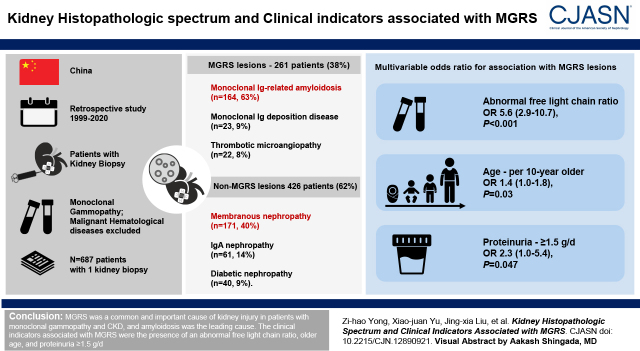
Visual Abstract
Keywords: monoclonal gammopathy, MGRS, kidney biopsy, cohort studies, China
Abstract
Background and objectives
Patients with monoclonal gammopathy and concomitant kidney diseases are frequently found in clinical practice. Some of them are diagnosed with monoclonal gammopathy of renal significance (MGRS) due to the presence of monoclonal Ig–related kidney injuries. This study aimed to investigate the histopathologic spectrum and clinical characteristics associated with MGRS in a large cohort of patients with monoclonal gammopathy and biopsy-proven kidney diseases from a single Chinese nephrology referral center.
Design, setting, participants, & measurements
Patients who presented with monoclonal gammopathy (monoclonal spike on serum and/or urine immunofixation tests) and underwent kidney biopsy in the Peking University First Hospital from January 1, 1999 to December 31, 2020 were enrolled in this retrospective study. Patients with malignant hematologic diseases were excluded. Clinical and laboratory data were collected from the electronic medical record system. Comparisons of patients with and without MGRS and with and without amyloidosis were performed. The clinical characteristics associated with MGRS were identified using multivariable logistic regression.
Results
A total of 700 patients with monoclonal gammopathy and kidney biopsy were identified. Thirteen patients with repeat kidney biopsies were analyzed separately. For the remaining 687 patients with one kidney biopsy, 261 patients (38%) had MGRS lesions, and the rest (426 patients, 62%) had non-MGRS kidney diseases. Ig-related amyloidosis accounted for the most MGRS cases (n=164, 63%), followed by monoclonal Ig deposition disease (n=23, 9%) and thrombotic microangiopathy (n=22, 8%). In the non-MGRS group, membranous nephropathy was the most common diagnosis (n=171, 40%). In the multivariable logistic regression model, the presence of abnormal serum free light chain ratio, older age, and greater proteinuria were independently associated with MGRS.
Conclusions
Monoclonal Ig amyloidosis is the leading cause of MGRS in Chinese patients with monoclonal gammopathy. The presence of abnormal free light chain ratio, older age, and greater proteinuria were associated with MGRS.
Introduction
Monoclonal Ig is produced by clonal proliferating plasma cells or B cells. The hematologic disorders include benign diseases such as monoclonal gammopathy of undetermined significance (MGUS), premalignant diseases such as smoldering multiple myeloma, or malignant diseases such as multiple myeloma. MGUS is defined as the presence of <30 g/L monoclonal Ig in serum and <10% of monoclonal plasma cells in the bone marrow without end organ damage related to the proliferating plasma cells (1,2). However, some patients with MGUS develop kidney diseases secondary to the monoclonal Igs by direct kidney deposition or an indirect mechanism as a interfering complement system, and these patients were defined as having monoclonal gammopathy of renal significance (MGRS). In 2018, MGRS was expanded to kidney injuries secondary to nephrotoxic monoclonal Ig in the absence of meeting any current B cell or plasma cell hematologic criteria for specific therapy, such as multiple myeloma, symptomatic Waldenström macroglobulinemia, or symptomatic chronic lymphocytic leukemia (3). MGRS includes a spectrum of kidney injuries with different substructures and involving different kidney compartments.
Patients with monoclonal gammopathy are predominantly among the elderly. In a previous population-based study in Olmsted County, Minnesota, the prevalence of MGUS was 3% in those age >50 years and 5% in those age >70 years (4). A similar prevalence increase of age-related monoclonal gammopathy was observed in China (5). CKD is common in all populations, with an estimated mean global prevalence of 13% (95% confidence interval [95% CI], 12% to 15%) (6), and it is accelerated by hypertension, diabetes, and obesity, which are also associated with aging (7). Therefore, monoclonal gammopathy and CKD are both prevalent in the elderly population, and some patients with CKD and monoclonal gammopathy could have MGRS and need specific antiplasma cell or B cell treatment to preserve kidney function. The diagnosis of MGRS requires kidney biopsy (8); however, the distribution of various MGRS-related kidney injuries and clinical indicators associated with MGRS was not well described in Chinese patients with monoclonal gammopathy and concomitant CKD.
In this study, we retrospectively studied the kidney histopathologic spectrum and clinical indicators associated with MGRS in a large cohort of patients with monoclonal gammopathy and concomitant kidney diseases from a single Chinese nephrology referral center.
Materials and Methods
Study Design and Population
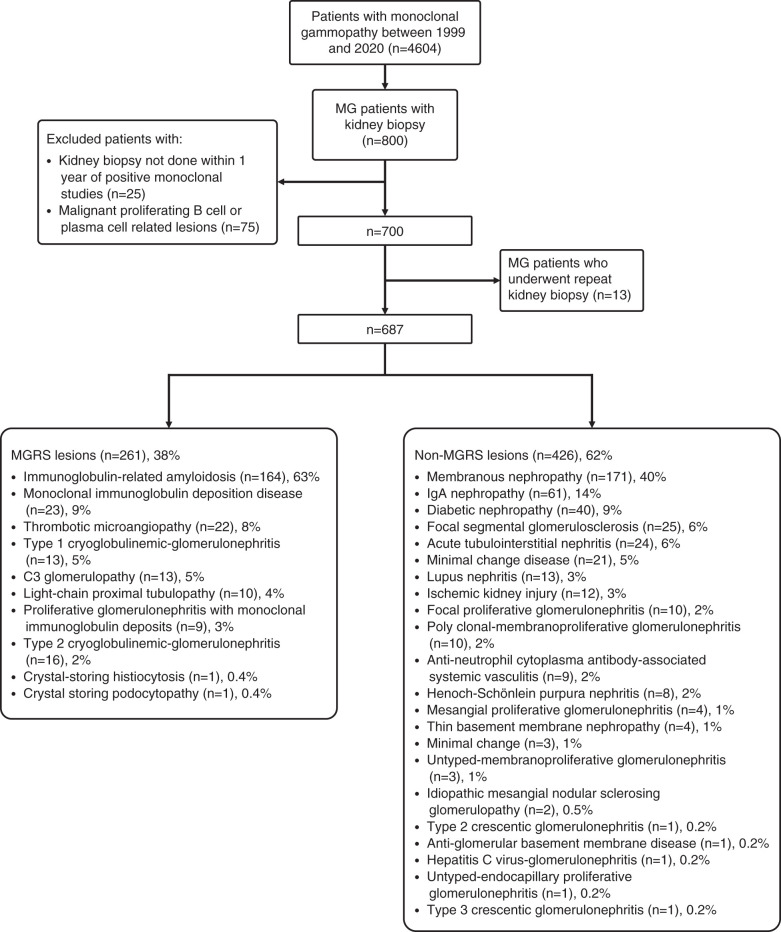
We screened all patients who had at least one positive serum or urine immunofixation result tested in the Peking University First Hospital from January 1, 1999 to December 31, 2020. We further checked the patients’ medical records and selected the patients with monoclonal gammopathy who underwent at least one kidney biopsy in the Peking University First Hospital. The overall clinical indications for kidney biopsy were 24-hour urine protein ≥1 g, Fanconi syndrome, or rising serum creatinine level. The clinical indications for kidney biopsy in patients with monoclonal gammopathy were 24-hour urine protein ≥0.5 g, complete or partial Fanconi syndrome, or rising serum creatinine. Then, a kidney biopsy was performed if the patients agreed and did not have contraindications for kidney biopsy. Patients who underwent repeated kidney biopsies were excluded due to misdiagnosed first kidney biopsy or developing monoclonal Ig during follow-up. Before or at the same time as kidney biopsy, patients who met hematologic criteria of multiple myeloma, Waldenström macroglobulinemia, chronic lymphocyte leukemia, or other hematologic malignancies were excluded. Patients with positive immunofixation over 12 months before or after the kidney biopsy were also excluded. The details for patient selection are shown in Figure 1.
Figure 1.
Study flow chart of patients with monoclonal gammopathy (MG) who underwent kidney biopsies and pathological spectrum from 1999 to 2020. MGRS, monoclonal gammopathy of renal significance.
Informed consent was obtained from each patient. Written informed consent for publication was obtained from the patients, and a copy of the written consent is available upon request. The research was in compliance with the Declaration of Helsinki and approved by the ethics committee of the Peking University First Hospital.
Clinical and Laboratory Assessment
The clinical and laboratory data at kidney biopsy are set as the baseline for analysis, including age, sex, history of hypertension and diabetes mellitus, proteinuria, hematuria, serum albumin, serum creatinine, eGFR, serum complement 3 (C3), complement 4, monoclonal Ig, serum free light chain level, and bone marrow biopsy. C3 hypocomplementemia and hypoalbuminemia were considered with cutoffs of 60 mg/dl and 30 g/L, respectively. The eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation. Abnormal free light chain ratio was identified if patients with eGFR≥60 ml/min per 1.73 m2 had a ratio outside the range of 0.27–1.65 or patients with an eGFR<60 ml/min per 1.73 m2 had a ratio outside the range of 0.37–3.10 (9). Abnormal bone marrow samples are defined as abnormal plasma cell or B cell clone, which had light chain–restricted expression or abnormal cluster of differentiation expression detected in bone marrow flow cytometry. All missing data are shown in Table 1, and patients with missing data were not included in the analysis of the corresponding indicators.
Table 1.
Characteristics of patients with monoclonal gammopathy who underwent kidney biopsy from 1999 to 2020 in the Peking University First Hospital
| Characteristics | Overall, n=687 | Monoclonal Gammopathy of Renal Significance Lesions, n=261 | Nonmonoclonal Gammopathy of Renal Significance Lesions, n=426 | P Value |
|---|---|---|---|---|
| Age, yr | 56 (49–66) | 58 (51–66) | 56 (47–66) | 0.28 |
| Men, n (%) | 457 (67) | 162 (62) | 295 (69) | 0.05 |
| Hypertension, n (%) | 439 (64) | 145 (56) | 294 (69) | <0.001 |
| Diabetes mellitus, n (%) | 154 (22) | 38 (15) | 116 (27) | <0.001 |
| Serum studies | ||||
| Albumin, g/dl | 2.9 (2.2–3.5) | 2.8 (2.2–3.3) | 3.0 (2.3–3.7) | 0.006 |
| Albumin <3.0 g/dl, n (%) | 381 (55) | 159 (61) | 222 (52) | 0.02 |
| Hemoglobin, g/dl | 12.0 (10.2–13.8) | 12.1 (10.2–13.9) | 12.0 (10.2–13.6) | 0.36 |
| Creatinine, mg/dl | 1.8 (0.8–2.1) | 1.8 (0.8–2.2) | 1.8 (0.8–2.0) | 0.80 |
| eGFR, ml/min per 1.73 m2 | 63 (32–91) | 60 (28–89) | 64 (35–92) | 0.25 |
| Complement 3, mg/dl | 88±27 (600/687) | 85 (65–105, 235/261) | 89±25 (365/426) | 0.02 |
| Complement 3 <60, mg/dl, n (%) | 91/600 (15) | 44/235 (19) | 47/365 (13) | 0.05 |
| Complement 4, mg/dl | 25 (18–29, 591/687) | 24 (17–30, 230/261) | 25 (19–29, 361/426) | 0.61 |
| Urinary studies | ||||
| Urinary protein, g/d | 4.9 (1.5–6.5) | 5.0 (2.3–6.6) | 4.9 (1.2–6.5) | 0.01 |
| Proteinuria ≥1.5 g/d, n (%) | 511 (74) | 212 (81) | 299 (70) | 0.001 |
| Hematuria | 371 (54) | 117 (45) | 254 (60) | <0.001 |
| Hematologic studies | ||||
| κ-free light chain, mg/L | 106 (20–68, 235/687) | 167 (15–133, 103/261) | 57 (21–58, 132/426) | 0.81 |
| λ-free light chain, mg/L | 98 (26–93, 235/687) | 130 (24–114, 103/261) | 73 (27–75, 132/426) | 0.19 |
| Free light chain-κ/λ ratio | 7.6 (0.4–1.6, 235/687) | 16.0 (0.2–2.9, 103/261) | 1.1 (0.6–1.2, 132/426) | 0.32 |
| Abnormal free light chain ratio | 95/235 (40) | 66/103 (64) | 29/132 (22) | <0.001 |
| Received bone marrow biopsy, n (%) | 521 (76) | 230 (88) | 291 (68) | <0.001 |
| Plasma cell percentage | 2.1 (0.5–2.5, 518/687) | 2.9 (1.0–4.0, 230/518) | 1.5 (0.5–2.0, 288/518) | <0.001 |
| Plasma cell percentage ≥10%, n (%) | 10/518 (2) | 8/230 (4) | 2/288 (1) | 0.02 |
| Abnormal bone marrow biopsy specimen, n (%) | 232/359 (65) | 121/146 (83) | 111/213 (52) | <0.001 |
Values for continuous variables are described as mean ± SD or median (interquartile range) depending on the distribution, and for categoric variables, they are described as count (percentage).
MGRS kidney injuries were classified according to the 2018 consensus by the International Kidney and Monoclonal Gammopathy (IKMG) Research Group (8). Thrombotic microangiopathy was considered as an MGRS lesion only if no other causes of thrombotic microangiopathy (such as atypical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, drugs, or underlying autoimmune diseases) were identified (10,11).
Because Ig-related amyloidosis is the most common pathologic phenotype of MGRS lesions, we performed a further comparison between Ig-related amyloidosis and other MGRS lesions.
Kidney Histopathology Assessment
Kidney biopsy was examined by standard direct immunofluorescence, light microscopy, and electron microscopy. Immunofluorescence staining on frozen tissue included IgG, IgA, IgM, C3, C1q, fibrin-related antigens, albumin, κ, and λ. Kidney biopsy specimens were fixed in 4% buffered formaldehyde for light microscopy. Consecutive serial 3-μm sections were used for histologic staining, including hematoxylin and eosin, periodic acid–silver methenamine, periodic acid–Schiff staining, and Masson trichrome. Immunofluorescence or immunohistochemistry staining on paraffin tissue after enzyme digestion, immunoelectron microscopy, and mass spectrometry were performed if necessary. Slides were reviewed by two experienced kidney pathologists independently. Differences between the two pathologists were resolved by rereviewing the slides to reach a consensus.
Statistical Analyses
SPSS 24.0 statistical software was used for statistical analysis. The Kolmogorov–Smirnov test was used for the normality test for continuous variables. Continuous variables were expressed as mean ± SD if normally distributed and median (interquartile range [IQR]) if not. Categorical variables were presented as number and percentage. Continuous data were compared using the t test for normally distributed variables. Non-normally distributed variables were compared using the Mann–Whitney U test. Differences between groups of semiquantitative data were tested with the Mann–Whitney U test. Categorical variables were compared using the chi-squared test. Multivariable logistic regression was used to analyze the odds ratios (ORs) for developing MGRS. P=0.05 was considered statistically significant. We chose the number of variables in the multivariable model on the basis of the rule of ten events per one predicting variable.
Results
Study Populations
From 1999 to 2020, 4604 patients with monoclonal gammopathy were identified, and 800 of them underwent at least one kidney biopsy. One hundred patients were excluded due to meeting hematologic criteria for specific therapy or positive immunofixation over 12 months before or after the kidney biopsy. Among the 700 patients, 13 patients underwent repeated kidney biopsies, and they were excluded due to misdiagnosed first kidney biopsy or developing monoclonal Ig during follow-up. Thus, we identified 687 patients who met the inclusion criteria (Figure 1). In those patients, 261 patients (38%) had MGRS lesions. There were no patients who had premalignant conditions, such as smoldering multiple myeloma or smoldering Waldenström macroglobulinemia, at the time of or before kidney biopsy in our study. In the nonamyloidosis MGRS group, 11 patients with nonamyloidosis MGRS were diagnosed with the help of immunofluorescence staining on paraffin tissue, and eight patients with nonamyloidosis MGRS were diagnosed with the help of immunoelectron microscopy staining. Four patients were diagnosed with the help of both. Fifty-nine patients with amyloidosis MGRS were diagnosed with the help of mass spectrometry, including five patients with early-stage amyloidosis who also received immunoelectron microscopy staining.
Monoclonal Ig-related amyloidosis was the most common MGRS type (n=164, 63%), followed by monoclonal Ig deposition disease (n=23, 9%) and thrombotic microangiopathy (n=22, 8%). Six patients had MGRS accompanied with non-MGRS lesions, and all were included in the MGRS group for statistics. Membranous nephropathy was the most common type (n=171, 40%) in the non-MGRS lesion group, followed by IgA nephropathy (n=61, 14%) and diabetic nephropathy (n=40, 9%). Other lesions are summarized in Figure 1.
Baseline Characteristics and Pathologic Manifestations of Patients with Monoclonal Gammopathy of Renal Significance versus Patients without Monoclonal Gammopathy of Renal Significance
The clinical and laboratory data of patients who underwent kidney biopsy are summarized in Table 1. In comparison with patients without MGRS, patients with MGRS lesions were less likely to have hypertension (56% versus 69%; P<0.001), diabetes (15% versus 27%; P<0.001), and hematuria (45% versus 60%; P<0.001). Patients with MGRS had a higher level of 24-hour urinary protein (5.0; IQR, 2.3–6.6 g/d versus 4.9; IQR, 1.2–6.5 g/d; P=0.01) with a higher percentage of proteinuria >1.5 g/d (81% versus 70%; P=0.001), a lower serum albumin (2.8; IQR, 2.2–3.3 g/dl versus 3.0; IQR, 2.3–3.7 g/dl; P=0.006), and higher prevalence of hypoalbuminemia (61% versus 52%; P=0.02). Patients with MGRS also had a lower serum C3 (85; IQR, 65–105 [235 of 261] mg/dl versus 89±25 [365 of 426] mg/dl; P=0.02) but did not show obvious C3 hypocomplementemia. Among the 235 (34%) patients with free light chain data, we found that patients in the MGRS group had a significantly higher abnormal free light chain ratio (64% versus 22%; P<0.001). We also found that patients with MGRS had a higher possibility to receive a bone marrow biopsy (88% versus 68%; P<0.001) and high probability to have an abnormal bone marrow biopsy specimen (121 of 146 [83%] versus 111 of 213 [52%]; P<0.001). Patients with MGRS had a higher percentage of plasma cells (2.9; IQR, 1.0–4.0 [230 of 518] versus 1.5; IQR, 0.5–2.0 [288 of 518]; P<0.001) and were more likely to have a plasma cell percentage over 10% (eight of 230 [3%] versus two of 288 [1%]; P=0.02) in the bone marrow specimen. Other clinical parameters, such as age, sex, eGFR, serum creatinine, serum complement 4, and hemoglobin, showed no significant differences between patients with MGRS and patients without MGRS. More details of monoclonal gammopathy type information are shown in Supplemental Table 1.
We incorporated factors such as age, sex, proteinuria ≥1.5 g/d, diagnosis of diabetes mellitus, hematuria, abnormal free light chain ratio, hypertension, C3 hypocomplementemia, and hypoalbuminemia into the multivariable logistic model; 201 patients with a complete sample of clinical data were included in the regression model, in which 91 of the 201 were patients with MGRS. The distribution of age samples is from 20 to 83 years old. We divided the age indicators into seven groups per 10 years. The clinical indicators associated with MGRS lesions were the presence of an abnormal free light chain ratio (OR, 5.57; 95% CI, 2.90 to 10.69; P<0.001), age per 10 years older (OR, 1.37; 95% CI, 1.04 to 1.79; P=0.03), and proteinuria ≥1.5 g/d (OR, 2.33; 95% CI, 1.01 to 5.37; P=0.05) (Table 2).
Table 2.
Clinical characteristics associated with a diagnosis of monoclonal gammopathy of renal significance in patients with monoclonal gammopathy
| Indicators | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Age per 10 yr older | 1.37 (1.04 to 1.79) | 0.03 |
| Men | 0.54 (0.27 to 1.09) | 0.09 |
| Diabetes mellitus | 0.63 (0.30 to 1.33) | 0.23 |
| Proteinuria ≥1.5 g/d | 2.33 (1.01 to 5.37) | 0.05 |
| Abnormal free light chain ratio | 5.57 (2.90 to 10.69) | <0.001 |
| Hematuria | 1.09 (0.57 to 2.11) | 0.79 |
| Hypertension | 0.69 (0.32 to 1.48) | 0.34 |
| Complement 3 hypocomplementemia | 1.81 (0.80 to 4.07) | 0.16 |
| Hypoalbuminemia | 0.63 (0.29 to 1.36) | 0.24 |
Associations are tested using multivariable linear regression, and all of the characteristics included in the model are listed in the table.
Clinical Characteristics of Patients with Immunoglobulin Amyloidosis versus Nonamyloidosis Monoclonal Gammopathy of Renal Significance
We further compared clinical data between patients with amyloidosis and patients without amyloidosis. Patients with amyloidosis were significantly older (60; IQR, 53–67 versus 54; IQR, 47–62; P<0.001), more likely to have hypoalbuminemia (76% versus 36%; P<0.001), and more likely to have nephrotic proteinuria (74% versus 41%; P<0.001). The percentages of patients with hypertension (38% versus 86%; P<0.001), diabetes mellitus (9% versus 24%; P=0.001), hematuria (30% versus 69%; P<0.001), stage 3 CKD (13% versus 33%; P<0.001), stage 4 CKD (12% versus 24%; P=0.02), and stage 5 CKD (5% versus 19%; P<0.001) were significantly lower. Patients with amyloidosis had a significantly higher level of 24-hour urinary protein (5.7; IQR, 3.4–7.2 versus 3.9; IQR, 0.9–5.3; P<0.001), a higher percentage of proteinuria >1.5 g/d (93% versus 62%; P<0.001), and a lower level of serum albumin (2.5; IQR, 2.1–3.0 versus 3.2±0.7; P<0.001). The eGFR was higher in patients with amyloidosis (72; IQR, 45–97 versus 41; IQR, 18–59; P<0.001). Among the 103 patients with MGRS and free light chain data, we found that patients with amyloidosis had a higher percentage of abnormal free light chain ratio (76% versus 52%; P<0.001). For the patients who underwent bone marrow biopsy, we found that patients with amyloidosis had a higher plasma cell percentage (3.7; IQR, 1.0 of 5.1 [144 of 164] versus 1.6; IQR, 0.5–2.0 [86 of 97]; P<0.001), were more likely to have a plasma cell percentage over 10% (eight of 144 [6%] versus zero of 86 [0%]; P=0.03), and were more likely to have an abnormal bone marrow biopsy specimen (75 of 82 [91%] versus 46 of 64 [72%]; P=0.002) (Table 3).
Table 3.
Characteristics of patients with amyloidosis compared with other causes of monoclonal gammopathy of renal significance
| Characteristics | Amyloidosis-Related Monoclonal Gammopathy of Renal Significance Lesions, n=164 | Nonamyloidosis Monoclonal Gammopathy of Renal Significance Lesions, n=97 | P Value |
|---|---|---|---|
| Age, yr | 60 (53–67) | 54 (47–62) | <0.001 |
| Men, n (%) | 104 (63) | 58 (60) | 0.56 |
| Hypertension, n (%) | 62 (38) | 83 (86) | <0.001 |
| Diabetes mellitus, n (%) | 15 (9) | 23 (24) | 0.001 |
| CKD, n (%) | |||
| Stage 3 | 22 (13) | 32 (33) | <0.001 |
| Stage 4 | 20 (12) | 23 (24) | 0.02 |
| Stage 5 | 8 (5) | 18 (19) | <0.001 |
| Serum studies | |||
| Albumin, g/dl | 2.5 (2.1–3.0) | 3.2±0.7 | <0.001 |
| Albumin <3.0 g/dl, n (%) | 124 (76) | 35 (36) | <0.001 |
| Hemoglobin, g/dl | 13.0 (11.9–14.3) | 10.6 (8.6–12.1) | <0.001 |
| Creatinine, mg/dl | 1.5 (0.7–1.4) | 2.5 (1.2–3.0) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 72 (45–97) | 41 (18–59) | <0.001 |
| Complement 3, mg/dl | 95 (74–113, 144/164) | 71±24 (91/97) | <0.001 |
| Complement 4, mg/dl | 28 (19–33, 140/164) | 19±10 (90/97) | <0.001 |
| Urinary studies | |||
| Urinary protein, g/d | 5.7 (3.4–7.2) | 3.9 (0.9–5.3) | <0.001 |
| Proteinuria ≥1.5 g/d, n (%) | 152 (93) | 60 (62) | <0.001 |
| Hematuria, n (%) | 50 (30) | 67 (69) | <0.001 |
| Nephrotic proteinuria ≥3.5 g/d, n (%) | 122 (74) | 40 (41) | <0.001 |
| Hematologic studies | |||
| Clonality | |||
| Monoclonal | 156 (95) | 91 (94) | Reference |
| Biclonal | 8 (5) | 6 (6) | 0.65 |
| κ-free light chain, mg/L | 193 (11–40, 51/164) | 143 (29–162, 52/97) | <0.001 |
| λ-free light chain, mg/L | 151 (34–135, 51/164) | 110 (20–92, 52/97) | 0.12 |
| Free light chain-κ/λ ratio | 25.4 (0.1–0.7, 51/164) | 6.8 (0.6–4.9, 52/97) | <0.001 |
| Abnormal free light chain ratio, n (%) | 39/51 (76) | 27/52 (52) | <0.001 |
| Received bone marrow biopsy | 144 (88) | 86 (89) | 0.84 |
| Plasma cell, % | 3.7 (1.0–5.1, 144/164) | 1.6 (0.5–2.0, 86/97) | <0.001 |
| Plasma cell ≥10%, n (%) | 8/144 (6) | 0/86 (0) | 0.03 |
| Abnormal bone marrow biopsy specimen, n (%) | 75/82 (91) | 46/64 (72) | 0.002 |
Values for continuous variables are described as mean ± SD or median (interquartile range) depending on the distribution, and for categoric variables, they are described as count (percentage).
Discussion
Over the last few decades, the prevalence of CKD and monoclonal gammopathy has increased rapidly. The monoclonal Igs have been increasingly recognized to cause kidney damage. In fact, monoclonal Igs can induce damages to multiple end organs. A new concept of monoclonal gammopathy of clinical significance was introduced to characterize these situations (12). MGRS is a special monoclonal gammopathy of clinical significance with kidney-dominant or isolated kidney damages by the monoclonal Igs. Here, we conducted the largest retrospective MGRS cohort study.
MGRS accounted for 2%–10% of the MGUS population according to previous studies (13–15). Compared with MGUS, which does not require treatment, patients with MGRS should receive specific antiplasma cell or B cell treatment, such as chemotherapy with bortezomib or high-dose melphalan with autologous peripheral blood stem cell transplantation, in order to slow the progression of kidney function and preserve other organs according to the IKMG consensus (16). These specific treatments are also used in the treatment of multiple myeloma with adverse effects and are usually expensive (17). Therefore, it is essential to identify patients with MGRS from patients without MGRS. MGRS can only be diagnosed using kidney biopsy. In our cohort, MGRS accounted for 38% of patients with monoclonal gammopathy and CKD who underwent kidney biopsy, which was similar to the Mayo clinic report (40%), indicating that MGRS is an important and common cause of CKD in these patients (18). Our study showed that the presence of an abnormal free light chain ratio, age (per 10 years older), and proteinuria ≥1.5 g/d were identified as independent clinical indicators associated with MGRS by multivariable logistic model, which is similar, but not the same as the Mayo Clinic study result that the presence of an abnormal free light chain ratio, proteinuria ≥1.5 g/d, and hematuria as potential clinical indicators associated with MGRS. These results could help nephrologists and hematologists to make more appropriate decisions to recommend patients to receive an invasive kidney biopsy. In our hospital, the free light chain test has only been available in recent years in China. Up to now, this test was only performed in a few independent laboratories, which made the free light chain test expensive, and it cannot be reimbursed by medical insurance. However, it is still difficult for patients with MGRS to test for free light chain due to economic reasons. Still, in the multivariable logistic regression analysis of clinical indictors of MGRS in patients with monoclonal gammopathy, all of the 91 patients with MGRS and 110 patients had free light chain test. By comparison of the two groups and multivariable logistic regression analysis, the abnormal free light chain test was associated with MGRS. Therefore, despite the lack of free light chain tests, our study and the Mayo Clinic study both supported the importance of the abnormal free light chain ratio as an indicator of MGRS. Unlike the Mayo Clinic study, hematuria was not a clinical indicator associated with MGRS in our study, and the percentage of patients with hematuria in our MGRS group was even less. One main possible reason is that GN is still the main cause of kidney injury in China in patients with kidney biopsies, especially IgA nephropathy, accounting for 14% in the non-MGRS group. Hematuria is the main manifestation of GN. Therefore, hematuria might not be a suitable predictive indicator of MGRS in Chinese patients with CKD and concomitant monoclonal gammopathy. In our cohort, we also identified age per 10 years older as a clinical indictor of MGRS, which indicates that the older Chinese patients with monoclonal gammopathy are more likely to be diagnosed with MGRS, considering all variables in the regression model.
Consistent with previous studies (18,19), we found that amyloidosis was the most common MGRS type in our cohort. Patients with amyloidosis have more severe proteinuria and a lower percentage of hypertension. In the non-MGRS lesion group, membranous nephropathy was the most common histopathologic type compared with arteriosclerosis in the Mayo Clinic study. This might be due to the reasons that both membranous nephropathy and monoclonal gammopathy occurred dominantly in the elderly and that membranous nephropathy is more common in China, especially in north China, with a possible link to environment pollution (20). Occasionally, MGRS, such as light-chain amyloidosis, was accompanied with non-MGRS lesions, such as IgA nephropathy and membranous nephropathy.
C3 glomerulopathy and thrombotic microangiopathy are special MGRS lesions that monoclonal Ig acted indirectly on by inferring the complement system. Functional tests are needed to verify the indirect mechanism, which is relatively difficult. In our study, we followed the latest consensus of MGRS that “the diagnosis of MGRS can be established only by performing a kidney biopsy that either demonstrates the presence of monotypic Ig deposits or infers their involvement in the case of C3 glomerulonephritis or thrombotic microangiopathy with a circulating monoclonal Ig” (8). In our study, patients with C3 glomerulopathy or thrombotic microangiopathy combined with monoclonal gammopathy were considered as having MGRS after exclusion of other causes, such as diarrhea-positive hemolytic uremic syndrome or thrombotic thrombocytopenia purpura. In addition, in our cohort, two patients with C3 glomerulopathy had functional tests to verify the indirect mechanism of monoclonal Ig, and one was published (21). Another two patients with C3 glomerulopathy showed kidney complete or partial remission after bortezomib-based chemotherapy, which might support a role of monoclonal Ig in the pathogenesis of C3 glomerulopathy. Other patients who did not receive chemotherapy were diagnosed before the new consensus of MGRS, the patients declined, or patients had contraindications for chemotherapy. We will perform in vitro functional experiments for other patients in our future study.
MGRS was a common and important cause of kidney injury in patients with monoclonal gammopathy and CKD, and amyloidosis was the leading cause. The clinical indicators associated with MGRS were the presence of an abnormal free light chain ratio, age per 10 years older, and proteinuria ≥1.5 g/d.
Disclosures
M.-h. Zhao reports consultancy agreements with AstraZeneca, GSK, INFLARX, Novartis, and Roche; honoraria from the Chinese Medical Association and the Chinese Society of Nephrology; and serving as an executive member of the Asian-Pacific Society of Nephrology and as Vice President of the Chinese Society of Nephrology. All remaining authors have nothing to disclose.
Funding
This study was supported by National Natural Science Foundation of China grants 81470956 and 81500543.
Supplementary Material
Acknowledgments
The funders had a role in data collection, analysis, and reporting.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
Z.-h. Yong and X.-j. Yu conceptualized the study; J.-x. Liu, S.-x. Wang, Z.-h. Yong, X.-j. Yu, M.-h. Zhao, and F.-d. Zhou were responsible for data curation; Z.-h. Yong and X.-j. Yu were responsible for investigation; Z.-h. Yong and X.-j. Yu were responsible for formal analysis; Z.-h. Yong and X.-j. Yu were responsible for methodology; J.-x. Liu, M.-h. Zhao, and F.-d. Zhou were responsible for project administration; J.-x. Liu, S.-x. Wang, X.-j. Yu, M.-h. Zhao, and F.-d. Zhou were responsible for resources; Z.-h. Yong and X.-j. Yu were responsible for validation; Z.-h. Yong was responsible for visualization; S.-x. Wang, X.-j. Yu, and M.-h. Zhao were responsible for funding acquisition; J.-x. Liu, X.-j. Yu, M.-h. Zhao, and F.-d. Zhou provided supervision; Z.-h. Yong and X.-j. Yu wrote the original draft; and X.-j. Yu and M.-h. Zhao reviewed and edited the manuscript.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12890921/-/DCSupplemental.
Supplemental Table 1. Type of monoclonal gammopathy of patients with MGRS versus patients without MGRS.
References
- 1.Kyle RA: Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med 64: 814–826, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M; International Myeloma Working Group : Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24: 1121–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA; International Kidney and Monoclonal Gammopathy Research Group : Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood 120: 4292–4295, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ 3rd: Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354: 1362–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Yu XJ, Wang MJ, Yong ZH, Ma YY, Wang SX, Zhou FD, Zhao MH: Proliferative glomerulonephritis with monoclonal IgG3λ deposits: A case report of a rare cause of monoclonal gammopathy of renal significance. Kidney Med 1: 221–225, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD: Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 11: e0158765, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, Dispenzieri A, Fervenza FC, Fermand JP, Gibbs S, Gillmore JD, Herrera GA, Jaccard A, Jevremovic D, Kastritis E, Kukreti V, Kyle RA, Lachmann HJ, Larsen CP, Ludwig H, Markowitz GS, Merlini G, Mollee P, Picken MM, Rajkumar VS, Royal V, Sanders PW, Sethi S, Venner CP, Voorhees PM, Wechalekar AD, Weiss BM, Nasr SH: The evaluation of monoclonal gammopathy of renal significance: A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol 15: 45–59, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchison CA, Harding S, Hewins P, Mead GP, Townsend J, Bradwell AR, Cockwell P: Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1684–1690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindran A, Go RS, Fervenza FC, Sethi S: Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int 91: 691–698, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Yui JC, Garceau D, Jhaveri KD, Wanchoo R, Bijol V, Glezerman I, Hassoun H, Dispenzieri A, Russell SJ, Leung N: Monoclonal gammopathy-associated thrombotic microangiopathy. Am J Hematol 94: E250–E253, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G: Monoclonal gammopathy of clinical significance: A novel concept with therapeutic implications. Blood 132: 1478–1485, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Merlini G, Palladini G: Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology (Am Soc Hematol Educ Program) 2012: 595–603, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ciocchini M, Arbelbide J, Musso CG: Monoclonal gammopathy of renal significance (MGRS): The characteristics and significance of a new meta-entity. Int Urol Nephrol 49: 2171–2175, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Steiner N, Göbel G, Suchecki P, Prokop W, Neuwirt H, Gunsilius E: Monoclonal gammopathy of renal significance (MGRS) increases the risk for progression to multiple myeloma: An observational study of 2935 MGUS patients. Oncotarget 9: 2344–2356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, Drayson MT, Dispenzieri A, Leung N; International Kidney and Monoclonal Gammopathy Research Group : How I treat monoclonal gammopathy of renal significance (MGRS). Blood 122: 3583–3590, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Landgren O, Iskander K: Modern multiple myeloma therapy: Deep, sustained treatment response and good clinical outcomes. J Intern Med 281: 365–382, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Klomjit N, Leung N, Fervenza F, Sethi S, Zand L: Rate and predictors of finding monoclonal gammopathy of renal significance (MGRS) lesions on kidney biopsy in patients with monoclonal gammopathy. J Am Soc Nephrol 31: 2400–2411, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie S, Wang M, Wan Q, Kong Y, Ou J, Jia N, Zhang X, Luo F, Liu X, Wang L, Cao Y, Chen R, Zhao M, Chan DYL, Wang G: Kidney biopsy in patients with monoclonal gammopathy: A multicenter retrospective cohort study. Front Med (Lausanne) 8: 687149, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF: Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 27: 3739–3746, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li LL, Li ZY, Wang SX, Yu XJ, Tan Y, Wang Y, Yu F, Zhao MH: Monoclonal immunoglobulin mediates complement activation in monoclonal gammopathy associated-C3 glomerulonephritis. BMC Nephrol 20: 459, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.