Abstract
Coronavirus disease 2019 (COVID-19), which is attributable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been causing a worldwide health issue. Airways colonization by Candida spp. is prevalent among patients on automatic ventilation in intensive care units (ICUs). This research aimed to ascertain the risk factors and roles of Candida spp. respiratory tract colonization, and Candida lung infection during the progression of COVID-19 pneumonia in critically ill patients. In total, Candida spp. were recovered in 69 from 100 immunosuppressed patients with COVID-19. Bronchoscopy was used to collect the Bronchoalveolar lavage (BAL) specimens. For the identification of Candida spp. PCR sequencing was done using the ITS1 and ITS4 primers. The amplification of the HWP1 gene was conducted to identify the Candida albicans complex. The antifungal activities of fluconazole, itraconazole, voriconazole, amphotericin B and caspofungin against Candida spp. were evaluated using the Clinical and Laboratory Standards Institute M60. In 63.77% of the patients, Candida respiratory colonization at D0 and D14 had no impact on the severity of COVID-19. In comparison to C. albicans strains, Candida respiratory disorder with C. glabrata had influenced the severity of COVID-19 for critically ill patients following adjustment for the risk factors of COVID-19 (P < 0.05). Amphotericin B and caspofungin showed superior activity against all Candida spp. All antifungal agents showed 100% sensitivity against the two C. africana strains. Our observation on patients who used automatic ventilation, respiratory colonization by Candida spp. was not seen to influence the infection or death caused by COVID-19. Amphotericin B and caspofungin showed superior activity against all Candida spp. and were recommended for the treatment regime of pulmonary candidiasis associated with COVID-19 infection. Although “Candida pneumonia” is rarely being reported in critically ill patients, Candida airway colonization mainly by Candida albicans is common especially among patients with diabetes, malignancies, and kidney disorders.
Keywords: COVID-19, SARS-CoV-2, Candida, Bronchoalveolar lavage, Mechanical ventilation, Antifungal agents
Abbreviation used
- Coronavirus disease 2019
(COVID-19)
- Minimum inhibitory concentration
(MIC)
- Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2)
- Intensive care unit
(ICU)
- Bronchoalveolar lavage
(BAL)
- Mechanical ventilation
(MV)
- Ventilator-associated pneumonia
(VAP)
1. Introduction
The novel coronavirus SARS-coV-2, which emerged in Wuhan in November 2019, has increasingly spread causing a global pandemic that infected more than 494 million people, resulting in severe social and economic ramifications, and claimed more than 6,183,000 lives by April 6, 2022 [1]. Coronavirus disease 2019 (COVID-19), which is attributable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been the cause of global health threats [2,3]. Bacterial and fungal co-infections are among various factors that play roles in morbidity and mortality in COVID-19 patients, particularly among those suffering from acute respiratory distress syndrome (ARDS). Furthermore, the wide use of corticosteroids and the irrational use antibiotics coupled with the tissue damage caused by SARS CoV-2, may facilitate invasion by commensal yeast causing deep seated invasive fungal infections. Patients with severe COVID-19 are at risk for healthcare-associated infections (HAIs), including Candida bloodstream infections. There have been reports on increasing incidence of candidemia in critically ill COVID-19 cases. High mortality rate is being reported among patients with COVID-19-associated candidemia (CAC). The mortality rate among patients with CAC reaches up to 83% despite antifungal therapy. The above highlights the clinical significance of severe COVID-19 that underscores the importance of rapid diagnosis and timely initiation of antifungal treatment [[4], [5], [6], [7]]. Moreover, the undefined standard of pharmacological therapy for COVID-19, including the invasive nature and multi-drug treatment methods, as well as some pathological oral conditions can aggravate SARS-CoV-2, particularly in those patients with a immune-compromised system or a long-term usage of pharmacotherapies that expose them to increased risk for developing mucosal candidiasis [8]. Bronchial colonization by Candida spp. is prevalent among patients who use automatic ventilation in the intensive care unit (ICU). Candida colonization has been found in approximately 30% of people who used mechanical ventilation (MV) for longer than 48 h and in 50% of those diagnosed with ventilator-associated pneumonia (VAP) [9,10]. Isolation of Candida spp. via the respiratory tract is linked to longer periods of MV, ICU admission, and hospital stay, with attendant poorer outcomes [[11], [12], [13]]. Except for highly immunocompromised patients, who are prone to fungal pneumonia, Candida spp. in lower airways shall be interpreted with cautions as the causative agents of lung disease [[14], [15], [16], [17], [18]]. Colonization of the respiratory tract by Candida spp. can have a significant effect on the progression of COVID-19 pneumonia. Evaluation for secondary fungal infections in COVID-19 patients, as well as their initiating agents, is critical for effective management of COVID-19 infection. Additionally, understanding the antifungal susceptibility profile of Candida spp. would be essential in treatment of COVID-19 patients. This research aimed to evaluate antifungal susceptibility patterns and the role of Candida spp. respiratory tract colonization, risk factors, and Candida lung infection during the progression of COVID-19 pneumonia in critically ill patients.
2. Materials and methods
2.1. Study areas and subjects
This descriptive study was performed on COVID-19 patients who were diagnosed based on clinical symptoms, radiological signs, and positive molecular test results and admitted to Shahid Beheshti Hospital in Kashan, Iran. Bronchoscopy was used to collect the bronchoalveolar lavage (BAL) specimens. The collected specimens were initially subjected to microscopic examination using 10% KOH solution to detect budding yeasts or pseudohyphae. Parts of the specimens were cultured on Sabouraud's Dextrose Agar (SDA) 2% (Merck, Denmark) and incubated at 35 °C for seven days. A few of the colonies grown on SDA were also mixed with sterile saline and 3% glycerol in 0.5 ml microtubes and stored at −70 °C [[19], [20], [21]].
The study included adult immunosuppressed patients with COVID-19 pneumonia who used invasive MV for more than four days. Other inclusion criteria were history of the regulation of immune status, even once; immunocompromised status; patients with neutropenia; use of corticosteroid at doses >2 mg/kg of dexamethasone; hospitalization in the ICU for more than four days; and use of invasive ventilation. Signs and symptoms of inflammation and other ICU-acquired complications were assessed regularly. The exclusion criteria were: Non-ICU patients with confirmed COVID-19, Age ≤18 year, and COVID-19 patients with Non-Invasive Ventilation. Verbal consent was obtained from patients before being enrolled in this study. The Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran has approved this study (ethics code: IR.TUMS.SPH.REC.1399.329).
2.2. Molecular identification of isolates
2.2.1. Extraction of genomic DNA
According to the manufacturer's instructions, genomic DNA was extracted directly from BAL specimens using a high-purity polymerase chain reaction (PCR) template purification package (Roche, Germany). Briefly, 200 μl of specimens were mixed with 200 μl of binding buffer and 40 μl of proteinase K. The mixture was incubated at 70 °C for 10 min followed by the addition of 100 μl of isopropanol. A high-purity filter tube was inserted into a collection tube, and the setup was mixed using a vortex. The sample was pipetted into the upper buffer reservoir of the filter tube. The whole high-purity filter tube assembly was placed in a standard table-top centrifuge and centrifuged at × 8000 g for 1 min. The filter tube was then removed and the rest of the setup was discarded; keeping the collection tube containing the filtrate Subsequently, 500 μl inhibitor removal buffer was added to the supernatant and was centrifuged for 1 min at 8000×g. Finally, the supernatant was removed from the collection tube, 500 μl wash buffer was added to it, and centrifuged for 1 min at 8000×g.
The flow-through was scrapped, and the whole high purity assembly was centrifuged at full speed for another 30 s. The elution buffer was added, and the DNA was precipitated in 100 μl TE. Brief centrifugation (15,000 g for 1 min) was used to separate the cell debris, and 1 μl of the supernatant was used for the PCR. The extracted DNA was stored at −20 °C.
2.2.2. Amplification of internal transcribed spacers
We used the PCR to detect Candida spp. The PCR reaction was run in a cumulative volume of 25 μl, containing 1 μl of each of reverse and forward primers, 2 μl of prototype DNA, 12.5 μl of master mix (Amplicon, Denmark), and water until it reached the final volume. The amplification was done using the internal transcribed spacers 1 (ITS1) and ITS4 primers based on the following protocol: 10 min of primary denaturation at 95 °C, 40 cycles of denaturation for 20 s at 95 °C, annealing for 20 s at 62 °C, an expansion for 20 s at 72 °C, and a final extension for 5 min at 72 °C. Eventually, the products were run on a 2% agarose gel. The HWP1 gene amplification using the paired primers HWP1–F (5′- GCTACCACTTCAGAATCATCATC-3′) and HWP1-R (5′- GCACCTTCAGTCGTAGAGACG-3′) was done as described previously for Candida albicans complex [14,22].
2.3. Antifungal susceptibility assay
The Clinical and Laboratory Standards Institute (CLSI) M60 approach was used to assess the minimum inhibitory concentrations (MIC) of fluconazole, itraconazole, voriconazole, caspofungin, and amphotericin B. Antifungal agent powders were bought from Sigma, USA. The serial dilution of routine antifungals was prepared in concentrations ranging from 0.0125 to 32/64 mg/ml, depending on the drug. The 100 μl of each agent was dispensed in a 96-well microplate. Growth and negative controls were included. The negative control was prepared using the 200 μl of RPMI1640 medium. The plates were incubated at 35 °C for 24 h. Candida parapsilosis ATCC 22019 was checked for quality control. It should be mentioned that each test was carried out twice [16,23].
2.4. Statistical analysis
Statistical analysis was carried out using SPSS software (version 16.0). Descriptive test was performed to describe the demographic characteristics, and chi-square test was performed to demonstrate any statistically significant relationship between the variables explored in this study. The MICs range and MICs 90 of all antifungals were calculated.
3. Results
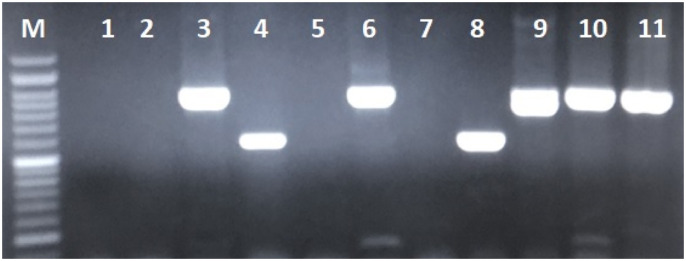
Candida colonization was confirmed in 69 (69%) of the 100 COVID-19 patients under MV. Of these, 37/69 (53.6%) patients were males; the mean age of all patients at presentation was 61.1 years (range = 21–88 years). Based on the PCR sequencing results, C. albicans (55; 79.7%) was the most common spp. followed by, C. glabrata (12; 17.4%). The co-infection of C. albicans and C. glabrata was seen in two cases (2.9%). In this research, two (2.9%) Candida africana were detected by the HWP1 gene amplification (Fig. 1 ), and no Candida dubliniensis was found.
Fig. 1.
Agarose gel of PCR amplification with HWP1 gene; Lanes 3, 6, 9–11: C. albicans ∼900 bp; Lanes 4, 8: C. africana ∼600 bp.
On the first day of admission, D0, all 69 patients using MV had Candida spp. airway colonization, while there was no substantial difference in the cause for ICU entry (P > 0.05). Moreover, at D0, C. albicans was responsible for 79.7% of Candida respiratory tract colonization. In 63.77% of patients, Candida respiratory colonization had no impact on the severity of COVID-19 (P > 0.05) between D0 and D14. In comparison to C. albicans strains, Candida respiratory tract colonization with C. glabrata had influenced the severity of COVID-19 in critically ill patients following adjustment for the risk factors of COVID-19 (P < 0.05).
The most common underlying diseases among patients with Candida colonization included diabetes (28 cases), malignancy (8 cases), kidney disorders (11 cases), cardiovascular diseases (7 cases), and one 1 case each of pregnancy and hyperthyroidism. Whereas patients with Candida colonization had diabetes (40.6%) and kidney disorders (16%) as their main underlying diseases, headache (97.1%), fever (85.5%), myalgia (91.6%), arthralgia (49.3%), gastrointestinal symptoms (71%), and dyspnea (100%) were most frequent symptoms at presentation depending on patients’ status of Candida colonization. Table 1 .
Table 1.
The major presenting symptoms in COVID-19 patients with Candida spp.
| Number of patients Characteristic, no (%) | 69 |
|---|---|
| Age at the time of diagnosis-years* | 61.1 (range = 21–88 years) |
| Sex | No |
| Male | 32 (46.4%) |
| Female | 37 (53.6%) |
| Total COVID-19 patients | 679 |
| ICU patients | 100 (14.2%) |
| Mechanical ventilation (MV) with colonization | 69/100 (69%) |
| Underlying cause of immunosuppression | |
| Malignancy | 8 (11.6%) |
| Diabetes Mellitus | 28 (40.6%) |
| Kidney disorder | 11 (16%) |
| Hyperthyroidism | 1 (1.4%) |
| Pregnancy | 1 (1.4%) |
| Cardiovascular disease | 7 (10.1%) |
| Signs and symptoms | |
| Headache | 67 (97.1%) |
| Fever | 59 (85.5%) |
| Myalgia | 63 (91.6%) |
| Arthralgia | 34 (49.3%) |
| Gastrointestinal | 49 (71%) |
| Dyspnea | 69 (100%) |
| Blood group | |
| A | 26 (37.7%) |
| AB | 5 (7.2%) |
| B | 20 (29%) |
| O | 18 (26.1%) |
| Extension | |
| BAL | 69 (100%) |
The clinical course and disease outcome of patients with and without Candida colonization is being been demonstrated in Table 2 (see Table 3).
Table 2.
Characteristics of patients, clinical course, and outcome in Candida and non-Candida colonization cases.
| Variable | Candida colonization (n = 69) | No Candida colonization (n = 31) | P-valuea |
|---|---|---|---|
| COVID-19 infection | 69/69 (100) | 31/31 (100) | 1 |
| Age, yr, median (range) | 61.1 (21–88) | 56.6 (26–89) | 0.67 |
| Sex, F, n (%) | 37/69 (53.6) | 13/31 (41.9) | 0.24 |
| Blood group, A, n (%) | 26/69 (37.7) | 8/31 (25.8) | 0.18 |
| Systemic corticosteroid use, n (%) | 46/69 (66.6) | 19/31 (61.3) | 0.77 |
| Interval from ICU admission to ICU discharge, median (range), d | 13.1 (5–35) | 10.9 (3–14) | 0.21 |
| ICU patients | 69/69 (100) | 31/31 (100) | 1 |
| Mechanical ventilation, n (%) | 69/69 (100) | 22/31 (70.9) | 0.09 |
| Candidemia | 3/69 (4.3) | 0/31 (0.0) | 0.05 |
| Urine culture | 17/69 (24.6) | 5/31 (16.2) | 0.19 |
| Mortality, n (%) | 45/69 (65.2) | 19/31 (61.3) | 0.61 |
Fischer's exact test; Mann-Whitney test for continuous data.
Table 3.
Characteristics of patients, clinical findings, signs and symptoms, laboratory findings, and outcome in patients colonized with C. albicans, patients colonized with C. glabrata and non-colonized patients.
| Variable | Candida albicans colonization (n = 55) | Candida glabrata colonization (n = 12) | No Candida colonization (n = 31) |
|---|---|---|---|
| Colonization | 55/69 (79.7) | 12/69 (17.4) | 31/31 (100) |
| Age, yr, median (range) | 56.1 (21–88) | 67.9 (44–83) | 56.6 (26–89) |
| Sex, F, n (%) | 30/55 (54.5) | 6/12 (50) | 13/31 (41.9) |
| Diabetes Mellitus, n (%) | 21/55 (38.2) | 6/12 (50) | 4/31 (12.9) |
| Kidney disorder, n (%) | 5/55 (9.1) | 6/12 (50) | 2/31 (6.4) |
| Malignancy, n (%) | 8/55 (14.5) | 0/12 (0.0) | 0/31 (0.0) |
| Cardiovascular disease, n (%) | 5/55 (9.1) | 2/12 (16.7) | 1/31 (3.2) |
| Candidemia, n (%) | 1/55 (1.8) | 2/12 (16.7) | 0/31 (0.0) |
| Urine culture, n (%) | 10/55 (18.2) | 7/12 (58.3) | 5/31 (16.2) |
| Headache | 53/55 (96.4) | 12/12 (100) | 24/31 (77.4) |
| Fever | 46/55 (83.6) | 12/12 (100) | 27/31 (87.1) |
| Myalgia | 51/55 (92.7) | 11/12 (91.7) | 19/31 (61.3) |
| Arthralgia | 25/55 (45.4) | 9/12 (75) | 7/31 (22.6) |
| Gastrointestinal | 43/55 (78.2) | 5/12 (41.7) | 13/31 (41.9) |
| Dyspnea | 55/55 (100) | 12/12 (100) | 27/31 (87.1) |
| WBC, mm3, median | 8.48 × 103/mm3 | 9.9 × 103/mm3 | 10.7 × 103/mm3 |
| FBS, mg/dl, median (range) | 189 (75–507) | 164 (36–470) | 101 (84–266) |
| BUN, mg/dl, median (range) | 34.4 (8–100) | 55.8 (11–103) | 31.2 (12–82) |
| CRP, mg/dl, median (range) | 96.1 (2–382) | 109 (21–344) | 85.6 (1–339) |
| ESR, mm/hr., median (range) | 48.2 (10–107) | 59.2 (15–109) | 48.5 (6–109) |
| Interval from ICU admission to ICU discharge, median (range), d | 10.2 (5–30) | 16.8 (7–35) | 10.9 (3–14) |
| Mortality, n (%) | 33/55 (60) | 11/12 (91.7) | 19/31 (61.3) |
Table 4 summarizes the MIC range and the MIC 90 of all antifungals. Amphotericin B and caspofungin showed superior activity against all Candida spp. For C. albicans: (isolates no:4,6–17,47-51,57,63) were resistant to voriconazole MIC≥ 16 μg/Ml; resistant to fluconazole MIC≥32 μg/Ml (isolates no: 6–17,25,26,48–51); to caspofungin MIC≥ 32 μg/Ml (isolates no: 2,8,16,17,57,63); and itraconazole MIC≥32 μg/Ml (isolates no: 2,6, 7–17,21-24,46–51,54,55) were seen. For C. glabrata resistant to voriconazole MIC≥ 8 μg/Ml (isolates no:3,5,18,19,21,58,66,68); fluconazole MIC≥ 32 μg/Ml (isolates no: all of isolates), caspofungin ≥8 μg/Ml (isolates no: 18,58) and itraconazole MIC≥32 μg/Ml (isolates no:3,5,18,19,21,58,59,66,68) were seen. All antifungal agents showed 100% sensitivity (range to 0.03–0.5) against Two C. africana strains. Table 5 .
Table 4.
MIC range and MIC 90 of five antifungals against Candida species.
| Species (n) | Amphotericin B μg/mL | Voriconazole μg/mL | Itraconazole μg/mL | Fluconazole μg/mL |
Caspofungin μg/mL |
|
|---|---|---|---|---|---|---|
| C. albicans | MICs Range | 0.03–1 | 0.03–16 | 0.03–32 | 0.125–32 | 0.03–32 |
| MIC90 | 0.03 | 16 | 16 | 32 | 0.03 | |
| C. glabrata | MICs Range | 0.03–1 | 0.03–16 | 0.03–32 | 32 | 0.125–16 |
| MIC90 | 0.5 | 8 | 2 | 32 | 0.125 |
Table 5.
MIC interpretation of five antifungal drugs against Candida spp. recovered from COVID-19 patients.
| Antifungal agents | C. albicans N = 55% | C. glabrata N = 12% | C. africana N = 2% | |
|---|---|---|---|---|
| Amphotericin B | S | 51 (92.7) | 10 (83.4) | 2 (100) |
| R | 4 (7.3) | 2 (16.6) | 0 (0) | |
| Itraconazole | S | 30 (54.5) | 3 (25) | 2 (100) |
| R | 25 (45.5) | 9 (75) | 0 (0) | |
| Voriconazole | S | 35 (63.6) | 4 (33.4) | 2 (100) |
| R | 20 (36.4) | 8 (66.6) | 0 (0) | |
| Fluconazole | S | 37 (67.3) | 0 (0) | 2 (100) |
| R | 18 (32.7) | 12 (100) | 0 (0) | |
| Caspofungin | S | 49 (89.1) | 10 (83.4) | 2 (100) |
| R | 6 (10.1) | 2 (16.6) | 0 (0) |
S: susceptible; R: resistance.
4. Discussion
Although microbial colonization is an important factor in the development of secondary infections, Candida pneumonia– as a secondary infection following airways colonization –is seldom reported even in the intensive care unit (ICU). Thus, the common consensus is that anti-Candida therapy is rarely necessary in most cases and it should be managed as airways colonization in which Candida spp. are being isolated [24]. Some studies have reported that Candida colonization in respiratory tracts (RT) might be an independent risk factor for the development of ventilator-associated pneumonia (VAP). Colonization can even change the antibiotic resistance patterns of pathogenic bacteria by polymicrobial biofilm formation [25,26]. Therefore, the significance of Candida colonization in RT remains controversial, and many clinical conditions need to be interpreted with caution. In this research, C. albicans (55; 79.7%) was the most common spp. followed by C. glabrata (12; 17.4%) and two (2.9%) C. africana (detected by the HWP1 gene amplification) as the etiologic agents of pulmonary Candida colonization associated with COVID-19 infection. The co-infection of C. albicans and C. glabrata was seen in two cases. Our observation on patients who used automatic ventilation, respiratory colonization by Candida spp. was not seen to influence the infection or death caused by COVID-19. From other reports, the rate of Candida spp. isolation in the RT is relatively high, especially in those with mechanical ventilation (MV) [25]. However, there are still controversies on whether Candida spp. can solely cause VAP due to the following reasons: (1) regardless of the causative pathogenic microorganism, the diagnosis of VAP is still challenging due to the lack of pathological evidence. The diagnostic criteria for a clinically suspected VAP are non-specific, and it is difficult to distinguish between colonization and infection [27]. (2) Generally, the understanding of the essence of bacterial and fungal co-existence in many cases is shallow. Many microbiology laboratories do not conduct further analysis when fast-growing Candida spp. are being isolated from RT samples. Further, only filamentous fungi isolation was being reported by some institutions [28]. (3) It is widely accepted that the cutoff counts of pathogenic bacteria for VAP diagnosis is 103 CFU/mL (protected specimen brush sample) or 104 CFU/mL (bronchoalveolar lavage fluid sample), but such consensus has not yet been reached for Candida; Candida pneumonia must be diagnosed by histopathology [27]. Thus, reporting Candida pneumonia is generally quite rare in the ICU, and the guidelines for the management of Candida spp. of both the IDSA and ESCMID do not recommend commencement of antifungal treatment without clear histological evidence of infection [24,29]. Reports of clinical studies from some centers have highlighted the isolation rate of Candida from the RT of ICU patients using MV to be as high as 50% with a prolonged median hospital stay (59.9 vs. 38.6 days, p = 0.006) or even increased the hospital mortality (34.2 vs. 21.0%, p = 0.003) [30]. Moreover, it might be associated with persistent immunosuppression and inflammation [31]. Candida airways colonization and its concomitant secretory inflammation may worsen the host's cellular immune function, especially in immunosuppressed hosts with severe monocyte and lymphocyte dysfunction that results in a decreased effective clearance of bacteria and fungi and may increase the incidence of VAP [32]. A report of a longitudinal cohort analysis published more than 10 years ago found that Candida spp. bronchial colonization was an independent risk factor for the establishment of Pseudomonas aeruginosa VAP (9 vs. 4.8% in non-colonized patients, P = 0.048). Likewise, the results of a retrospective single-center case-control study indicated that antifungal treatment of patients with Candida airway colonization was able to inhibit P. aeruginosa VAP [17]. Findings from recent research have revealed that Candida airway colonization was independently related to Acinetobacter baumannii VAP [18]. In another prospective cohort study, the FUNGIBACT, that examined 146 patients under MV for more than 96 h. After adjusting for the immune index mHLA-DR, the findings revealed that there was no correlation between airway Candida colonization and the incidence of VAP [HR: 0.98; 95% CI (0.59–1.65), p = 0.95] [33].
The co-occurrence of viral and fungal species is possible and both organisms can detect and react to a variety of diffusible signaling molecules created in the niches in which they co-exist. Increased host tissue damage and inflammation may result from fungi and COVID-19 interaction. However, in a murine model, Ader et al. found that animals colonized by direct tracheal inoculation of live Candida spp. with a protocol developed to acquire Candida spp. colonization without epithelial injury was immune to P. aeruginosa pneumonia [34]. Besides, in a cohort investigation, usage of nebulized amphotericin B in people with Candida spp. airway colonization who used mechanical ventilation did not affect the incidence rate of VAP or ICU mortality despite the increase in the rate of Candida spp. decolonization. Furthermore, micafungin treatment of people with multiple Candida spp. colonization, new sepsis of unknown etiology, and multiple organ failure could not decrease the incidence rate of VAP in comparison with the placebo [35]. Regarding the results, amphotericin B and caspofungin showed superior activity against all Candida spp. and were recommended for the treatment regime of pulmonary candidiasis associated with COVID-19 infection. Various degrees of resistance to voriconazole, itraconazole and fluconazole were seen in C. albicans and C. glabrata strains. Antifungal agents showed 100% sensitivity against the two C. africana strains. In another study, 100%, 30%, and 40% of the Candida auris isolates were resistant to FCZ, combination of FCZ and voriconazole, and combination of FCZ and AMB, respectively, and only one Candida glabrata isolate was resistant against echinocandin [8,[36], [37], [38], [39], [40], [41], [42]].
5. Conclusion
In this study, the use of automatic ventilation, respiratory colonization, or infection with Candida spp. was not recognized to influence variables of the infection or death caused by COVID-19. Although “Candida pneumonia” is rarely being reported in critically ill patients, Candida airway colonization mainly by Candida albicans is common especially among patients with diabetes, malignancies, and kidney disorders. In this study, amphotericin B and caspofungin showed superior activity against all Candida spp.
Authors’ contribution
Study concept and design and technical supervision: Erami M, Hashemi SJ; Obtaining the specimens from patients and interpretation: Erami M, Heravi M, Yarahmadi M, Amiri S, Fakhrehi M, Raissi V; Acquisition of data and drafting of the manuscript: Raiesi O, Getso M, Mehri N; Critical revision of the manuscript: Raiesi O, Getso M; Procedure: Erami M, Raiesi O.
Financial support
This work was supported by the Tehran University of Medical Sciences, Tehran, Iran (grant number: 9711353002).
CRediT authorship contribution statement
Mahzad Erami: Investigation, Data curation, Conceptualization. Omid Raiesi: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Mansooreh Momen-Heravi: Methodology, Investigation, Data curation. Muhammad Ibrahim Getso: Writing – review & editing, Writing – original draft. Mojtaba Fakhrehi: Formal analysis, Data curation. Narges Mehri: Methodology, Investigation, Data curation. Mohammad Yarahmadi: Formal analysis, Data curation. Sasan Amiri: Data curation. Vahid Raissi: Data curation. Seyed Jamal Hashemi: Visualization, Validation, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Seyed Jamal Hashemi reports financial support was provided by Tehran University of Medical Sciences.
References
- 1.Mohseni M., Raissi V., Sharifan Y., Barikro K., Amiri S., Mohseni M.S., et al. Therapeutic status of famotidine in COVID-19 patients: a review. Infect. Disord. - Drug Targets. 2022 doi: 10.2174/1871526522666220107125511. [DOI] [PubMed] [Google Scholar]
- 2.Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M., et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296:E15–e25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabanejad Z., Darvish S., Borjian Boroujeni Z., Asadi S.S., Mesri M., Raiesi O., et al. Seroepidemiological study of novel coronavirus disease (CoVID-19) in Tehran, Iran. Infection Epidemiology and Microbiology. 2021;7 0. [Google Scholar]
- 4.Kayaaslan B, Kaya Kalem A, Asilturk D, Kaplan B, Dönertaş G, Hasanoglu I, et al. Incidence and risk factors for COVID‐19 associated candidemia (CAC) in ICU patients. Mycoses. 2022:1–9. doi: 10.1111/myc.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayaaslan B., Eser F., Kaya Kalem A., Bilgic Z., Asilturk D., Hasanoglu I., et al. Characteristics of candidemia in COVID‐19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non‐COVID‐19 patients. Mycoses. 2021;64:1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci M., Barreiros G., Guimarães L.F., Deriquehem V.A., Castiñeiras A.C., Nouér S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID‐19 pandemic. Mycoses. 2021;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arastehfar A., Shaban T., Zarrinfar H., Roudbary M., Ghazanfari M., Hedayati M.-T., et al. Candidemia among Iranian patients with severe COVID-19 admitted to ICUs. Journal of Fungi. 2021;7:280. doi: 10.3390/jof7040280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi M., Ahmadikia K., Mahmoudi S., Kalantari S., Jamalimoghadamsiahkali S., Izadi A., et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63:771–778. doi: 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelbaum O., Chasan R. Candidemia in the intensive care unit. Clin. Chest Med. 2017;38:493–509. doi: 10.1016/j.ccm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Wu H.-H., Chen Y.-T., Shih C.-J., Lee Y.-T., Kuo S.-C., Chen T.-L. Association between recent use of proton pump inhibitors and nontyphoid salmonellosis: a nested case-control study. Clin. Infect. Dis. 2014;59:1554–1558. doi: 10.1093/cid/ciu628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillman K.M., Bristow P.J., Chey T., Daffurn K., Jacques T., Norman S.L., et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28:1629–1634. doi: 10.1007/s00134-002-1496-y. [DOI] [PubMed] [Google Scholar]
- 12.Shokohi T., Soteh M.H., Pouri Z.S., Hedayati M., Mayahi S. Identification of Candida species using PCR-RFLP in cancer patients in Iran. Indian J. Med. Microbiol. 2010;28:147–151. doi: 10.4103/0255-0857.62493. [DOI] [PubMed] [Google Scholar]
- 13.Khalandi H., Masoori L., Farahyar S., Delbandi A.A., Raiesi O., Farzanegan A., et al. Antifungal Activity of capric acid, nystatin, and fluconazole and their in vitro interactions against candida isolates from neonatal oral thrush. Assay Drug Dev. Technol. 2020;18:195–201. doi: 10.1089/adt.2020.971. [DOI] [PubMed] [Google Scholar]
- 14.Romeo O., Criseo G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn. Microbiol. Infect. Dis. 2008;62:230–233. doi: 10.1016/j.diagmicrobio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Fattahi A., Lotfali E., Masoumi-Asl H., Sayyahfar S., Kalani M., Khourgami M.R., et al. Candidemia and its risk factors in neonates and children. Archives of Pediatric Infectious Diseases. 2020;8:1–5. [Google Scholar]
- 16.Wayne P. 2017. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. CLSI Document M60 3 Rd Ed. [Google Scholar]
- 17.Green I.M., Margoni I., Nair S.P., Petridis H. Adhesion of methicillin-resistant Staphylococcus aureus and Candida albicans to parylene-C-coated polymethyl methacrylate. Int. J. Prosthod. 2019;32:193–195. doi: 10.11607/ijp.5918. [DOI] [PubMed] [Google Scholar]
- 18.Dadar M., Tiwari R., Karthik K., Chakraborty S., Shahali Y., Dhama K. Candida albicans-Biology, molecular characterization, pathogenicity, and advances in diagnosis and control–An update. Microb. Pathog. 2018;117:128–138. doi: 10.1016/j.micpath.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Boroujeni Z.B., Shamsaei S., Yarahmadi M., Getso M.I., Khorashad A.S., Haghighi L., et al. Distribution of invasive fungal infections: molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: a 3-year experience with 490 patients under intensive care. Microb. Pathog. 2021;152 doi: 10.1016/j.micpath.2020.104616. [DOI] [PubMed] [Google Scholar]
- 20.Raiesi O., Hashemi S.J., Ardehali M.M., Ahmadikia K., Getso M.I., Pakdel F., et al. Molecular identification and clinical features of fungal rhinosinusitis: a 3-year experience with 108 patients. Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105018. [DOI] [PubMed] [Google Scholar]
- 21.Raiesi O., Hashemi S.J., Getso M.I., Ardi P., Ardehali M.M., Raissi V., et al. First report of chronic invasive fungal rhinosinusitis in a patient with ovarian cancer caused by Didymella pedeiae and successful treatment with voriconazole: a case report. Current Medical Mycology. 2021;7:55. doi: 10.18502/cmm.7.1.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamsaei S., Falahati M., Farahyar S., Raiesi O., Haghighi L., Farahani H.E., et al. Acute invasive fungal rhinosinusitis: molecular identification and update in management of frozen section biopsy. Microb. Pathog. 2021;159 doi: 10.1016/j.micpath.2021.105125. [DOI] [PubMed] [Google Scholar]
- 23.Performance Standards for Antifungal Susceptibility Testing of Yeasts. 2017. https://clsi.org/media/1895/m60ed1_sample.pdf CLSI supplement M60. 1 st ed. 1–12. [Google Scholar]
- 24.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamet M., Pavon A., Dalle F., Pechinot A., Prin S., Quenot J.-P., et al. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med. 2012;38:1272–1279. doi: 10.1007/s00134-012-2584-2. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilska R.A., Rumbaugh K.P. Biofilm models of polymicrobial infection. Future Microbiol. 2015;10:1997–2015. doi: 10.2217/fmb.15.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barenfanger J., Arakere P., Cruz R.D., Imran A., Drake C., Lawhorn J., et al. Improved outcomes associated with limiting identification of Candida spp. in respiratory secretions. J. Clin. Microbiol. 2003;41:5645–5649. doi: 10.1128/JCM.41.12.5645-5649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Loeches I., Antonelli M., Cuenca-Estrella M., Dimopoulos G., Einav S., De Waele J.J., et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 30.Delisle M.-S., Williamson D.R., Perreault M.M., Albert M., Jiang X., Heyland D.K. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J. Crit. Care. 2008;23:11–17. doi: 10.1016/j.jcrc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Jiao Y., Zhang J., Xu J., Cheng Q., Li Y., et al. Microbial etiology and prognostic factors of ventilator-associated pneumonia: a multicenter retrospective study in Shanghai. Clin. Infect. Dis. 2018;67:S146–S152. doi: 10.1093/cid/ciy686. [DOI] [PubMed] [Google Scholar]
- 32.Delisle M.-S., Williamson D.R., Albert M., Perreault M.M., Jiang X., Day A.G., et al. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can. Respir. J. J. Can. Thorac. Soc. 2011;18:131–136. doi: 10.1155/2011/827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timsit J.-F., Schwebel C., Styfalova L., Cornet M., Poirier P., Forrestier C., et al. Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med. 2019;45:834–843. doi: 10.1007/s00134-019-05622-0. [DOI] [PubMed] [Google Scholar]
- 34.Ader F., Jawhara S., Nseir S., Kipnis E., Faure K., Vuotto F., et al. Short term Candida albicans colonization reduces Pseudomonas aeruginosa-related lung injury and bacterial burden in a murine model. Crit. Care. 2011;15:1–9. doi: 10.1186/cc10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Geest P.J., Dieters E.I., Rijnders B., Groeneveld J.A. Safety and efficacy of amphotericin-B deoxycholate inhalation in critically ill patients with respiratory Candida spp. colonization: a retrospective analysis. BMC Infect. Dis. 2014;14:1–9. doi: 10.1186/s12879-014-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antinori S., Bonazzetti C., Gubertini G., Capetti A., Pagani C., Morena V., et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun. Rev. 2020;19:102564. doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ventoulis I., Sarmourli T., Amoiridou P., Mantzana P., Exindari M., Gioula G., et al. Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. Journal of Fungi. 2020;6:98. doi: 10.3390/jof6030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. 2020. A National Strategy to Diagnose COVID-19 Associated Invasive Fungal Disease in the ICU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posteraro B., Torelli R., Vella A., Leone P.M., De Angelis G., De Carolis E., et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. Journal of Fungi. 2020;6:163. doi: 10.3390/jof6030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hatmi A.M., Mohsin J., Al-Huraizi A., Khamis F. COVID-19 associated invasive candidiasis. J. Infect. 2020;82:45–46. doi: 10.1016/j.jinf.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically Ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020;26:2694. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raiesi O., Shabandoust H., Getso M., Raissi V., Rezaei A.A. Candida auris: a new emerging fungal monster. Archives of Clinical Infectious Diseases. 2019;14 [Google Scholar]