Introduction
Pseudo-hyperaldosteronism is a syndrome featuring hypokalemia, metabolic alkalosis, and suppressed renin in the absence of elevated aldosterone levels. It can be due to enzyme deficiency or inhibition, channelopathy, or constitutive activation of the mineralocorticoid receptor (Figure 1A). In this clinical narrative, we present a case of pseudo-hyperaldosteronism and review a schema for diagnosis and management.
Figure 1.
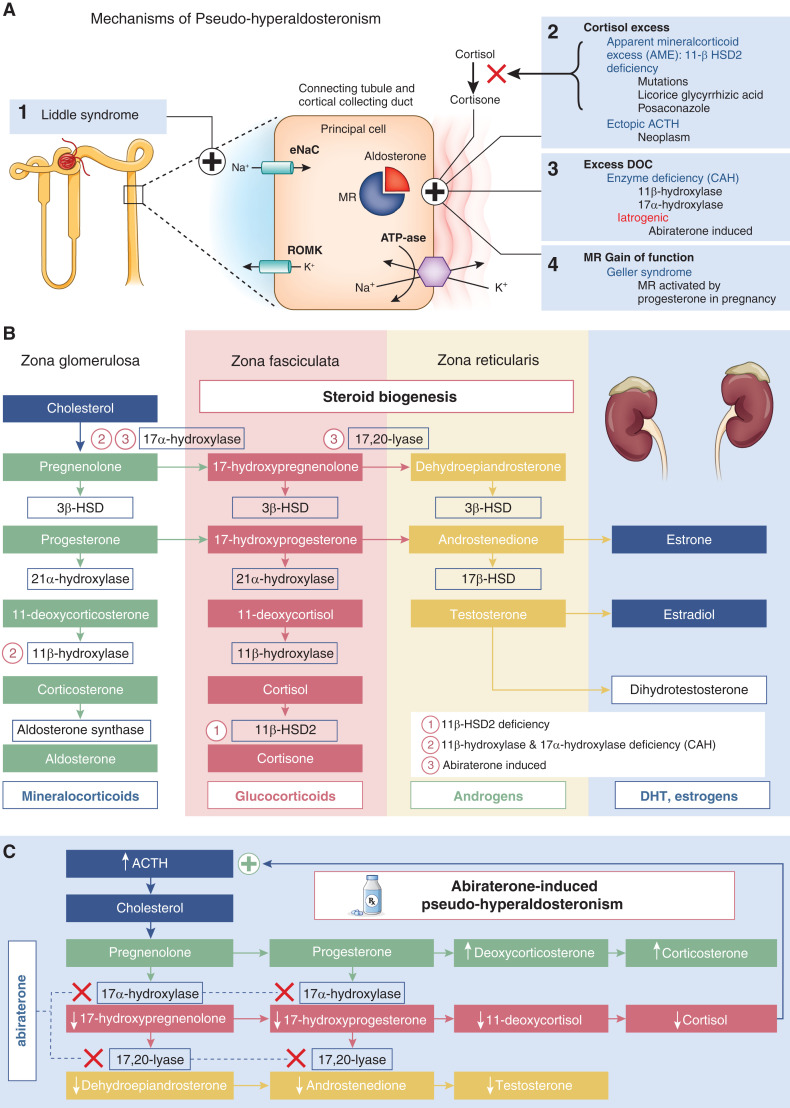
Mechanisms of pseudo-hyperaldosteronism and steroid biogenesis pathways. (A) Mechanisms of pseudo-hyperaldosteronism. (1) Primary channelopathy. Liddle syndrome, an autosomal dominant mutation of the gene encoding for the epithelial sodium channel (ENaC) causing constitutive ENaC activation, leads to excessive sodium (Na+) reabsorption in the distal tubule. (2) Excess cortisol activation of the mineralocorticoid receptor (MR) is from (a) apparent mineralocorticoid excess due to 11β-hydroxysteroid dehydrogenase type 2 (11-βHSD2) deficiency from an autosomal recessive mutation or ingestions, like black licorice (glicyrrhizic acid), posaconazole, or itraconazole, which prevent conversion of cortisol to the inert form cortisone, or (b) ectopic adrenocorticotrophic hormone (ACTH) that stimulates excess cortisol secretion exceeding 11-βHSD2 deactivation capacity. (3) Excess deoxycorticosterone (DOC) activating the MR is from (a) congenital adrenal hyperplasia (CAH), an autosomal recessive mutation causing 11β-hydroxylase and 17α-hydroxylase deficiencies during steroid biogenesis, or (b) abiraterone-induced inhibition of 17α-hydroxylase and C17,20-lyase resulting in low testosterone and cortisol levels, loss of ACTH inhibition, and subsequent DOC excess. (4) MR gain of function. Geller syndrome, an autosomal dominant mutation causing constitutive activation of MR by progesterone, was unmasked during pregnancy. (B) Steroid biogenesis pathways. (C) Mechanism of abiraterone-induced pseudo-hyperaldosteronism. DHT, dihydrotestosterone; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; ROMK, renal outer medullary potassium channel.
Patient
A 74-year-old man with metastatic, castration-resistant prostate cancer is admitted with weakness and leg cramps. Home medications include abiraterone 1 g daily, prednisone 5 mg daily (decreased from 5 mg twice daily), and spironolactone 25 mg daily. The examination is significant for BP of 152/94 mm Hg.
Initial laboratory values include sodium (Na) of 139 mmol/L, potassium (K+) of 2.3 mmol/L, bicarbonate of 29 mEq/L, creatinine of 1.2 mg/dl, BUN of 39 mg/dl, and magnesium of 1.9 mg/dl. Urine laboratory values include Na+ of 34 mmol/L, K+ of 48 mmol/L, creatinine of 36 mg/dl, and potassium-creatinine ratio of 133 mEq/g (elevated) with fractional excretion of potassium (FeK) of 70% and fractional excretion of sodium (FeNa) of 0.8%. The nephrology team is consulted when the patient remains hypokalemic despite receiving 170 mEq of potassium chloride daily.
Differential Diagnosis of Hypokalemia
The clinical presentation and laboratory findings narrow the differential for hypokalemia to kidney potassium wasting. Normal serum magnesium eliminates urinary loss from hypomagnesemia. Elevated serum bicarbonate rules out renal tubular acidosis. The presence of hypertension is less suggestive of surreptitious diuretics, Gitelman syndrome, or Bartter syndrome. The constellation of hypertension, hypokalemia, and metabolic alkalosis suggests excess mineralocorticoid activity, with further workup guided by renin and aldosterone levels (1).
Aldosterone is produced by the adrenal zona glomerulosa in response to angiotensin II or independent of RAAS by hyperkalemia. Aldosterone stimulates synthesis and activity of basolateral Na+/K+-ATPase, maintaining high levels of intracellular K+ and low levels of intracellular Na+. It also stimulates epithelial sodium channel (ENaC) synthesis and insertion at the apical membrane in the connecting tubule, leading to increased Na+ resorption. Increased Na+ reabsorption increases the electrochemical gradient, promoting secretion of K+ into the lumen. The resultant volume expansion suppresses renin.
Excess Mineralocorticoid Activity Workup
Serum aldosterone (6.5 ng/dl) and renin activity (1 ng/ml per hour) return to normal. Primary hyperaldosteronism is characterized by an aldosterone-renin ratio of >20 and serum aldosterone of >10 ng/dl (2). By comparison, our patient’s aldosterone-renin ratio is 6.5 with low serum aldosterone and low-normal serum renin activity, raising suspicion for nonaldosterone mineralocorticoid activity. Subsequent laboratory values reveal elevated adrenocorticotrophic hormone (ACTH) at 121 pg/ml (normal 10–60) and low morning serum cortisol of 2.3 μg/dl without response to cosyntropin stimulation (3.1 μg/dl). A diagnosis was made.
Differential Diagnosis of Pseudo-Hyperaldosteronism
Although serum renin and aldosterone levels vary, the pattern of hypertension and suppressed renin without elevated aldosterone suggests pseudo-hyperaldosteronism, a heterogeneous collection of diseases characterized by nonaldosterone mineralocorticoid activity. In excess, cortisol and deoxycorticosterone, an aldosterone precursor, can activate the mineralocorticoid receptor (Figure 1A). Mutations of the mineralocorticoid receptor itself or its target channel ENaC can also induce aldosterone-independent hypertension.
Mineralocorticoid Receptor Gain of Function
Geller syndrome is a rare autosomal dominant disease unmasked during pregnancy. A missense mutation of the mineralocorticoid receptor gene causes constitutive activation of the mineralocorticoid receptor by progesterone (Figure 1A).
Primary Kidney Channelopathy
Liddle syndrome is an autosomal dominant mutation of the gene encoding the ENaC channel of the cortical collecting tubule, causing constitutive activation and excessive Na reabsorption (Figure 1A) (3). Diagnosis is confirmed by genetic testing. Liddle syndrome is treated with a low-Na+ diet and ENaC blockade with amiloride or triamterene.
Cortisol Excess
Aldosterone and cortisol bind the mineralocorticoid receptor with similar affinity. Although cortisol circulates at significantly higher levels than aldosterone, the mineralocorticoid receptor is protected from cortisol activation by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). This enzyme converts cortisol to cortisone, which does not stimulate the mineralocorticoid receptor; 11β-HSD2 activity can be reduced from enzyme deficiency or inhibition, or it can be overwhelmed in the setting of excess cortisol or ectopic ACTH secretion (Figure 1A).
Congenital 11β-HSD2 deficiency or classic apparent mineralocorticoid excess is a rare autosomal recessive condition from HSD11B2 gene mutations. Patients present in childhood with polydipsia, hypertension, and poor growth. Nonclassic apparent mineralocorticoid excess results from partial 11β-HSD2 deficiency or substances that inhibit the enzyme 11β-HSD2, such as glycyrrhizic acid found in black licorice (4) or itraconazole (5), among others. Conditions of cortisol excess in the setting of 11β-HSD2 deficiency or inhibition are suggested by a high urinary cortisol-cortisone ratio. Treatment is largely mineralocorticoid antagonism.
Overproduction of cortisol, whether ACTH independent (a variety of conditions exist) or ACTH dependent (such as Cushing disease), also results in apparent mineralocorticoid excess as elevated cortisol levels overwhelm the 11β-HSD2 enzyme. In this case, urinary cortisone will be elevated (6).
Deoxycorticosterone Excess
Both 11β-hydroxylase deficiency and 17α-hydroxylase deficiency are rare autosomal recessive forms of congenital adrenal hyperplasia; 11β-hydroxylase deficiency decreases conversion of 11-deoxycortisol to cortisol, causing ACTH elevation through loss of negative feedback and accumulation of deoxycorticosterone as well as sex steroids, and 17α-hydroxylase is the first enzymatic step in converting progesterone and pregnenolone to active sex hormones (Figure 1B). Deficiency results in lower cortisol and sex hormone production, ACTH elevation via loss of cortisol-mediated negative feedback, and increased deoxycorticosterone production. Deoxycorticosterone is a less potent mineralocorticoid than aldosterone, but in excess, it binds to the mineralocorticoid receptor. Diagnosis is made via elevated 11-deoxycortisol or elevated ACTH–stimulated 11-deoxycortisol levels. Glucocorticoids suppress ACTH and the downstream mineralocorticoid effects.
Abiraterone is a selective, irreversible CYP17A1 inhibitor used to treat castration-resistant prostate cancer. The CYP17A1 enzyme has two activities, 17α-hydroxylase and C17,20-lyase, both of which are required for androgen synthesis. Abiraterone is used as a potent inhibitor of androgen production, limiting disease progression (7). Similar to 17α-hydroxylase deficiency, CYP17A1 inhibition by abiraterone results in pseudo-hyperaldosteronism due to cortisol synthesis blockade, leading to high ACTH levels and accumulation of steroid precursors, such as deoxycorticosterone and corticosterone (Figure 1C) (8). Because corticosterone has glucocorticoid activity, adrenal insufficiency is prevented.
Wrap-Up and Treatment
The patient is diagnosed with abiraterone-induced pseudo-hyperaldosteronism. Prednisone is increased back to 5 mg twice daily, and eplerenone replaces spironolactone. Two months later, serum K+, bicarbonate, BP, and ACTH levels normalize.
This patient exhibited signs of mineralocorticoid excess thought to be triggered by recent reduction in prednisone and loss of negative feedback on ACTH. The low cortisol reflects abiraterone-induced blockade of pregnenolone and progesterone conversion to cortisol precursors (Figure 1C). As increased corticosterone should provide sufficient glucocorticoid activity to prevent symptoms of adrenal insufficiency, phase 1 and 2 trials for abiraterone were performed without glucocorticoid supplementation. However, the majority of patients required eplerenone or glucocorticoids to curb mineralocorticoid excess symptoms (8).
Abiraterone is approved for use only with glucocorticoids. In efficacy studies as a prostate cancer treatment, abiraterone was administered with either prednisone or prednisolone 10 mg daily (8). Eplerenone is recommended when hypertension is present and has similar efficacy to glucocorticoids in reducing mineralocorticoid excess (9). Eplerenone is preferred over spironolactone, as spironolactone has androgen receptor activity. Amiloride increases urinary Na+ excretion in combination with hydrochlorothiazide for additional antihypertensive effect (10). Sodium restriction can complement mineralocorticoid receptor antagonism.
Pseudo-hyperaldosteronism, characterized by symptoms of mineralocorticoid excess and low-normal renin without elevated aldosterone, may be secondary to mineralocorticoid receptor activation via channelopathy, mineralocorticoid receptor gain of function, enzyme deficiencies, or inhibition by licorice or medications. Abiraterone can cause pseudo-hyperaldosteronism due to inhibition of 17α-hydroxylase, resulting in mineralocorticoid excess, and it is remediable with steroids and eplerenone.
Disclosures
S. Ambruso reports consultancy agreements with AstraZeneca, honoraria from Kidney International Reports, and serving as a scientific advisor or member of Kidney International Reports. All remaining authors have nothing to disclose.
Funding
Dr. Rizzolo reports funding from National Institutes of Diabetes and Digestive and Kidney Diseases grant 5T32DK007135-46.
Acknowledgments
The authors acknowledge Stuart Linas for his review of this manuscript.
This case was previously presented as an abstract at the 2021 American Society of Nephrology meeting.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
S.L. Ambruso conceptualized the study; S.L. Ambruso, N.M. Beck, and K. Rizzolo wrote the original draft; and S.L. Ambruso, N.M. Beck, and K. Rizzolo reviewed and edited the manuscript.
References
- 1.Palmer BF: A physiologic-based approach to the evaluation of a patient with hypokalemia. Am J Kidney Dis 56: 1184–1190, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH, Fineberg NS: The diagnosis of primary aldosteronism and separation of two major subtypes. Arch Intern Med 153: 2125–2129, 1993 [PubMed] [Google Scholar]
- 3.Nussberger J: Investigating mineralocorticoid hypertension. J Hypertens Suppl 21: S25–S30, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Sabbadin C, Bordin L, Donà G, Manso J, Avruscio G, Armanini D: Licorice: From pseudohyperaldosteronism to therapeutic uses. Front Endocrinol (Lausanne) 10: 484, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck KR, Telisman L, van Koppen CJ, Thompson GR 3rd, Odermatt A: Molecular mechanisms of posaconazole- and itraconazole-induced pseudohyperaldosteronism and assessment of other systemically used azole antifungals. J Steroid Biochem Mol Biol 199: 105605, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Stewart PM: Tissue-specific Cushing’s syndrome, 11beta-hydroxysteroid dehydrogenases and the redefinition of corticosteroid hormone action. Eur J Endocrinol 149: 163–168, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Auchus RJ, Yu MK, Nguyen S, Mundle SD: Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist 19: 1231–1240, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pia A, Vignani F, Attard G, Tucci M, Bironzo P, Scagliotti G, Arlt W, Terzolo M, Berruti A: Strategies for managing ACTH dependent mineralocorticoid excess induced by abiraterone. Cancer Treat Rev 39: 966–973, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Gill D, Gaston D, Bailey E, Hahn A, Gupta S, Batten J, Alex A, Boucher K, Stenehjem D, Agarwal N: Efficacy of eplerenone in the management of mineralocorticoid excess in men with metastatic castration-resistant prostate cancer treated with abiraterone without prednisone. Clin Genitourin Cancer 15: e599–e602, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedussi F, Galli D, Fragni M, Valcamonico F, Rossini E, Dalla Volta A, Vezzoli S, Roca E, Ferrari V, Lazzari B, Memo M, Sigala S, Berruti A: Amiloride is effective in the management of abiraterone-induced mineralocorticoid excess syndrome without interfering with its antineoplastic activity. Pharmacology 100: 261–268, 2017 [DOI] [PubMed] [Google Scholar]