation of these drugs impacted the number of days alive and out of the hospital through 30 days.
Patients ≥18 years of age with a confirmed diagnosis of COVID-19 who were chronically using ACEIs/ARBs were eligible for the trial. Patients using more than 3 antihypertensive agents, those using sacubitril/valsartan for heart failure, and those hospitalized for heart failure in the previous 12 months were not eligible. Patients with a clinical indication to stop ACEI/ARB treatment, such as hypotension, acute kidney injury, and/or shock were also excluded. Eligible patients were randomized using a 1:1 allocation ratio to either discontinue or continue ACEI/ARB therapy for 30 days. For patients randomized to the ACEI/ARB discontinuation arm, other drugs could replace these agents at the discretion of the treating physician. Beta-blockers were maintained in patients already using them for heart failure. Patients were treated for COVID-19 according to current local standards of supportive care. Study compliance was assessed based on medical prescriptions throughout hospitalization and after discharge via a final phone call at 30 days.
Disease severity
The main characterization of patients regarding COVID-19 at baseline was according to mild or moderate disease severity at hospital presentation.12 , 13 The definition of disease severity was based on the initial description of illness severity in a large cohort that included more than 44,000 people with COVID-19 from China that showed that illness severity can range from mild to critical.4 This definition is also cited in the Centers for Disease Control and Prevention clinical guidance as the reference for classification of COVID-19 clinical severity at presentation.14It is a classification that includes baseline lung imaging with chest computed tomography (CT), oxygen saturation, and necessity of organ support. Mild disease was defined as blood oxygen saturation ≥94% and lung infiltrates on initial chest CT scan ≤50%. Moderate disease was defined as blood oxygen saturation <94%, lung infiltrates on initial CT scan >50%, or a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300.4 , 15 , 16 The evaluation of COVID-19 manifestation on chest CT was performed through a systematic and visual assessment by the site radiologist using the Radiological Society of North America (RNSA) classification to report chest CT findings related to COVID-19. The analysis of the impact of disease severity at presentation on clinical outcomes was prespecified.
Disease Progression
Disease progression was defined as worsening of clinical severity during hospitalization in relation to baseline. Patients were considered to have disease progression if their clinical status progressed from mild disease at presentation to moderate or severe disease, or if they progressed from moderate disease at presentation to severe disease. Severe disease was defined as invasive mechanical ventilation, hemodynamic instability, or multiple organ dysfunction or failure.
Outcomes
The primary outcome was the number of days alive and out of the hospital from randomization through 30 days. This outcome was calculated for each patient by subtracting the days in the hospital and days from death until the end of 30-day follow-up. Secondary outcomes included the following: length of hospital stay (days), death (during the 30-day follow-up period), in-hospital death, cardiovascular death, COVID-19 progression (worsening of clinical severity during hospitalization in relation to baseline severity), acute myocardial infarction, new heart failure or worsening of preexisting heart failure, hypertensive crisis, transient ischemic attack, stroke, myocarditis, pericarditis, thromboembolic phenomena, arrhythmias requiring treatment, respiratory failure requiring mechanical ventilation, shock requiring vasopressors, kidney failure requiring hemodialysis, troponin level, B-type natriuretic peptide level, and D-dimer level.
Statistical Analysis
The primary analysis was expressed as the mean ratio of number of days alive and out of the hospital at 30 days. The primary outcome was analyzed according to disease severity at presentation and according to the presence of disease progression. The analyses were performed using generalized additive models for location, scale, and shape with β binomial distribution inflated at zero.
The results are presented as mean ratios between study groups with 95% confidence intervals (CIs). A mean ratio greater than 1.0 indicates that patients randomized to discontinuing ACEIs/ARBs had more days alive and out of the hospital over the 30-day follow-up; a mean ratio less than 1.0 indicates that patients randomized to continuing ACEIs/ARBs had more days alive and out of the hospital. The primary analysis followed the intention-to-treat principle. A sensitivity analysis “on treatment” for the primary outcome and secondary outcomes according to disease severity was also performed including only patients who were adherent to the study intervention until the time of death or through 30 days.
In this manuscript, the effect of treatment assignment on outcomes was analyzed according to disease severity. Interaction test between treatment effect and disease severity at baseline for the primary outcome was performed using bootstrapping. Mortality and cardiovascular events at 30 days were compared between study groups using logistic regression, and we report odds ratios with the respective 95% CIs with interaction tests between disease severity and study treatment strategy. Mortality at 30 days was assessed according to clinical severity at presentation and progression of clinical severity during hospitalization. Continuous variables are described as medians (25th, 75th percentiles) or mean ± standard deviation, according to normality of the distribution. Categorical variables are described by absolute and relative frequencies. A P-value <.05 was considered statistically significant. All analyses were performed using R version 4.0.5 (R Core Team, 2021).
RESULTS
Baseline Characteristics
A total of 659 patients with a confirmed diagnosis of COVID-19 were randomized and classified as having mild or moderate disease severity at hospital presentation (Table 1 ). At presentation, 376 patients (57.1%) had mild and 283 (42.9%) had moderate COVID-19 disease. Patients with moderate disease were more likely to be obese (body mass index >30 kg/m2) (59.3% vs 46.9%), have diabetes (37.5% vs 27.7%), and have higher rates of COVID-19 related clinical symptoms including fever (72.9% vs 66.9%), cough (74.2% vs 67.3%), and dyspnea (65.4% vs 44.9%) than those with mild disease.
Table 1.
Baseline characteristics according to disease severity at presentation†
| COVID-19 clinical severity* at hospitalization |
||||
|---|---|---|---|---|
| Mild |
Moderate |
|||
| Continuation (n = 183) | Discontinuation (n = 193) | Continuation (n = 142) | Discontinuation (n = 141) | |
| Age, median (25th, 75th), yrs | 55.1 (45.0, 66.1) | 53.0 (44.1, 60.7) | 56.1 (46.3, 66.1) | 57.0 (49.1, 65.1) |
| Female sex, no./No. (%) | 75/183 (41.0%) | 82/193 (42.5%) | 55/142 (38.7%) | 54/141 (38.3%) |
| BMI >30 kg/m2, no./No. (%) | 80/181 (44.2%) | 95/192 (49.5%) | 78/141 (55.3%) | 88/139 (63.3%) |
| ARB use at hospital admission, no./No. (%) | 165/183 (90.2%) | 151/193 (78.2%) | 120/142 (84.5%) | 113/141 (80.1%) |
| ACEI use at hospital admission, no./No. (%) | 18/183 (9.8%) | 42/193 (21.8%) | 22/142 (15.5%) | 28/141 (19.9%) |
| Medical history, no./No. (%) | ||||
| Hypertension | 183/183 (100.0%) | 193/193 (100.0%) | 142/142 (100.0%) | 141/141 (100.0%) |
| Asthma | 7/183 (3.8%) | 9/193 (4.7%) | 4/142 (2.8%) | 6/141 (4.3%) |
| Kidney disease | 2/183 (1.1%) | 4/193 (2.1%) | 2/142 (1.4%) | 1/141 (0.7%) |
| Diabetes | 46/183 (25.1%) | 58/193 (30.1%) | 53/142 (37.3%) | 53/141 (37.6%) |
| Heart failure | 3/183 (1.6%) | 2/193 (1.0%) | 4/142 (2.8%) | 0/141 (0.0%) |
| Coronary heart disease | 7/183 (3.8%) | 7/193 (3.6%) | 7/142 (4.9%) | 9/141 (6.4%) |
| Clinical characteristics at hospital admission | ||||
| Symptom duration, median (25th, 75th), days | 6.0 (4.0, 8.50 | 7.0 (4.0, 8.0) | 7.0 (5.0, 9.0) | 6.0 (3.0, 9.0) |
| Fever with temperature >37.5°C, no./No. (%) | 125/180 (69.4%) | 122/189 (64.6%) | 108/140 (77.1%) | 94/137 (68.6%) |
| Heart rate, median (25th, 75th), beats/min | 88.0 (79.0, 100.0) | 91.0 (80.8, 102.0) | 92.0 (82.2, 101.0) | 90.0 (82.0, 105.0) |
| Systolic blood pressure, median (25th, 75th), mmHg | 134.0 (124.5, 147.0) | 137.0 (125.0, 149.0) | 139.5 (126.2, 150.0) | 140.0 (123.0, 150.0) |
| Respiratory rate, median (25th, 75th), breaths/min | 18.0 (18.0, 20.0) | 18.0 (17.0, 20.0) | 20.0 (18.0, 22.0) | 19.5 (17.8, 22.0) |
| Oxygen saturation <94% on room air, no./No. (%) | 0/177 (0.0%) | 0/187 (0.0%) | 85/133 (63.9%) | 88/140 (62.9%) |
| Cough, no./No. (%) | 115/183 (62.8%) | 138/193 (71.5%) | 102/142 (71.8%) | 108/141 (76.6%) |
| Dyspnea, no./No. (%) | 78/183 (42.6%) | 91/193 (47.2%) | 95/142 (66.9%) | 90/141 (63.8%) |
| Lung involvement on initial chest CT scan,† no./No. (%) | ||||
| ≤25% | 99/173 (57.2%) | 112/185 (60.5%) | 54/135 (40.0%) | 52/132 (39.4%) |
| 26-50% | 74/173 (42.8%) | 73/185 (39.5%) | 51/135 (37.8%) | 39/132 (29.5%) |
| >50% | 0/173 (0.0%) | 0/185 (0.0%) | 30/135 (22.2%) | 41/132 (31.1%) |
| Time from hospital admission to randomization, median (25th, 75th), days | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (2.0, 3.8) | 2.0 (1.0, 3.0) |
| Time from symptom start to randomization, median (25th, 75th), days | 8.0 (6.0, 11.0) | 9.0 (7.0, 11.0) | 9.0 (7.0, 12.0) | 9.0(6.0, 11.0) |
| Duration of ACEI or ARB use median (25th, 75th), yrs | 5.0 (3.0, 8.0) | 4.2 (2.0, 8.0) | 5.0 (3.0, 7.2) | 5.0 (3.0, 10.0) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; COVID-19, coronavirus disease 2019; CT, computed tomography.
Mild defined as blood oxygen saturation ≥94% and lung infiltrates ≤50%. Moderate defined as blood oxygen saturation <94%, or lung infiltrates >50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Estimated by visual assessment performed by a radiologist.
The in-hospital adherence rate to the study intervention (calculated based on the number of doses of ACEIs or ARBs) was 96.4% in the discontinuation group and 94.8% in the continuation group. At the end of the study, 30 days after randomization, the adherence rates (assessed via a phone call) in patients with mild disease at presentation were 137/193 (70.9%) for discontinuation and 172/183 (93.9%) for continuing ACEIs/ARBs. In patients with moderate disease at baseline, the adherence rate for discontinuation was 100/141 (70.9%) and 130/141 (92.1%) for continuing ACEIs/ARBs.
Laboratory Results at Baseline
Laboratory tests at hospital presentation are described in Table 2 . Patients with moderate disease had a higher median value of C-reactive protein (median 5.8 mg/dL [25th, 75th: 2.5, 10.0]) than those with mild disease (median 3.2 mg/dL [1.1, 6.4]). A similar pattern was seen for D-dimer levels.
Table 2.
Laboratory values at hospital admission*
| COVID-19 clinical severity† at hospitalization |
||
|---|---|---|
| Mild (n = 376) | Moderate (n = 283) | |
| Troponin above ULN, no./No. (%) | 26/376 (6.9%) | 13/283 (4.6%) |
| D-dimer above ULN, no./No. (%) | 151/376 (40.2%) | 151/283 (53.4%) |
| Leukocytes, median (25th, 75th), × 109/L | 5400.0 (4300.0, 6910.0) (n = 361) | 6000.0 (4600.0, 7710.0) (n = 277) |
| Sodium, median (25th, 75th), mmol/L | 138.0 (136.0, 140.0) (n = 292) | 137.0 (134.0, 139.0) (n = 247) |
| Lymphocytes, median (25th, 75th), × 109/L | 1240.0 (920.0, 1682.5) (n = 340) | 1193.0 (880.0, 1640.0) (n = 262) |
| Creatinine, median (25th, 75th), mg/dL | 1.0 (0.8, 1.1) (n = 357) | 1.0 (0.8, 1.2) (n = 280) |
| C-reactive protein, median (25th, 75th), mg/dL | 3.2 (1.1, 6.4) (n = 349) | 5.8 (2.5, 10.0) (n = 266) |
| Potassium, median (25th, 75th), mg/dL | 4.0 (3.7, 4.4) (n = 294) | 4.0 (3.7, 4.4) (n = 246) |
COVID-19, coronavirus disease 2019; ULN, upper limit of normal.
Reference ranges: 1000 to 5000 × 109/L for lymphocytes; 0.8 to 1.2 mg/dL for creatinine; <10 mg/L for C-reactive protein; and 3.5 to 5.0 mmol/L for potassium. Upper limit of normal for troponin: troponin I: 0.16 ng/mL; troponin T: 14 ng/L. Upper limit of normal for D-Dimer: 500 μg/L (D-dimer was not age adjusted).
Mild defined as blood oxygen saturation ≥94% and lung infiltrates ≤50%. Moderate defined as blood oxygen saturation <94%, or lung infiltrates >50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Concomitant Medications
Baseline medications at hospital admission and COVID-19 treatments are described in Table 3 . ARBs were used in 84% of patients with mild disease and 82.3% with moderate disease. ACEIs were used in 16% of patients with mild disease and 17.7% with moderate disease. In terms of other COVID-19 treatments, azithromycin was used in 90.2% of patients with mild disease and 91.2% with moderate disease. Anticoagulation and corticosteroids were more often used in patients with moderate disease than in those with mild disease.
Table 3.
Baseline medications
| COVID-19 clinical severity* at hospitalization |
||
|---|---|---|
| Mild (n = 376) | Moderate (n = 283) | |
| Medication use at hospital admission | ||
| ARB | 316/376 (84.0%) | 233/283 (82.3%) |
| ACEI | 60/376 (16.0%) | 50/283 (17.7%) |
| Diuretics† | 117/376 (31.1%) | 89/283 (31.4%) |
| Statin | 75/376 (19.9%) | 64/283 (22.6%) |
| Calcium channel blocker | 67/376 (17.8%) | 54/283 (19.1%) |
| β-Blocker | 47/376 (12.5%) | 49/283 (17.3%) |
| Antiplatelet‡ | 35/376 (9.3%) | 31/283 (11.0%) |
| Insulin | 12/376 (3.2%) | 15/283 (5.3%) |
| Oral anticoagulants§ | 12/376 (3.2%) | 6/283 (2.1%) |
| Concomitant therapy | ||
| Azithromycin | 339/376 (90.2%) | 258/283 (91.2%) |
| Anticoagulation|| | 223/376 (59.3%) | 217/283 (76.7%) |
| Antiviral¶ | 153/376 (40.7%) | 124/283 (43.8%) |
| Chloroquine or hydroxychloroquine | 60/376 (16.0%) | 70/283 (24.7%) |
| Tocilizumab | 10/376 (2.7%) | 14/283 (4.9%) |
| Corticosteroid# | 153/376 (40.7%) | 173/283 (61.1%) |
| Any antibiotics | 365/376 (97.1%) | 277/283 (97.9%) |
Data presented as no./No. (%).
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COVID-19, coronavirus disease 2019.
Mild defined as blood oxygen saturation ≥94% and lung infiltrates ≤50%. Moderate defined as blood oxygen saturation <94%, or lung infiltrates >50%, or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300; and severe, invasive mechanical ventilation or hemodynamic instability or multiple organ dysfunction or failure.
Furosemide, hydrochlorothiazide, or spironolactone.
Aspirin, clopidogrel, or ticagrelor.
Warfarin, rivaroxaban, apixaban, dabigatran, or edoxaban.
Enoxaparin, unfractioned heparin, warfarin, rivaroxaban, apixaban, dabigatran, or edoxaban. The differentiation between therapeutic and prophylactic anticoagulation was based on the dose.
Oseltamivir, ribavirin, or lopinavir-ritonavir.
Prednisone, dexamethasone, hydrocortisone, or methylprednisolone.
Disease progression
Of the 376 patients classified as having mild disease at presentation, 169 (44.9%) progressed to having moderate disease during hospitalization and 20 (5.3%) progressed to having severe disease. Of the 283 patients classified as having moderate disease at presentation, 44 (15.5%) progressed to having severe disease.
At 30 days, the mortality rate was 1.59% in patients classified as having mild disease at presentation (6/376) and 4.24% (12/283) in those classified as having moderate disease at presentation. The mortality rate was 30% (6/20) in patients who progressed from mild to severe disease and 25% (11/44) in those who progressed from moderate to severe disease. There were no deaths in 30 days among patients who progressed from mild to moderate disease during hospitalization.
Treatment effect according to clinical severity at presentation
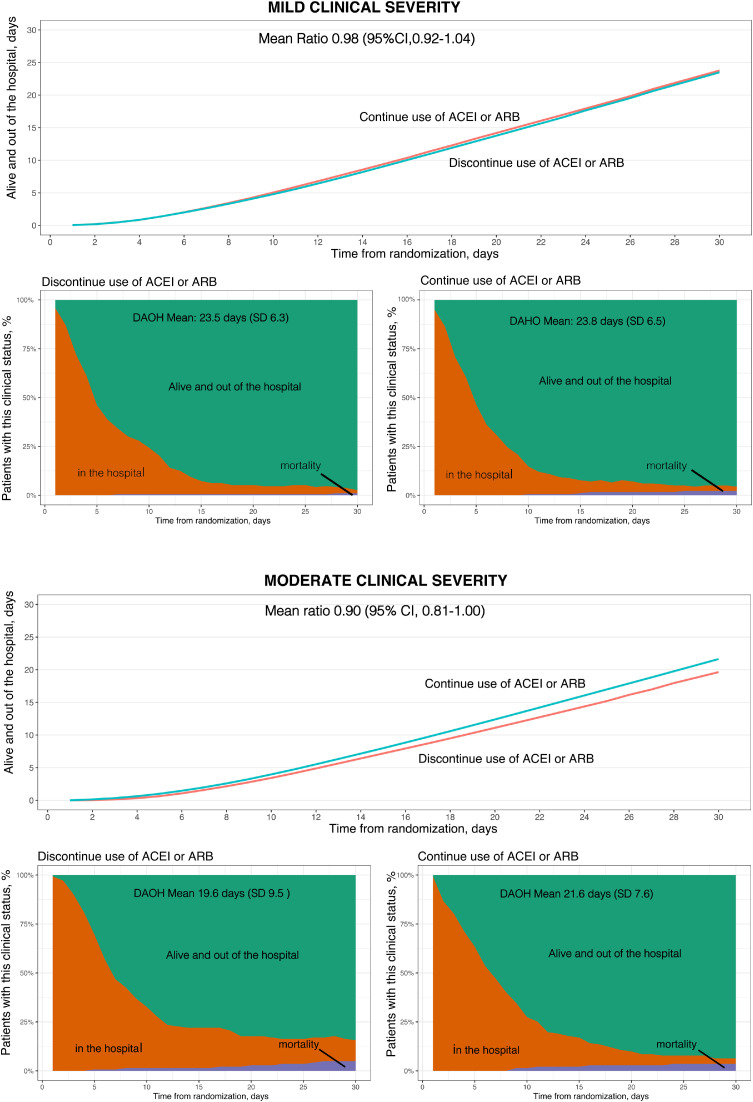
There was a statistically significant interaction of treatment effect on the primary outcome according to baseline disease severity (P= .01). In patients with mild disease, the mean number of days alive and out of the hospital was 23.5 (SD 6.3) in the discontinuation group and 23.8 (SD 6.5) in the continuation group; the mean ratio was 0.98 (95% CI 0.92-1.04) ( Table 4 ; Figure 1 a).
Table 4.
Primary and secondary outcomes at 30 days according to clinical severity* (intention-to-treat population)
| Mild |
Moderate |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Discontinue ACEI/ARB | Continue ACEI/ARB | Absolute Difference (95% CI) | Effect Size (95% CI) | Discontinue ACEI/ARB | Continue ACEI/ARB | Absolute Difference (95% CI) | Effect Size (95% CI) | Interaction P-value | |
| Primary Outcome | |||||||||
| Days alive and out of the hospital | |||||||||
| Mean (SD) | 23.5 ± 6.3 (n = 193) | 23.8 ± 6.5 (n = 183) | 0.00 (-1.09 to 1.09) | MR 1.00 (0.95-1.05) | 19.6 ± 9.5 (n = 141) | 21.6 ± 7.6 (n = 142) | -2.48 (-4.34 to -0.62) | MR 0.88 (0.80-0.97) | 0.01 |
| Median (25th, 75th) | 26.0 (21.0, 28.0) (n = 193) | 26.0 (22.5, 28.0) (n = 183) | 24.0 (19.0, 26.0) (n = 141) | 24.0 (19.2, 27.0) (n = 142) | |||||
| Secondary Outcomes | |||||||||
| Length of hospitalization, days | |||||||||
| Mean (SD) | 6.4 ± 6.0 | 5.9 ± 5.8 | 0.77 (-0.59 to 2.12) | MR 1.12 (0.89-1.36) | 9.7 ± 8.7 | 7.8 ± 6.7 | 2.20 (0.27-4.13) | MR 1.27 (1.01-1.54) | 0.39 |
| Median (25th, 75th) | 4.0 (2.0, 9.0) (n = 193) | 4.0 (2.0, 7.5) (n = 183) | 6.0 (4.0, 11.0) (n = 141) | 6.0 (3.0, 10.0) (n = 142) | |||||
| Death at 30 days, no./No. (%) | 2/193 (1.0%) | 4/183 (2.2%) | -1.15 (-3.70 to 1.41) | OR 0.47 (0.08–2.59) | 7/141 (5.0%) | 5/142 (3.5%) | 1.44 (-3.25 to 6.14) | OR 1.43 (0.44-4.62) | 0.291 |
| In-hospital death, no./No. (%) | 2/193 (1.0%) | 3/183 (1.6%) | -0.60 (-2.93 to 1.73) | OR 0.63 (0.10-3.80) | 7/141 (5.0%) | 4/142 (2.8%) | 2.15 (-2.35 to 6.65) | OR 1.80 (0.52-6.30) | 0.346 |
| CV death, no./No. (%) | 1/193 (0.5%) | 0/183 (0.0%) | 0.50 (-0.95 to 1.95) | OR 2.86 (0.11-71.26) | 1/141 (0.7%) | 1/142 (0.7%) | 0.01 (-2.37 to 2.39) | OR 1.01 (0.10-9.88) | 0.604 |
| COVID-19 progression, no./No. (%) | 103/193 (53.4%) | 86/183 (47.0%) | 6.37 (-3.72 to 16.46) | OR 1.29 (0.86-1.94) | 25/141 (17.7%) | 19/142 (13.4%) | 4.35 (-4.08 to 12.78) | OR 1.40 (0.73-2.67) | 0.842 |
| Respiratory failure requiring invasive mechanical ventilation, no./No. (%) | 9/193 (4.7%) | 9/183 (4.9%) | -0.25 (-4.57 to 4.07) | OR 0.95 (0.37-2.44) | 23/141 (16.3%) | 16/142 (11.3%) | 5.04 (-2.97 to 13.06) | OR 1.53 (0.77-3.05) | 0.417 |
| Shock requiring vasopressors, no./No. (%) | 9/193 (4.7%) | 9/183 (4.9%) | -0.74 (-4.71 to 3.22) | OR 0.82 (0.29-2.32) | 23/141 (16.3%) | 16/142 (11.3%) | 4.33 (-3.42 to 12.08) | OR 1.48 (0.73-3.01) | 0.358 |
| CV outcomes, no./No. (%) | |||||||||
| Acute MI | 8/193 (4.1%) | 5/183 (2.7%) | 1.41 (-2.26 to 5.09) | OR 1.54 (0.49-4.79) | 17/141 (12.1%) | 10/142 (7.0%) | 5.01 (-1.81 to 11.84) | OR 1.81 (0.80-4.10) | 0.821 |
| New or worsening HF | 7/193 (3.6%) | 7/183 (3.8%) | -0.20 (-4.03 to 3.63) | OR 0.95 (0.33-2.75) | 7/141 (5.0%) | 9/142 (6.3%) | -1.37 (-6.75 to 4.00) | OR 0.77 (0.28-2.13) | 0.787 |
| Other outcomes, no./No. (%) | |||||||||
| Acute kidney failure requiring hemodialysis | 4/193 (2.1%) | 2/183 (1.1%) | 0.98 (-1.53 to 3.49) | OR 1.92 (0.35-10.59) | 7/141 (5.0%) | 7/142 (4.9%) | 0.03 (-5.02 to 5.09) |
OR 1.01 (0.34-2.95) | 0.533 |
| Thromboembolic events | 1/193 (0.5%) | 3/183 (1.6%) | -1.12 (-3.22 to 0.98) | OR 0.31 (0.03-3.03) | 5/141 (3.5%) | 1/142 (0.7%) | 2.84 (-0.51 to 6.19) | OR 5.18 (0.60-44.95) | 0.079 |
| Stroke or TIA | 2/193 (1.0%) | 2/183 (1.1%) | -0.06 (-2.13 to 2.02) | OR 0.95 (0.13-6.80) | 1/141 (0.7%) | 1/142 (0.7%) | 0.00 (-1.95 to 1.96) | OR 1.01 (0.06-16.26) | 0.972 |
| BNP above ULN | 14/193 (7.3%) | 17/183 (9.3%) | -2.04 (-7.61 to 3.54) | OR 0.76 (0.37-1.60) | 14/141 (9.9%) | 22/142 (15.5%) | -5.56 (-13.30 to 2.17) | OR 0.60 (0.29-1.23) | 0.648 |
| D-dimer | 63/116 (54.3%) | 54/109 (49.5%) | 4.77 (-8.28 to 17.82) | OR 1.21 (0.72-2.04) | 44/63 (69.8%) | 43/69 (62.3%) | 7.52 (-8.58 to 23.62) | OR 1.40 (0.68-2.89) | 0.750 |
| Treated arrhytmias | 2/193 (1.0%) | 4/183 (2.2%) | -1.15 (-3.70 to 1.41) | OR 0.47 (0.08-2.59) | 6/141 (4.3%) | 4/142 (2.8%) | 1.44 (-2.86 to 5.74) | OR 1.53 (0.42-5.55) | 0.278 |
| Myocarditis† | 0 (0%) | 0 (0%) | — | — | 0 (0%) | 0 (0%) | — | — | — |
| Pericarditis† | 0/193 (0.0%) | 0/183 (0.0%) | — | — | 1/141 (0.7%) | 0/142 (0.0%) | — | — | — |
| Hypertensive crisis† | 0/193 (0.0%) | 1/183 (0.5%) | — | — | 1/141 (0.7%) | 2/142 (1.4%) | — | — | — |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; CI, confidence interval; COVID-19, coronavirus disease 2019; CV, cardiovascular; HF, heart failure; MI, myocardial infarction; MR, mean ratio; OR, odds ratio; SD, standard deviation; TIA, transient ischemic attack; ULN, upper limit of normal.
*The treatment effect of discontinuing versus continuing ACEIs/ARBs on outcomes was analyzed according to disease severity. Interaction test between treatment effect and disease severity at baseline for the primary outcome was performed using bootstrapping. Mortality and cardiovascular events at 30 days were compared using logistic regression, reporting odds ratio with the respective 95% CIs with interaction tests between disease severity groups and study treatment. Mortality at 30 days was assessed according to clinical severity at presentation and progression of clinical severity during hospitalization.
†The effect size of treatment assignment on these outcomes according to disease severity at presentation was not calculated due to low number of events.
Figure 1.
A, Primary outcome: days alive and out of the hospital at 30 days in patients with mild disease severity at presentation. B, Primary outcome: days alive and out of the hospital at 30 days in patients with moderate disease severity at presentation
In patients with moderate disease, the mean number of days alive and out of the hospital was 19.6 (SD 9.5) in the discontinuation group and 21.6 (SD 7.6) in the continuation group; the mean ratio was 0.90 (95% CI 0.81-1.00) (Table 4; Figure 1b). Similar results were seen for the on-treatment population (Table 5 ).
Table 5.
Primary and secondary outcomes at 30 days according to clinical severity* (on-treatment population)†
| Mild |
Moderate |
P-value for interaction | |||||
|---|---|---|---|---|---|---|---|
| Discontinue ACEI/ARB | Continue ACEI/ARB | Effect size (95% CI) | Discontinue ACEI/ARB | Continue ACEI/ARB | Effect size (95% CI) | P-value | |
| Primary outcome | |||||||
| Days alive and out of the hospital | |||||||
| Mean (SD) | 23.2 ± 7.0 (n = 137) | 24.2 ± 5.8 (n = 172) | 0.97 (0.91-1.04) | 18.9 ± 10.1 (n = 100) | 22.2 ± 6.9 (n = 130) | 0.82 (0.73-0.91) | 0.01 |
| Median (IQR) | 26.0 (21.0, 28.0) (n = 137) | 26.0 (23.0, 28.0) (n = 172) | 24.0 (17.8, 26.0) (n = 100) | 24.0 (20.0, 27.0) (n = 130) | |||
| Secondary outcomes | |||||||
| Length of hospitalization | |||||||
| Mean (SD) | 6.6 ± 6.7 | 5.6 ± 5.3 | 1.25 (0.91-1.60) | 10.3 ± 9.3 | 7.2 ± 5.9 | 1.45 (1.11-1.80) | 0.42 |
| Median (IQR) | 4.0 (2.0, 9.0) (n = 137) | 4.0 (2.0, 7.0) (n = 172) | 6.0 (4.0, 11.0) (n = 100) | 6.0 (3.0, 9.0) (n = 130) | |||
| Death at 30 days, no./no. (%) | 2/137 (1.5%) | 3/172 (1.7%) | 0.83 (0.14-5.07) | 6/100 (6.0%) | 4/130 (3.1%) | 2.01 (0.55-7.33) | 0.437 |
| In-hospital death, no./no. (%) | 2/137 (1.5%) | 2/172 (1.2%) | 1.26 (0.18-9.06) | 6/100 (6.0%) | 3/130 (2.3%) | 2.70 (0.66-11.08) | 0.537 |
| CV Death, no./no. (%) | 1/137 (0.7%) | 0/172 (0.0%) | 3.79 (0.15-94.73) | 1/100 (1.0%) | 1/130 (0.8%) | 1.30 (0.13-12.83) | 0.596 |
| COVID-19 progression, no./no. (%) | 72/137 (52.6%) | 78/172 (45.3%) | 1.33 (0.85-2.09) | 20/100 (20.0%) | 14/130 (10.8%) | 2.07 (0.99-4.34) | 0.320 |
| Respiratory failure requiring invasive mechanical ventilation, no./no. (%) | 8/137 (5.8%) | 6/172 (3.5%) | 1.72 (0.58-5.07) | 18/100 (18.0%) | 11/130 (8.5%) | 2.37 (1.07-5.29) | 0.636 |
| Shock requiring vasopressors, no./no. (%) | 8/137 (5.8%) | 6/172 (3.5%) | 1.80 (0.56-5.80) | 18/100 (18.0%) | 11/130 (8.5%) | 2.22 (0.99-4.97) | 0.774 |
| CV outcomes, no./no. (%) | |||||||
| Acute MI | 3/137 (2.2%) | 4/172 (2.3%) | 0.94 (0.21-4.27) | 15/100 (15.0%) | 7/130 (5.4%) | 3.10 (1.21-7.93) | 0.189 |
| New or worsening HF | 3/137 (2.2%) | 6/172 (3.5%) | 0.62 (0.15-2.52) | 6/100 (6.0%) | 7/130 (5.4%) | 1.12 (0.36-3.45) | 0.518 |
| Other outcomes, no./no. (%) | |||||||
| Acute kidney failure requiring hemodialysis | 4/137 (2.9%) | 2/172 (1.2%) | 2.56 (0.46-14.17) | 7/100 (7.0%) | 5/130 (3.8%) | 1.88 (0.58-6.12) | 0.773 |
| Thromboembolic events, no./no. (%)‡ | 0/137 (0.0%) | 3/172 (1.7%) | — | 4/100 (4.0%) | 0/130 (0.0%) | — | — |
| Stroke or TIA | 2/137 (1.5%) | 2/172 (1.2%) | 1.26 (0.18-9.06) | 1/100 (1.0%) | 1/130 (0.8%) | 1.30 (0.08-21.09) | 0.984 |
| BNP above the ULN | 9/137 (6.6%) | 16/172 (9.3%) | 0.69 (0.29-1.60) | 9/100 (9.0%) | 20/130 (15.4%) | 0.54 (0.24-1.25) | 0.703 |
| D-dimer | 47/85 (55.3%) | 51/104 (49.0%) | 1.29 (0.72-2.28) | 31/46 (67.4%) | 39/65 (60.0%) | 1.38 (0.62-3.04) | 0.889 |
| Treated arrhytmias | 1/137 (0.7%) | 3/172 (1.7%) | 0.41 (0.04-4.03) | 5/100 (5.0%) | 4/130 (3.1%) | 1.66 (0.43-6.34) | 0.303 |
| Myocarditis‡ | 0 (0%) | 0 (0%) | — | 0 (0%) | 0 (0%) | — | — |
| Pericarditis‡ | 0 (0%) | 0 (0%) | — | 0 (0%) | 0 (0%) | — | — |
| Hypertensive crisis‡ | 0/137 (0.0%) | 1/172 (0.6%) | — | 1/100 (1.0%) | 1/130 (0.8%) | — | — |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; CI, confidence interval; COVID-19, coronavirus disease 2019; CV, cardiovascular; HF, heart failure; IQR, interquartile range; MI, myocardial infarction; SD, standard deviation; TIA, transient ischemic attack; ULN, upper limit of normal.
*The treatment effect of discontinuing versus continuing ACEIs/ARBs on outcomes was analyzed according to disease severity. Interaction test between treatment effect and disease severity at baseline for the primary outcome was performed using bootstrapping. Mortality and cardiovascular events at 30 days were compared using logistic regression, reporting odds ratio with the respective 95% CIs with interaction tests between disease severity groups and study treatment. Mortality at 30 days was assessed according to clinical severity at presentation and progression of clinical severity during hospitalization
†A sensitivity analysis “on treatment” for the primary outcome and secondary outcomes according to disease severity was performed including only patients who were adherent to the study intervention until the time of death or through 30 days.
‡The effect of treatment assignment on these outcomes according to disease severity was not calculated due to low number of events.
Primary outcome according to disease progression
Among patients who experienced progression of disease severity during hospitalization, the mean number of days alive and out of the hospital was 17.9 (SD 10.2) in the discontinuation group and 18.8 (SD 9.8) in the continuation group; the mean ratio was 0.96 (95% CI 0.85-1.06). In patients with no disease progression, the mean number of days alive and out of the hospital was 24.4 (SD 4.9) in the discontinuation group and 24.8 (SD 4.1) in the continuation group; the mean ratio was 0.98 (95% CI 0.95-1.01; P-interaction = .59).
Secondary outcomes according to disease severity at presentation
All secondary outcomes according to disease severity at presentation are shown in Table 4 and Figure 2 . Although there was not a statistically significant interaction, the point estimates of treatment effect on number of days in the hospital, mortality at 30 days, in-hospital mortality, respiratory failure, and shock favor the continuation of ACEIs/ARBs in patients with moderate disease at hospital presentation. Similar results were seen for the on treatment population as shown in Table 5.
Figure 2.
Secondary outcomes according to disease severity at presentation in the intention to treat population.
DISCUSSION
In this pre-specified analysis of the BRACE CORONA trial, patients hospitalized with moderate COVID-19 had worse outcomes than patients with mild disease. There was a statistically significant interaction of treatment effect by baseline COVID-19 disease severity. In patients with mild disease, there was little difference in the primary outcome between stopping or continuing ACEIs/ARBs; however, patients with moderate disease who continued ACEIs/ARBs had more days alive and out of the hospital at the end of 30 days than those who discontinued ACEIs/ARBs. In patients with moderate disease severity, those who continued ACEI/ARB therapy had lower rates of in-hospital and 30-day mortality, fewer days in the hospital, and a lower chance of progressing to respiratory failure with mechanical ventilation and shock requiring vasoactive drugs than those who discontinued ACEIs/ARBs. These findings were not seen in patients with mild disease at presentation.
Evaluation and management of COVID-19 depends on the severity of the disease.16 Different definitions have been used to classify COVID-19 severity.4 We used a comprehensive definition of disease severity that includes lung imaging with computed tomography, oxygen saturation, and necessity of organ support. In the BRACE CORONA trial, disease severity was systematically and objectively assessed though hospitalization.13 In our study, patients with severe disease at presentation were not included since patients requiring mechanical ventilation and those with hemodynamic instability, acute renal failure, and shock were not eligible for enrollment. The population included in BRACE CORONA was well distributed with regard to the percentage of patients with mild (50.3%) and moderate disease (49.7%). This balance provided a good opportunity to study the effect of RAS inhibition on days alive and out of the hospital in two representative populations according to disease severity and to evaluate disease progression and mortality.
At the beginning of the pandemic, concerns were raised regarding patients with hypertension diagnosed with COVID-19 due to their worse prognosis.5 , 17., 18., 19. Many of these patients were taking ACEIs or ARBs and some experimental studies suggested that by causing an increase in the expression of the ACE2 receptor, which is the pathway by which SARS-CoV-2 enters human cells, these therapies could be related to an increased susceptibility to infection with and/or severity of COVID-19.20 , 21 Nevertheless, some animal studies have demonstrated that the increased expression of the ACE2 receptor could prevent its complete neutralization by the virus, preventing a hyperinflammatory pulmonary state, by favoring the conversion of angiotensin II (proinflammatory) in angiotensin 1 to 7 (anti-inflammatory).22 , 23 Thus, since ACEIs and ARBs can block this pathway, discontinuation of these drugs (mainly antagonists of the AT1 receptor) in the course of COVID-19 could potentially lead to a worsening of disease severity.24 , 25 Our overall findings, together with the fact that the majority of patients included in BRACE CORONA were using ARBs at presentation, support this hypothesis.
BRACE CORONA was the largest randomized trial showing that among patients hospitalized due to COVID-19 there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus continue ACEIs/ARBs. Recently, 2 large observational studies and a meta-analysis have corroborated our findings. The systematic review findings of the 7 case-population and cohort studies on SARS-CoV-2 infection provide more evidence that therapy with ACE inhibitors or ARBs was not associated with an increase of the severity of COVID-19 disease or overall population mortality.25., 26., 27.
Two other published randomized controlled trials reported no harm associated with continuation of ACEIs or ARBs in patients with a recent COVID-19 infection. The REPLACE-COVID trial, a multicenter international, randomized controlled trial that included 152 patients, showed that discontinuing RAS inhibition had no effect on the global rank score, a non-parametric ranked outcome that hierarchically included death, mechanical ventilation or extracorporeal membrane oxygenation, renal replacement therapy or vasopressor therapy, and area under a modified Sequential Organ Failure Assessment (SOFA) score.26 The ACEI-COVID trial was a multicenter, randomized, controlled, open-label trial that included 204 patients and showed that discontinuation of chronic RAS inhibition did not significantly affect the maximum severity of disease within 30 days, the primary outcome measure of the study. However, an exploratory analysis suggested a favorable net effect of discontinuation of RAS inhibition on organs, suggesting better and faster recovery of high-risk elderly patients with COVID-19.27 This is contrary to what we have shown in our current subgroup analysis from BRACE CORONA. Our trial is characterized by a younger (mean age 55 years) patient population than the ACEI-COVID study; however, 25% of the patients were older than 65 years (169 patients). In addition, in the current prespecified analysis, we found no benefit in discontinuing ACEIs/ARBs in patients with moderate disease on the number of days alive and out of the hospital. Some of the possible reasons that the results from the ACEI-COVID trial differed from BRACE CORONA include a smaller sample size, post-hoc analyses, an older population, or simply the play of chance.
An important aspect to be taken into account when interpreting the results of these two trials is the rate of ACEIs/ARBs used by patients in the studies and an effect modification by ACEI versus ARB use. Experimental data in animals suggest that ACEIs and ARBs have differential effects on ACE2 expression and activity,8 although this might not be consistent across organs.28 In fact, it is reasonable that variations in their effect on ACE2 could induce ACEIs and ARBs to impose distinctively different results on COVID-19-related endpoints.29 The best anti-inflammatory effect by the RAS blockade cascade would occur with the blocking of ATR1 by the high-affinity ARBs,30 which could explain the benefit in continuing RAS inhibition in BRACE CORONA since around 80% of the population used ARBs versus 44% in the ACEI-COVID trial.
In this pre-specified analysis from BRACE CORONA, we included a substantial proportion of patients with moderate COVID-19 severity and showed that this group might benefit the most from continuation of ACEIs or ARBs after COVID-19 infection. These findings are aligned with the hypothesis that ACEIs and ARBs might have a protective role in more severe cases of COVID-19. These medications have well-known benefits for patients with hypertension and heart failure and stopping them could be deleterious. A recent retrospective study showed a significant association between ACEIs/ARBs and lower rates of death in patients with COVID-19, particularly in patients using ARBs.31 In light of our study results and most of the current evidence in the field, physicians should not discontinue ACEIs/ARBs because of COVID-19, particularly in patients with moderate COVID-19 disease. Whether starting ACEIs or ARBs in patients with COVID-19 who do not have a prior reason for treatment (like hypertension or heart failure) is beneficial or not is currently being investigated in ongoing studies (NCT04335786, NCT04311177, NCT04328012) that are testing the impact of the introduction of ACEI or ARB treatment versus placebo as a therapy option for COVID-19.
Our study has limitations. Although it was a pre-specified analysis, subgroup analyses of trials without an overall benefit of study intervention should be considered as hypothesis generating. Nevertheless, the continuation of ACEIs/ARBs is the standard regimen for patients without contraindications, and BRACE CORONA showed that this approach is safe and could further benefit patients admitted to the hospital with moderate disease. Importantly, we could not provide data on the serum creatinine values after baseline, average dose of ACEIs or ARBs used at baseline, and the reasons for non-adherence to the study intervention after hospital discharge. Finally, the relatively small number of patients taking ACEIs might restrict the expansion of our results in this population.32
In conclusion, patients with moderate COVID-19 disease at hospital presentation have worse outcomes than patients with mild disease. While in patients with mild disease there is no difference on outcomes between discontinuing or continuing ACEIs/ARBs, patients with moderate disease who continued ACEIs/ARBs appeared to have more days alive and out of the hospital at the end of 30 days than those who stopped their ACEIs/ARBs. Our findings provide evidence to support the continuation of ACEIs/ARBs, particularly in patients with moderate COVID-19 disease severity.
Funding
No extramural funding was used to support this work.
Conflict of interest
AVSM: Consulting fees from Pfizer, Bayer, Novartis, Daiichi-Sankyo, Zodiac, Roche, Janssen. PGMBS: Consulting fees Pfizer, Roche, Bayer. TCP: Nothing to report. RJM-B: Nothing to report. TMS: Nothing to report. LM: Nothing to report. AF: Consulting fees Pfizer, Bayer, Daiichi-Sankyo, Boehringer, Servier. GASA: Consulting fees from Bayer; Pfizer; Servier; Astra Zeneca; Daichii Sankyo. DCA: Boehringer Ingelheim, AstraZeneca, Bayer, Servier. ASS: Nothing to report. OFS: Research support from Boehringer Ingelheim. Consulting fees Pfizer, Bayer, Daiichi-Sankyo, Boehringer Ingelheim. CMG: Nothing to report. CBG: Research Grants: AstraZeneca, FDA, NIH, GSK, Medtronic, Novartis, Apple, Boehringer Ingelheim, BMS/ Pfizer, Janssen. Consulting: AstraZeneca, Espero, GSK, Medtronic, Novartis, Boehringer Ingelheim, Boston Scientific, BMS/ Pfizer, Daiichi Sankyo, Merck, Roche, Eli Lilly, Janssen. JHA: Research support from Boehringer Ingelheim, Bristol-Myers Squibb, CryoLife, CSL Behring, GlaxoSmithKline, US Food and Drug Administration, US National Institutes of Health, XaTek; Consulting fees or honoraria from AbbVie, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Novo Nordisk, Pfizer, Portola, Quantum Genomics, US Veterans Administration, XaTek, Zafgen. RDL: Research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer; Consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola.
Disclosures
AVSM: Consulting fees from Pfizer, Bayer, Novartis, Daiichi-Sankyo, Zodiac, Roche, Janssen. PGMBS: Consulting fees Pfizer, Roche, Bayer. TCP: Nothing to report. RJM-B: Nothing to report. TMS: Nothing to report. LM: Nothing to report. AF: Consulting fees Pfizer, Bayer, Daiichi-Sankyo, Boehringer, Servier. GASA: Consulting fees from Bayer; Pfizer; Servier; Astra Zeneca; Daichii Sankyo. DCA: Boehringer Ingelheim, AstraZeneca, Bayer, Servier. ASS: Nothing to report. OFS: Research support from Boehringer Ingelheim. Consulting fees Pfizer, Bayer, Daiichi-Sankyo, Boehringer Ingelheim. CMG: Nothing to report. CBG: Research Grants: AstraZeneca, FDA, NIH, GSK, Medtronic, Novartis, Apple, Boehringer Ingelheim, BMS/ Pfizer, Janssen. Consulting: AstraZeneca, Espero, GSK, Medtronic, Novartis, Boehringer Ingelheim, Boston Scientific, BMS/ Pfizer, Daiichi Sankyo, Merck, Roche, Eli Lilly, Janssen. JHA: Research support from Boehringer Ingelheim, Bristol-Myers Squibb, CryoLife, CSL Behring, GlaxoSmithKline, US Food and Drug Administration, US National Institutes of Health, XaTek; Consulting fees or honoraria from AbbVie, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Novo Nordisk, Pfizer, Portola, Quantum Genomics, US Veterans Administration, XaTek, Zafgen. RDL: Research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer; Consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola.
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drager LF, Pio-Abreu A, Lopes RD, et al. Is Hypertension a Real Risk Factor for Poor Prognosis in the COVID-19 Pandemic? Curr Hypertens Rep. 2020;22:43. doi: 10.1007/s11906-020-01057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Rea F, Ludergnani M, et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud G, Kaiming W, Anissa V, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenzato L, Botton J, Drouin J, et al. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Offringa-Hup AK, Logtenberg SJ, et al. Discontinuation of Antihypertensive Medications on the Outcome of Hospitalized Patients With Severe Acute Respiratory Syndrome-Coronavirus 2. Hypertens. 2021;78:165–173. doi: 10.1161/HYPERTENSIONAHA.121.17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes RD, Macedo AVS, Moll-Bernardes RJ, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Am Heart J. 2020;226:49–59. doi: 10.1016/j.ahj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes RD, Macedo AV, PGdBE Silva, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.24, 2022. Centers for Disease Control and Prevention.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html#clinical-presentation Accessed March. [Google Scholar]
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi R, Lynch J, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 17.Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.South AM, Tomlinson L, Edmonston D, et al. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10:410. doi: 10.1007/s11906-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Y, Liu Q, Li N, et al. Angiotensin II receptor blocker as a novel therapy in acute lung injury induced by avian influenza A H5N1 virus infection in mouse. Sci China Life Sci. 2015;58:208–211. doi: 10.1007/s11427-015-4814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarzani R, Giulietti F, Di Pentima C, et al. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am J Physiola Lung Cell Mol Physiol. 2020;319:L325–L336. doi: 10.1152/ajplung.00189.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278. doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JB, Hanff TC, Corrales-Medina V, et al. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol. J Clin Hypertens. 2020;22:1780–1788. doi: 10.1111/jch.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer A, Schreinlechner M, Sappler N, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9:863–872. doi: 10.1016/S2213-2600(21)00214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wysocki J, Lores E, Ye M, et al. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HY, Peng S, Ye Z, Li P, Li Q, Shi X, Zeng R, Yao Y, He F, Li J, et al. Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension. Front Med. 2021:1–9. doi: 10.1007/s11684-021-0850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancia G. COVID-19, hypertension, and RAAS blockers: the BRACE-CORONA trial. Cardiovasc Res 2020;116:e198-e199. [DOI] [PMC free article] [PubMed]