Visual Abstract
Keywords: ADPKD, clinical trial, diabetes insipidus, diuretics, receptors, vasopressin, hydrochlorothiazide, metformin
Abstract
Background and objectives
The vasopressin V2 receptor antagonist tolvaptan is the only drug that has been proven to be nephroprotective in autosomal dominant polycystic kidney disease (ADPKD). Tolvaptan also causes polyuria, limiting tolerability. We hypothesized that cotreatment with hydrochlorothiazide or metformin may ameliorate this side effect.
Design, setting, participants, & measurements
We performed a clinical study and an animal study. In a randomized, controlled, double-blind, crossover trial, we included 13 tolvaptan-treated patients with ADPKD. Patients were treated for three 2-week periods with hydrochlorothiazide, metformin, or placebo in random order. Primary outcome was change in 24-hour urine volume. We also measured GFR and a range of metabolic and kidney injury markers.
Results
Patients (age 45±8 years, 54% women, measured GFR of 55±11 ml/min per 1.73 m2) had a baseline urine volume on tolvaptan of 6.9±1.4 L/24 h. Urine volume decreased to 5.1 L/24 h (P<0.001) with hydrochlorothiazide and to 5.4 L/24 h (P<0.001) on metformin. During hydrochlorothiazide treatment, plasma copeptin (surrogate for vasopressin) decreased, quality of life improved, and several markers of kidney damage and glucose metabolism improved. Metformin did not induce changes in these markers or in quality of life. Given these results, the effect of adding hydrochlorothiazide to tolvaptan was investigated on long-term kidney outcome in an animal experiment. Water intake in tolvaptan-hydrochlorothiazide cotreated mice was 35% lower than in mice treated with tolvaptan only. Combination treatment was superior to “no treatment” on markers of disease progression (kidney weight, P=0.003 and cystic index, P=0.04) and superior or equal to tolvaptan alone.
Conclusions
Both metformin and hydrochlorothiazide reduced tolvaptan-caused polyuria in a short-term study. Hydrochlorothiazide also reduced polyuria in a long-term animal model without negatively affecting nephroprotection.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_03_21_CJN11260821.mp3
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease (1). Recently, studies have shown that the vasopressin V2 receptor antagonist tolvaptan slows the rate of growth in kidney volume and attenuates kidney function decline (2,3). As a side effect, tolvaptan also causes nephrogenic diabetes insipidus (4–6), resulting in polyuria with, on average, 6–8 L/d (7), which limits the clinical use of the only proven treatment for this disease.
Recent research suggested that metformin may be a vasopressin-independent activator of water transport in the rat inner kidney medulla (8) and showed that it decreased polyuria by 50% in tolvaptan-treated rats (9). The diuretic hydrochlorothiazide is an established treatment for nephrogenic diabetes insipidus, known to lower urine output by around 30% (10–12). Whether the addition of these treatments to tolvaptan is safe and whether it influences aquaresis are unknown.
In this study, we investigated the short-term effects of the addition of hydrochlorothiazide or metformin to tolvaptan on polyuria, tolerability, safety, and markers of progression in patients with ADPKD and on long-term polyuria and nephroprotection in a mouse ADPKD model.
Materials and Methods
The manuscript combines data from two experiments: a clinical short-term study in patients with ADPKD and an experimental longer-term study in an ADPKD mouse model; they are described separately.
Clinical Study
Trial Design and Participants.
This investigator-driven study was designed as a double-blind, randomized, controlled, crossover trial, and it was performed at the University Medical Center Groningen (UMCG). The clinical trial was in accordance with the Declaration of Helsinki and approved by the medical ethical committee of UMCG. All participants provided written informed consent before entry into the trial. The trial was listed in The Netherlands Trial Register before first inclusion (NL6546).
Initially, patients were eligible for inclusion if they were 18–50 years of age, had a diagnosis of ADPKD (13), had an eGFR ≥45 ml/min per 1.73 m2, were treated with tolvaptan (indication on the basis of criteria by the ERA-EDTA workgroup [14], the highest tolerated dose), and provided informed consent. The inclusion criteria age and baseline eGFR were broadened (age 18–55 years, amended October 10, 2018; eGFR ≥30 ml/min per 1.73 m2, amended June 3, 2019) to be able to include the projected number of participants.
Exclusion criteria were potential safety risk in the opinion of the investigators, unlikeliness to adequately comply with the trials’ procedures, concomitant use of medication or illnesses likely to confound end point assessments, pregnancy or breastfeeding, and known contraindications or allergies to the study medication. Specific examples of exclusion criteria can be found in Supplemental Material.
Randomization and Interventions.
An independent pharmacist used a computer program to randomize participants; this is further detailed in Supplemental Material. All patients, investigators, and health care providers were blinded to treatment allocation. Patients were randomized to receive hydrochlorothiazide (in the morning, 12.5 mg in week 1 and 25 mg in week 2, with placebo in the evening), metformin (500 mg twice a day in week 1 and 1000 mg twice a day in week 2), or matching double-blind placebo treatment (in the morning and evening) for 2 weeks each.
Procedures.
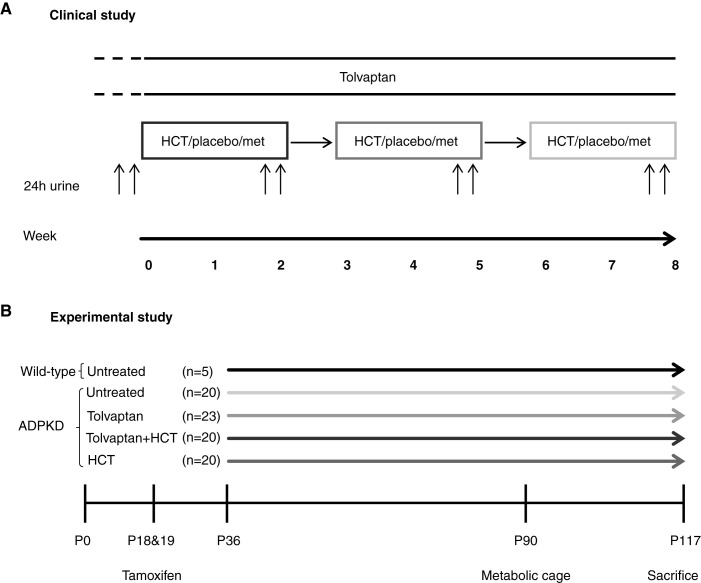
Figure 1A shows an overview of the trial design. During a screening visit, patients were instructed to adhere to their regular low-sodium (<6 g of salt per day) diet. A detailed description of the study procedures can be found in Supplemental Material.
Figure 1.
Design of the clinical and experimental study. (A) Design of the clinical randomized, controlled, crossover trial that included 13 patients with autosomal dominant polycystic kidney disease (ADPKD). Treatments are in random order. (B) Design of the experimental study that included 88 mice, of which 83 had ADPKD. Mice were treated with tamoxifen at postnatal day 18 (P18) and P19 to inactivate Pkd1. Drug treatment was started at P36: tolvaptan, tolvaptan plus hydrochlorothiazide (HCT), HCT, or no treatment. Animals were euthanized at P117. met, metformin.
Outcomes and Measurements.
The primary outcome in the trial was change in 24-hour urine volume, taking the average of the two urine volumes per period. Prespecified secondary outcomes were the effect of treatment on plasma copeptin (a surrogate marker of vasopressin), the effect on measured GFR, and tolerability of study medication. Outcome assessment was performed blinded to treatment allocation.
Some additional ad hoc outcomes were assessed to further analyze the method of action of the interventions. These outcomes were not prespecified, and results should therefore be interpreted as hypothesis generating rather than hypothesis testing. Electrolytes were assessed in plasma during the study. After study completion, several markers of damage to different parts of the nephron and of metabolic pathways were measured in thawed 24-hour urine samples: heart-type fatty acid binding protein; neutrophil gelatinase–associated lipocalin; β2-microglobulin; monocyte chemoattractant protein-1; and lactate, pyruvate, succinate, and pyruvate kinase M2 as metabolic markers. Several other markers of volume status and vasopressin activity were measured, such as fractional lithium excretion (15), aquaporin-2 excretion, and an estimate of extracellular volume using the single-shot iohexol clearance technique (16–19). Further details of the measurements can be found in Supplemental Material.
As another ad hoc outcome, the subjective effect of treatment on quality of life was assessed. Participants were asked three dichotomous questions (yes/no) at the end of each treatment period: whether compared with baseline, they experienced less urination, less thirst, and better quality of life.
Experimental Study
Study Design and Animals.
To test longer-term effects of adding hydrochlorothiazide to tolvaptan, we used a tamoxifen-inducible kidney epithelium–specific Pkd1 deletion mouse model (tam-KspCad-CreERT2; Pkd1lox2–1l/lox2–11) (20). Figure 1B shows the experimental protocol; details can be found in Supplemental Material. The animal experiment was approved by the Dutch Central Committee of Animal Experiments (reference no. AVD1050020186705).
Randomization and Interventions.
Drug treatment began on postnatal day 36 (P36). Mice were randomized into four groups stratified for body weight and sex: untreated, tolvaptan, hydrochlorothiazide, and hydrochlorothiazide-tolvaptan cotreatment. We also included wild-type mice that remained untreated. There were two or three animals per cage; the number of cages with two or three animals was divided equally among treatment groups. The investigators were blinded to treatment allocation during the experiment. Treatment groups received food pellets supplemented with either 0.15% tolvaptan-SD and/or 0.035% hydrochlorothiazide. Details on treatment and food pellets are shown in Supplemental Material.
Procedures.
Animals were euthanized at P117 or in the case of 20% weight loss (humane end point), whichever occurred first. Blood was obtained by cardiac puncture, and kidneys were embedded in paraffin for histomorphometry and immunohistochemistry. More details are in Supplemental Material.
Outcomes and Measurements.
There were two main outcomes. The primary outcome was aquaresis evaluated by monitoring daily water intake (per cage) and by 24-hour urine collection at P90 (per individual). The main secondary outcome was ADPKD severity at euthanasia measured by kidney weight and kidney weight–body weight ratio. More details are in Supplemental Material.
Statistical Analyses
For the clinical study, we calculated that enrollment of at least ten patients was needed in the trial to have 80% power to detect a 1.8-L reduction in urine volume on a 6-L pretreatment average (30%) with a two-tailed α of 0.025 (0.05/2, applying a Bonferroni correction for the two treatments) using a paired sample t test. To allow for dropouts, we aimed to include 12 patients. More details are in Supplemental Material.
For the animal experiment, we calculated that we needed to enroll six mice in each group to be able to detect a 6-ml/24 h (30%) reduction in urine volume during tolvaptan of 20.0±3.5 ml/24 h average (21). Twenty-two animals per group were needed to show noninferiority with regard to kidney weight–body weight ratio. Further information on power analyses can be found in Supplemental Material.
Continuous variables are presented as mean±SD or median (25th to 75th percentiles). Categorical data are presented as percentages. For all tests, a P value of 0.05 was adopted to indicate statistical significance.
In the clinical study, we performed generalized linear mixed models with study periods as fixed effects and patients as random effects to compare the measurements during study periods. We tested for potential carryover effects by modeling the interaction between period × treatment and tested for a potential period effect. Because of the non-Gaussian distribution of the results in the animal study, all data are represented as median (interquartile range). The Mann–Whitney U test was used to compare treatment groups. P values are compared with untreated cystic mice unless otherwise specified. For statistical analyses, we used SPSS version 23 (IBM Corp., Armonk, NY) or Stata SE 14 (StataCorp, College Station, TX) in the case of linear mixed model analyses.
Results
Clinical Study
Between October 2018 and September 2019, 25 eligible patients were asked to participate in the study. Thirteen agreed to a screening visit, of whom all 13 were subsequently enrolled in a randomized, controlled, crossover study (Figure 1A). Follow-up lasted until 8 weeks after final recruitment (November 2019). All patients completed all treatment periods and were included in the analysis. There were no dropouts or missing data for the primary or secondary outcomes. Patients were randomized to the treatment sequence hydrochlorothiazide-metformin-placebo (n=2), hydrochlorothiazide-placebo-metformin (n=2), metformin-hydrochlorothiazide-placebo (n=2), metformin-placebo-hydrochlorothiazide (n=2), placebo-hydrochlorothiazide-metformin (n=3), or placebo-metformin-hydrochlorothiazide (n=2). The baseline characteristics of these patients are presented in Table 1 (per treatment sequence in Supplemental Table 1). On average, they were 45±8 years of age, 54% was women, and measured GFR was 55±11 ml/min per 1.73 m2. Eleven patients (85%) used tolvaptan at the maximum dose of 90/30 mg, one patient used 60/30 mg, and another used 45/15 mg. All patients were able to increase the study medication to the maximum dose in the second week of every treatment period.
Table 1.
Baseline characteristics
| Characteristic | Total, n=13 |
|---|---|
| Age, yr | 45±8 |
| Women, N (%) | 7 (54) |
| Weight, kg | 91±18 |
| Height, m | 1.79±0.13 |
| Systolic BP, mm Hg | 125±13 |
| Diastolic BP, mm Hg | 77±7 |
| Measured GFR, ml/min per 1.73 m2 | 55±11 |
| Mayo risk class, N (%) a | |
| 1A/1B (low-risk disease) | 4 (31) |
| 1C/1D/1E (high-risk disease) | 9 (69) |
| 2 (atypical) | 0 (0) |
| RAASi user, N (%) | 13 (100) |
| Urine volume, L/24 h | 6.87±1.39 |
| Osmolar excretion, mOsm/24 h | 1044±362 |
| Sodium excretion, mEq/24 h | 169±58 |
| Urea excretion, g/24 h | 14.0±0.2 |
| Copeptin, pmol/L | 25.4 (19.1–28.7) |
Variables are presented as mean±SD, as median (interquartile range) in the case of non-normal distribution, or as percentage for categorical variables. RAASi, renin-angiotensin II-aldosterone system inhibitor.
Mayo autosomal dominant polycystic kidney disease classification predicts prognosis and is on the basis of total kidney volume indexed for height and age (42).
Primary Outcome.
Baseline urine volume was 6.9±1.4 L/24 h. At the end of the hydrochlorothiazide treatment period, urine volume had decreased to 5.1±1.5 L/24 h (P<0.001), and at the end of the metformin treatment period, urine volume was 5.4±1.5 L/24 h (P<0.001) (Figure 2). Both changes were significantly different from placebo (both P<0.001). The hydrochlorothiazide treatment effect on urine volume (percentage change) was negatively correlated with baseline measured GFR (i.e., a larger polyuria-reducing effect at higher baseline measured GFR; Pearson correlation, St. β =−0.56, P=0.05). There was no correlation of the hydrochlorothiazide treatment effect with Mayo class.
Figure 2.
Urine volume and plasma copeptin concentration during the various treatment phases. Treatments were given in random order. (A) The 24-hour urine volume over the different treatment periods. (B) Plasma copeptin over the different treatment periods. The difference between HCT metformin versus placebo was tested. BL, baseline. **P=0.01; ***P<0.01.
The interaction term treatment × period did not yield statistically significant results, indicating that there was no carryover effect and that there was no evidence of a period effect.
Secondary Outcomes.
Secondary outcomes are summarized in Table 2. At baseline, plasma copeptin was 25±8 pmol/L. Hydrochlorothiazide decreased plasma copeptin by 5.6 pmol/L (95% confidence interval, −9.0 to −2.3; P=0.001), and metformin induced no change. During hydrochlorothiazide treatment, there was a statistically significant decrease in GFR from 55±11 to 51±10 ml/min per 1.73 m2. During metformin treatment, there was no change. A list of adverse events can be found in Table 3. There were no serious adverse events during the study. Gastrointestinal side effects were more common during metformin treatment (nine participants). Plasma potassium was lower during hydrochlorothiazide treatment compared with placebo (3.6±0.4 versus 3.9±0.2 mmol/L; P=0.01). The incidences of other adverse events were similar between treatment periods.
Table 2.
Main study parameters during the various treatment phases
| End Points | Baseline | Hydrochlorothiazide | Placebo | Metformin | |||
|---|---|---|---|---|---|---|---|
| Mean | Mean | P Value | Mean | P Value | Mean | P Value | |
| Primary end point | |||||||
| Absolute urine volume, L/24 h | 6.87±1.40 | 5.13±1.46a | <0.001a | 6.34±1.62a | 0.002a | 5.40±1.51a | <0.001a |
| Secondary end points | |||||||
| Copeptin, pmol/L | 25±8 | 20±7a | 0.006a | 26±7 | 0.90 | 28±8 | 0.40 |
| Measured GFR, ml/min per 1.73 m2 | 55±11 | 51±10a | <0.001a | 55±12 | 0.90 | 54±11 | 0.20 |
Variables are presented as mean±SD. Changes were compared with baseline using generalized linear mixed models.
P<0.05.
Table 3.
Adverse events
| Event | Hydrochlorothiazide | Placebo | Metformin |
|---|---|---|---|
| Adverse events | 13 | 10 | 24 |
| Participants with adverse event | 6 (46) | 5 (38) | 9 (69) |
| Serious adverse events | 0 | 0 | 0 |
| Adverse event leading to withdrawal | 0 | 0 | 0 |
| Gastrointestinal | 1 (8) | 2 (15) | 9 (69)a |
| Nausea | 1 (8) | 2 (15) | 2 (15) |
| Vomiting | 0 | 0 | 3 (23) |
| Diarrhea | 0 | 0 | 4 (31) |
| Flatulence | 0 | 0 | 1 (8) |
| Eructation | 0 | 0 | 2 (15) |
| Decreased appetite | 0 | 0 | 4 (31) |
| Bloated feeling | 0 | 0 | 2 (15) |
| Stomachache | 0 | 0 | 1 (8) |
| Mild hypokalemia, 3.0–3.5 mmol/L | 4 (31) | 0 | 0 |
| Severe hypokalemia, <3.0 mmol/L | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 |
| General discomfort | 1 (8) | 1 (8) | 1 (8) |
| Fatigue | 0 | 1 (8) | 0 |
| Headache | 1 (8) | 1 (8) | 1 (8) |
| Dizziness | 1 (8) | 1 (8) | 0 |
| Dry eyes | 0 | 0 | 1 (8) |
| Dry mouth | 0 | 0 | 1 (8) |
| Itchy throat | 1 (8) | 0 | 0 |
| Laryngitis | 0 | 1 (8) | 0 |
| Knee pain | 1 (8) | 0 | 0 |
| Peripheral edema | 1 (8) | 0 | 0 |
| Kidney pain | 1 (8) | 0 | 0 |
| Palpitations | 0 | 1 (8) | 0 |
| Paroxysmal atrial fibrillation | 0 | 0 | 1 (8) |
Values represent N (percentage).
P=0.05 compared with placebo.
Other Outcomes.
The 24-hour excretion of creatinine was similar during the three treatment periods, indicating correct collections of 24-hour urine samples. Osmolar excretion (as measure of osmolar intake) did not change during hydrochlorothiazide or metformin treatment, suggesting a stable diet (additional information is in Supplemental Material).
The changes in outcomes relevant to the mechanism of action of hydrochlorothiazide and metformin are summarized in Table 4. During hydrochlorothiazide treatment, there were decreases in body weight, N-terminal pro-brain natriuretic peptide (NTproBNP), systolic BP, and measured GFR, consistent with a decrease in extracellular volume. Fractional lithium excretion decreased during both hydrochlorothiazide and metformin treatment, indicating an increase in sodium reabsorption in the proximal tubule. Urinary aquaporin-2 excretion did not change during any treatment period.
Table 4.
Change in various study parameters
| End Points | Mean Value | Changes Compared with Placebo | |||||
|---|---|---|---|---|---|---|---|
| Hydrochlorothiazide | Placebo | Metformin | Hydrochlorothiazide | Metformin | |||
| Mean (95% Confidence Interval) | P Value | Mean (95% Confidence Interval) | P Value | ||||
| Primary end point | |||||||
| Absolute urine volume, L/24 h | 5.13±1.46 | 6.34±1.62 | 5.40±1.51 | –1.2 (–1.7 to –0.8)a | <0.001a | –0.9 (–1.4 to –0.5)a | <0.001a |
| Secondary end points | |||||||
| Plasma copeptin, pmol/L | 20±7 | 26±7 | 28±8 | –5.6 (–9.0 to –2.3)a | 0.001a | 1.6 (–1.7 to 5.0) | 0.30 |
| Measured GFR, ml/min per 1.73 m2 | 51±10 | 55±12 | 54±11 | –3.8 (–5.5 to –2.2)a | <0.001a | −1.1 (–2.7 to 0.5) | 0.20 |
| Post hoc exploratory end points | |||||||
| Free water clearance, L/24 h | 1.60±1.25 | 2.88±1.76 | 2.13±1.42 | –1.3 (–1.7 to –0.9)a | <0.001a | –0.7 (–1.1 to –0.3)a | <0.001a |
| Plasma osmolality, mOsm/L | 288±4 | 292±5 | 289±4 | –3.4 (–5.0 to –1.7)a | <0.001a | –2.1 (–3.8 to –0.5)a | 0.001a |
| Plasma sodium, mEq/L | 139±2 | 141±2 | 140±2 | –1.7 (–2.9 to –0.4)a | 0.008a | −0.6 (–1.9 to 0.6) | 0.30 |
| Plasma NTproBNP, ng/L | 43±23 | 82±52 | 53±28 | –40 (–57 to –23)a | <0.001a | –27 (–45 to –9)a | 0.003a |
| Urine osmolality, mOsm/L | 204±54 | 167±64 | 180±60 | 38 (27 to 49)a | <0.001a | 13 (2 to 24)a | 0.03a |
| Osmole excretion, mOsm/24 h | 1020±307 | 1013±296 | 947±311 | 6 (–68 to 79) | 0.90 | −64 (–137 to 9) | 0.09 |
| FeLithium, % | 18.4±3.4 | 21.8±3.4 | 19.3±5.4 | –3.5 (–5.4 to –1.6)a | <0.001a | –2.4 (–4.4 to –0.5)a | 0.01a |
| Aquaporin-2 excretion, g/24 h | 4.67±4.50 | 5.19±6.60 | 6.78±8.42 | 0.2 (–0.6 to 1.0) | 0.60 | 0.8 (–0.1 to 1.6) | 0.07 |
| Weight, kg | 90.6±18 | 91.6±18 | 91.2±18 | –1.0 (–1.5 to –0.4)a | <0.001a | −0.4 (–0.9 to 0.1) | 0.10 |
| Systolic BP, mm Hg | 117±11 | 122±9 | 120±12 | –5.6 (–9.8 to –1.4)a | 0.009a | −2.1 (–6.2 to 2.1) | 0.30 |
| Extracellular volume, L | 14.5±3.4 | 15.1±2.9 | 14.7±2.8 | −0.6 (–1.2 to 0.0) | 0.05 | −0.4 (–1.0 to 0.2) | 0.20 |
Variables are presented as mean±SD. Changes were compared with placebo using generalized linear mixed models. Other end points were ad hoc and not prespecified; results should be interpreted as hypothesis generating rather than hypothesis testing. NTproBNP, N-terminal pro-brain natriuretic peptide; FeLithium, fractional lithium excretion.
P<0.05.
Seven (54%) participants experienced better quality of life during hydrochlorothiazide treatment as compared with baseline (P=0.001). Quality of life was not significantly different from baseline during metformin (P=0.90) or placebo treatment (P=0.50) (Supplemental Figure 1).
Supplemental Table 2 shows the changes in the urinary excretions of damage and metabolic markers. During hydrochlorothiazide treatment, albumin, β2-microglobulin, heart-type fatty acid binding protein, and neutrophil gelatinase–associated lipocalin did not change, whereas monocyte chemoattractant protein-1 decreased (P=0.02). Damage markers were measured in frozen samples, which may have influenced the results. However, previous research showed that values remain fairly stable during prolonged frozen storage at −80°C (22,23).
Experimental Study
Because tolvaptan-hydrochlorothiazide cotreatment was most promising in the clinical study, the effect on long-term aquaresis and nephroprotection of the tolvaptan-hydrochlorothiazide combination was studied in a tamoxifen-inducible Pkd1 gene inactivation mouse model for polycystic kidney disease (24).
There were no baseline differences in weight or sex distribution between these five groups. Increase in body weight was similar for the different treatment groups.
Primary Outcomes.
Daily water intake is shown in Figure 3. Water intake was highest in the tolvaptan-treated mice and lower when tolvaptan was combined with hydrochlorothiazide. At P90, 24-hour urine was collected, and the results were consistent with water intake (Figure 3). The tolvaptan-hydrochlorothiazide combination resulted in a 39% lower urine volume compared with tolvaptan alone (3.9 versus 6.4 ml/24 h; P=0.02) and an increase in urine osmolality (711 versus 490 mOsm/L; P<0.001).
Figure 3.
Aquaresis in mice. (A) Water intake (measured daily during the experiment), with the first measured time point at P37. (B) Urine volume measured at day 90 in a metabolic cage. Both tolvaptan (Tolv) and Tolv plus HCT were statistically significant different from untreated (P<0.001 for both; not shown). *P<0.05.
At euthanasia, median body weight was 21.0 g (interquartile range, 19.6–23.8), with no differences across treatment groups (P=0.60). During the experiment, eight of 88 mice had 20% weight loss and were therefore euthanized before P117 (two untreated mice, five hydrochlorothiazide-treated mice, and one tolvaptan-treated mouse).
Differences in parameters of cyst growth are shown in Figure 4. Untreated cystic mice had over three times higher kidney weight than wild-type mice (0.81 versus 0.25 g; P<0.001), and they also had higher kidney weight–body weight ratio, cystic index, and cyst count. All parameters of cyst growth in hydrochlorothiazide-treated cystic mice were similar to untreated mice. Although there was the suggestion of potential acceleration of disease in male mice only, these results did not reach statistical significance and were not found in female mice. Tolvaptan-treated mice had lower kidney weight compared with untreated cystic mice, although the difference was not statistically significant (0.68 versus 0.81 g; P=0.10). Tolvaptan-hydrochlorothiazide combination treatment resulted in the lowest kidney weight, lowest kidney weight-to-body weight ratio, cyst count, and cystic index. This was all statistically significant compared with untreated animals, with a trend toward significance compared with tolvaptan treatment.
Figure 4.
Disease progression in mice. (A) Total kidney weight (KW; left panel) and total KW-body weight (BW) ratio (right panel). (B) Transversal midslices of the right kidney stained with periodic acid–Schiff. (C) Analyses of transversal midslices for cyst count, total cystic area, and cystic index (proportion of total area consisting of cysts as a percentage). WT, wild type. *P=0.05 compared with untreated cystic mice, Mann–Whitney U test; **P=0.01 compared with untreated cystic mice, Mann–Whitney U test; ***P<0.001 compared with untreated cystic mice, Mann–Whitney U test.
When both sexes were studied separately, similar results were obtained, with the only difference being that tolvaptan-treated female mice had significantly lower kidney weight and kidney weight-to-body weight ratio when compared with untreated mice (Supplemental Figure 2).
Secondary Outcomes.
There were no differences between the groups in median plasma creatinine level (Supplemental Table 3). This lack of a difference in the various polycystic kidney disease treatment groups with the wild-type mice suggests no significant kidney function decline during the experiment.
Discussion
The short-term clinical study showed that addition of both hydrochlorothiazide and metformin to tolvaptan can decrease urine volume in tolvaptan-treated patients with ADPKD. Hydrochlorothiazide dosed at 25 mg every day was better tolerated than metformin 1000 mg twice a day.
There was a small placebo effect, evidenced by a limited reduction in urine volume during the placebo treatment period. This reduction was present regardless of treatment sequence. Also compared with placebo, metformin and hydrochlorothiazide reduced urine volume significantly; however, participants still remained polyuric. The antiaquaretic effect of metformin has not been investigated previously in humans with nephrogenic diabetes insipidus. In an animal experiment by Efe et al. (9), metformin decreased urine production by almost 50% in tolvaptan-treated animals (9). Metformin treatment led to vasopressin-independent upregulation of aquaporin-2 and increased water reabsorption in the collecting duct. In our clinical study, urine osmolality increased by 15% and urine production fell by 22% when patients with ADPKD on tolvaptan were cotreated with metformin. We saw no change in aquaporin-2 excretion.
During metformin treatment, two thirds of the participants reported gastrointestinal side effects. These side effects may have been aggravated in this study by the relatively rapid dose increase scheme to 1000 mg twice a day in 2 weeks (25,26) and by tolvaptan coprescription. These gastrointestinal side effects may also be the reason that patients did not report better quality of life compared with baseline, despite the decreases in urine volume and thirst that were subjectively experienced.
Interestingly, it has been suggested that metformin could also be a disease-modifying therapy in ADPKD (27–29). Two recent randomized controlled trials found metformin treatment to be safe and tolerable (30,31). In the short term, we found no significant changes in metabolic biomarkers or urinary damage markers when metformin was added to tolvaptan. Our trial was, however, not designed to study nephroprotection.
The antiaquaretic effect of hydrochlorothiazide was striking in animals as well as humans. It is consistent with earlier studies of hydrochlorothiazide in nephrogenic diabetes insipidus (10–12). The antiaquaretic effect of hydrochlorothiazide is believed to be a consequence of (mild) hydrochlorothiazide-induced extracellular volume contraction, which leads to increased sodium reabsorption in the proximal tubule and lower volume of preurine being delivered to the collecting duct (32). The decreases in body weight, NTproBNP, measured GFR, and systolic BP (and trend in extracellular volume) and the reduction in fractional lithium excretion (thus, increased proximal sodium absorption) that we measured in the clinical trial are all consistent with this mechanism. We found no increase in urinary aquaporin-2, which does not support the theory that hydrochlorothiazide exerts vasopressin-like effects on the collecting duct (33). The correlation we found with measured GFR suggests that the effect is most pronounced in patients with more preserved kidney function. This is an important finding because these are also the patients who experience the most side effects and could theoretically benefit most from the nephroprotective effect of tolvaptan (34,35).
This study shows that hydrochlorothiazide was well tolerated, and participants reported an improved quality of life while on this treatment. There were four cases of mild hypokalemia (3.0–3.5 mmol/L), but no cases of severe hypokalemia.
We found a significant decrease in copeptin after the addition of hydrochlorothiazide to tolvaptan, indicative of a decrease in plasma vasopressin. This is in line with other studies on hydrochlorothiazide (36,37) and could be explained by a decreased plasma osmolality that is also seen, as vasopressin is known to be more sensitive to an osmotic stimulus than to a volume stimulus (38).
In cystic mice, we showed that hydrochlorothiazide-tolvaptan cotreatment was superior to “no treatment” for treatment of polycystic kidney disease and perhaps even better than tolvaptan alone. It may be that tolvaptan-hydrochlorothiazide offers additional nephroprotection compared with tolvaptan alone. If so, this might be explained by the lower copeptin levels that we found in the clinical trial when hydrochlorothiazide was added to tolvaptan. The resultant altered balance between competitive antagonist (tolvaptan) and agonist (vasopressin) might be expected to lead to more effective antagonism and, thus, less cystogenesis. We caution that this should be interpreted as hypothesis generating rather than hypothesis testing. A recent case report in a single tolvaptan-treated patient with ADPKD suggested the opposite, namely that eGFR decline might have been accelerated by the addition of hydrochlorothiazide (39). More research with longer follow-up is needed to determine the effects on the rate of GFR decline before hydrochlorothiazide can be prescribed to all patients who use tolvaptan.
A limitation of our study is that it was performed in a relatively small number of patients. However, the paired design and highly statistically significant results counterbalance this limitation. In order to limit the number of visits and the number of 24-hour urine collections within the 8-week study period, we did not repeat baseline measurements after the washout periods. From the literature, it is known that 7 days should be sufficient to return to baseline values, and we found no evidence of carryover effects in the results after each treatment period. Nevertheless, not having additional baseline measurements is a limitation of this study (40,41). In the animal study, the differences in markers of cystic phenotype between untreated and tolvaptan-treated cystic mice did not reach statistical significance in the overall analyses. This was probably a consequence of the large variation and skewed distribution in the cystic phenotype within male mice (21). As drugs were administered in the food, differences in intake may have resulted in varying doses and thus partly account for the variation. Strengths of our study are the double-blind crossover design, the fact that patients were studied in detail, including multiple measurements of GFR using the gold standard iohexol method, and the eight 24-hour urine collections that were performed. Furthermore, we were able to support our short-term clinical findings with findings in a longer-term animal model.
On the basis of the data provided by this study, we conclude that hydrochlorothiazide and, to a lesser extent, metformin treatment can improve the tolerability of tolvaptan by reducing its aquaretic side effects. Whether adding hydrochlorothiazide to tolvaptan influences nephroprotection deserves further investigation in a study of longer duration. The results of such studies should be awaited before changing clinical practice.
Disclosures
R.T. Gansevoort reports consultancy agreements with AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceuticals, and Sanofi-Genzyme; received consultancy fees from Otsuka Pharmaceuticals, the manufacturer of tolvaptan (paid to the institution); research funding from AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceuticals, and Sanofi-Genzyme; honoraria from Bayer, Galapagos, Mironid, Otsuka Pharmaceuticals, and Sanofi-Genzyme; serving in an advisory or leadership role for American Journal of Kidney Diseases, CJASN, Journal of Nephrology, Kidney360, Nephrology Dialysis Transplantation, and Nephron Clinical Practice; and serving as a member of the steering committee of the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 and Replicating Evidence of Preserved Renal Function: Investigation of Tolvaptan Safety and Efficacy (REPRISE) trials that studied tolvaptan. K.R. Hallows reports consultancy agreements with Maze Therapeutics, Inc.; ownership interest in Bristol Myers Squibb; research funding from Esperion Therapeutics, Inc. and Otsuka Pharmaceutical Co., Ltd.; and serving on the editorial board of American Journal of Physiology Renal Physiology and as an associate editor of Frontiers in Renal and Epithelial Physiology. H.L. Heerspink reports ongoing consultancy agreements with AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, NovoNordisk, and Travere Pharmaceuticals; research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen research support (grant funding directed to employer); and speakers bureau for AstraZeneca. E. Meijer reports research funding from the Dutch Kidney Foundation, Ipsen, Otsuka Pharmaceuticals, and Sanofi (all money was paid directly to the institution); received consultancy fees from Otsuka Pharmaceuticals, the manufacturer of tolvaptan (paid to the institution); and other interests or relationships with the Dutch Kidney Foundation, Health Holland, Nieren.nl, NvN, and Werkgroep Erfelijke Nierziekten. D.J.M. Peters reports ownership interest in Bayer, BioCity Scotland, Innoser, and Mironid Ltd. J. Qiu reports research funding from International Graduate School 1874 (Diabetic Microvascular Complications—Diabetes Microvascular Complications [DIAMICOM]) of the German Research Foundation. D.J. Touw reports research funding from Astellas Pharma BV and Chiesi Pharmaceuticals BV, serving in an advisory or leadership role for Sanquin (Amsterdam, The Netherlands), and serving as a member of the Medical Advisory Board. All remaining authors have nothing to disclose.
Funding
The authors received unrestricted grants from Otsuka Pharmaceuticals (the manufacturer of a vasopressin V2 receptor antagonist). This study was sponsored by Dutch Kidney Foundation grant 18OKG04.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
R.T. Gansevoort and B.J. Kramers conceptualized the study; B.J. Kramers was responsible for data curation; R.T. Gansevoort, K.R. Hallows, I.W. Koorevaar, B.J. Kramers, H. Li, E. Meijer, J. Qiu, D.J. Touw, and M.D.A. van Gastel were responsible for investigation; I.W. Koorevaar and B.J. Kramers were responsible for formal analysis; R.T. Gansevoort, K.R. Hallows, H.L. Heerspink, I.W. Koorevaar, B.J. Kramers, W.N. Leonhard, E. Meijer, D.J.M. Peters, and H. van Goor were responsible for methodology; R.T. Gansevoort and E. Meijer were responsible for resources; R.T. Gansevoort and E. Meijer were responsible for funding acquisition; R.T. Gansevoort, K.R. Hallows, H.L. Heerspink, E. Meijer, D.J.M. Peters, and H. van Goor provided supervision; and R.T. Gansevoort, K.R. Hallows, H.L. Heerspink, I.W. Koorevaar, B.J. Kramers, W.N. Leonhard, H. Li, E. Meijer, D.J.M. Peters, J. Qiu, D.J. Touw, M.D.A. van Gastel, and H. van Goor reviewed and edited the manuscript.
Data Sharing Statement
Data were collected and stored using CastorEDC electronic case report forms, from which analyzable datasets can be produced. Data can be made available upon contacting the primary investigator. Additional related documents, such as the study protocol, will be available on request. The planned data use must not breach the ethical approval obtained for our study.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11260821/-/DCSupplemental.
Supplemental Figure 1. Quality of life.
Supplemental Figure 2. Differences in parameters of disease progression in male and female mice separately.
Supplemental Material. Methods and results.
Supplemental Table 1. Baseline characteristics per treatment sequence.
Supplemental Table 2. Changes in urinary excretion of kidney damage and metabolic markers.
Supplemental Table 3. Biochemistry analyses in mice.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 3.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Blair HA, Keating GM: Tolvaptan: A review in autosomal dominant polycystic kidney disease. Drugs 75: 1797–1806, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockenhauer D, Bichet DG: Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11: 576–588, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT: Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist tolvaptan. Am J Kidney Dis 73: 354–362, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Klein JD, Wang Y, Blount MA, Molina PA, LaRocque LM, Ruiz JA, Sands JM: Metformin, an AMPK activator, stimulates the phosphorylation of aquaporin 2 and urea transporter A1 in inner medullary collecting ducts. Am J Physiol Renal Physiol 310: F1008–F1012, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efe O, Klein JD, LaRocque LM, Ren H, Sands JM: Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight 1: e88409, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford JD, Kennedy GC, Hill LE: Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series. N Engl J Med 262: 737–743, 1960 [DOI] [PubMed] [Google Scholar]
- 11.Crawford JD, Kennedy GC: Chlorothiazid in diabetes insipidus. Nature 183: 891–892, 1959 [DOI] [PubMed] [Google Scholar]
- 12.Havard CW: Thiazide-induced antidiuresis in diabetes insipidus. Proc R Soc Med 58: 1005–1007, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, Devuyst O, Drechsler C, Eckardt KU, Emma F, Knebelmann B, Le Meur Y, Massy ZA, Ong AC, Ortiz A, Schaefer F, Torra R, Vanholder R, Więcek A, Zoccali C, Van Biesen W: Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: A position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31: 337–348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koomans HA, Boer WH, Dorhout Mees EJ: Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int 36: 2–12, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G: Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 6: 257–263, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Bröchner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30: 271–274, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Gaspari F, Perico N, Matalone M, Signorini O, Azzollini N, Mister M, Remuzzi G: Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 9: 310–313, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Bird NJ, Michell AR, Peters AM: Accurate measurement of extracellular fluid volume from the slope/intercept technique after bolus injection of a filtration marker. Physiol Meas 30: 1371–1379, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, Breuning MH, Verbeek S, de Heer E, Peters DJ: Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis 44: 225–232, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kanhai AA, Bange H, Verburg L, Dijkstra KL, Price LS, Peters DJM, Leonhard WN: Renal cyst growth is attenuated by a combination treatment of tolvaptan and pioglitazone, while pioglitazone treatment alone is not effective. Sci Rep 10: 1672, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leemasawatdigul K, Gappa-Fahlenkamp H: Effect of storage conditions on the stability of recombinant human MCP-1/CCL2. Biologicals 39: 29–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauta FL, Bakker SJ, Lambers Heerspink H, de Zeeuw D, van Oeveren W, Bilo H, de Jong PE, Gansevoort RT: Effect of frozen storage on urinary concentration of kidney damage markers. Am J Kidney Dis 59: 586–589, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Leonhard WN, Song X, Kanhai AA, Iliuta IA, Bozovic A, Steinberg GR, Peters DJM, Pei Y: Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine 47: 436–445, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL: Efficacy of metformin in type II diabetes: Results of a double-blind, placebo-controlled, dose-response trial. Am J Med 103: 491–497, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Blonde L, Dailey GE, Jabbour SA, Reasner CA, Mills DJ: Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: Results of a retrospective cohort study. Curr Med Res Opin 20: 565–572, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Seliger SL, Abebe KZ, Hallows KR, Miskulin DC, Perrone RD, Watnick T, Bae KT: A randomized clinical trial of metformin to treat autosomal dominant polycystic kidney disease. Am J Nephrol 47: 352–360, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takiar V, Nishio S, Seo-Mayer P, King JD Jr., Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ: Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A 108: 2462–2467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brosnahan GM, Wang W, Gitomer B, Struemph T, George D, You Z, Nowak KL, Klawitter J, Chonchol MB: Metformin therapy in autosomal dominant polycystic kidney disease: A feasibility study [published online ahead of print August 12, 2021]. Am J Kidney Dis 10.1053/j.ajkd.2021.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrone RD, Abebe KZ, Watnick TJ, Althouse AD, Hallows KR, Lalama CM, Miskulin DC, Seliger SL, Tao C, Harris PC, Bae KT: Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD). Kidney Int 100: 684–696, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earley LE, Orloff J: The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest 41: 1988–1997, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim GH, Lee JW, Oh YK, Chang HR, Joo KW, Na KY, Earm JH, Knepper MA, Han JS: Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol 15: 2836–2843, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Devuyst O, Chapman AB, Shoaf SE, Czerwiec FS, Blais JD: Tolerability of aquaretic-related symptoms following tolvaptan for autosomal dominant polycystic kidney disease: Results from TEMPO 3:4. Kidney Int Rep 2: 1132–1140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE: A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol 29: 2458–2470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoaf SE, Bramer SL, Bricmont P, Zimmer CA: Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a non-peptide AVP antagonist, and furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol 50: 213–222, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ware JS, Wain LV, Channavajjhala SK, Jackson VE, Edwards E, Lu R, Siew K, Jia W, Shrine N, Kinnear S, Jalland M, Henry AP, Clayton J, O’Shaughnessy KM, Tobin MD, Schuster VL, Cook S, Hall IP, Glover M: Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J Clin Invest 127: 3367–3374, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bankir L: Antidiuretic action of vasopressin: Quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res 51: 372–390, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Kramers BJ, van Gastel MDA, Meijer E, Gansevoort RT: Case report: A thiazide diuretic to treat polyuria induced by tolvaptan. BMC Nephrol 19: 157, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bankir L, Perucca J, Norsk P, Bouby N, Damgaard M: Relationship between sodium intake and water intake: The false and the true. Ann Nutr Metab 70[Suppl 1]: 51–61, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Maronde RF, Milgrom M, Vlachakis ND, Chan L: Response of thiazide-induced hypokalemia to amiloride. JAMA 249: 237–241, 1983 [PubMed] [Google Scholar]
- 42.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.