Abstract
Neuromuscular complications in paediatric patients with severe coronavirus disease 2019 (COVID-19) are poorly characterised. However, adult patients with severe COVID-19 reportedly present with frequent neuromuscular complications that mainly include critical illness polyneuropathy (CIP), critical illness myopathy (CIM), and focal neuropathies. We examined the records of all paediatric patients with severe COVID-19 who were mechanically ventilated and experienced neuromuscular complications from our single tertiary centre between March 2020 and August 2021. During this period, 4/36 (11%) patients admitted to the paediatric ICU who were mechanically ventilated experienced neuromuscular complications (one CIM, two focal neuropathies, and one CIP associated with plexopathy). In three of them, the gamma genetic variant of SARS-CoV-2 was identified. At the 4–5 month follow-up, three of our patients exhibited slight clinical improvement. We conclude that paediatric patients with severe COVID-19 may present neuromuscular complications similar to adults (11%), and their medium-term prognosis seems unfavourable.
Keywords: Brachial plexus neuropathies, COVID-19, Intensive care units, Neuromuscular manifestations, Peripheral nervous system, Severe acute respiratory syndrome
1. Introduction
Patients with severe coronavirus disease 19 (COVID-19), like other patients hospitalised in the Intensive Care Unit (ICU), can present with specific neuromuscular (NM) complications, with critical illness polyneuropathy CIP) and myopathy (CIM) being the most common [1]. In adults, severe COVID-19 is also reportedly associated with focal neuropathies and plexopathies at a significantly higher frequency than expected [2].
CIP and CIM have been reported in up to half the adults presenting with severe COVID-19 [1]. A prospective study observed that CIP was more frequent in patients who suffered COVID-19 than in a control group of critically ill patients [3]. Conversely, focal neuropathies and plexopathies have been observed in 5–15% of adult patients with COVID-19 who were hospitalised in the ICU [4,5]. These findings suggest that there is an association between severe COVID-19 and peripheral nerve involvement, at least in adults (Table 1 ).
Table 1.
Neuromuscular complications in adult patients with severe COVID-19.
| COVID-19 in ICU (N) | 256 | NR | 69 | 114 (74 with IMV) | 111 | 40 with VMI | |
|---|---|---|---|---|---|---|---|
| Patients with NM complications | Age mean, range | 54 39–69 |
53 40–76 |
58 50–77 |
60 53–67 |
64 55–70 |
50 40–59 |
| Men% | 80 | 100 | 73 | 35 | 100 | 100 | |
| Obesity | 53% | NR | 18% | mean BMI =28 | mean BMI = 35 |
60 | |
| Diabetes% | 46 | 28 | 18 | NR | 36 | NR | |
| ICU/ MV days, mean |
32/ NR |
NR / NR |
36 / NR |
24/ 30 |
27/ NR |
NR/ 15 |
|
| Prone |
100% | 100% | 45% | NR | NR | NR | |
| Total NM patients (N) | 15 upper limb MPNI | 7 MPNI | 11 MPNI | 36 ICUAW | 11 ICUAW | 5 ICUAW | |
| CIP/ CIM/ CIPM |
NR | NR | 1/ 0 |
36 with ICUAW |
7/ 4 |
2/ 1/ 1 |
|
| Brachial plexopathy | 12 | 2 | 0 | NR | NR | 0 | |
| Ulnar Np | 11 | 5 | 6 | NR | NR | 0 | |
| Sciatic Np | NR | 0 | 5 | NR | NR | 0 | |
| Other focal Np | NR | 2 | 17 | NR | NR | 1 | |
| Reference | Miller | Brugliera | Needham | Van Aerde | Frithiof | Bax |
CIM: critical illness myopathy, CIP: critical illness neuropathy, CIPM: critical illness neuromyopathy, ICU: intensive care unit, ICUAW: ICU acquired weakness, IMV invasive mechanical ventilation, MPNI: multiples peripheral nerve injury, MV: mechanical ventilation, N: number, NM: neuromuscular, Np Neuropathy; NR not reported.
However, in paediatric patients, it has been reported that patients hospitalised for COVID-19 may present with flaccid tetraparesis [6,7], characterised by variable myopathic and neuropathic signs in electrophysiological studies. Nevertheless, we found no reports of focal neuropathy or plexopathy in children with severe COVID-19 [8].
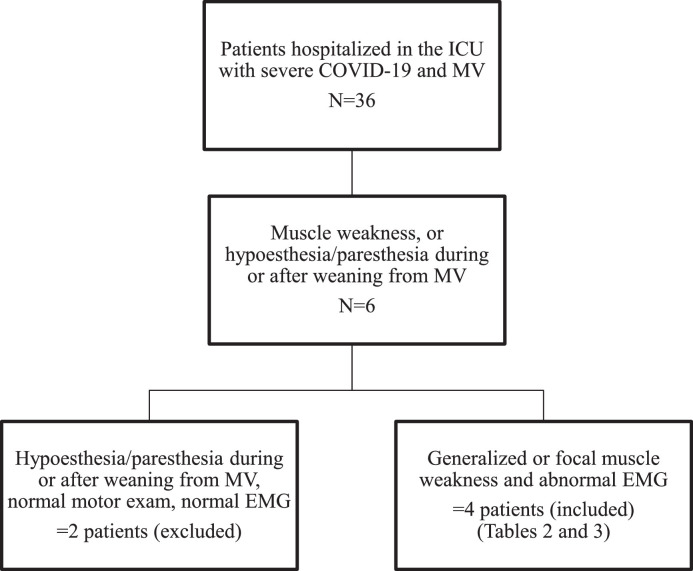
Between March 2020 and August 2021, 11% (4/36) of the children under the age of 18 years admitted to the paediatric ICU of the Sótero del Río Hospital for severe COVID-19 with mechanical ventilation (MV) requirement experienced NM complications. Given that NM complications associated with severe COVID-19 in paediatric patients have been poorly characterised, here, we report the details of these four patients in our centre (Fig. 1 ).
Fig. 1.
Selection flowchart of 36 paediatric patients with severe COVID-19
Thirty-six patients were hospitalized in the ICU. Six of them had sensitive or motor deficits, but only four of them had motor and EMG findings, which were confirmatory of a neuromuscular complication. ICU: intensive care unit, MV: mechanical ventilation.
This study was approved by the local ethics review board (Comité Etico Científico del Servicio de Salud Metropolitano Sur Oriente). Owing to the retrospective nature of this study, the requirement for informed consent was waived.
2. Case report
These four patients were admitted to the ICU between May and June 2021, with the diagnosis of acute respiratory distress syndrome due to COVID-19 and were treated with a dexamethasone and anticoagulation scheme for 10 days, according to our protocol. No other anti-inflammatory or immunomodulatory drugs were used as part of this protocol. The SARS-CoV-2 virus gamma variant was identified in three patients. The characteristics of each patient are presented in Tables 2 and 3 .
Table 2.
Neuromuscular complications in four paediatric patients with severe COVID-19: ICU stay characterisation.
| P | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | Male | Female | Female | Female |
| Age | 17 years | 15 years | 10 months | 15 years |
| Nutritional status | BMI= 55. Class III obesity |
BMI=30. Class I obesity |
Score z = 2.8. Obesity | BMI=51. Class III obesity |
| COVID-19 variant | gamma | other non-identified | gamma | gamma |
| CRP mg/L D-dimer ng/mL WBC (uL) at Adm |
145 1552 12.090 |
32.4 510 2.520 |
0.2 1482 3.640 |
77.3 666 8.940 |
| ANC (uL) ALC (uL) at Adm |
10.600 900 |
1.700 700 |
1.000 2.400 |
6.900 1.700 |
| Haematocrit (%) Platelet count (uL) at Adm |
40 225.000 |
41 151.000 |
33 119.000 |
40 188.000 |
| CK at Adm UI/L | 276 | 214 | 154 | 242 |
| Peak CK UI/L | 3400 | 214 | 542 | 362 |
| IMV NIMV (days) |
17 15 | 0 6 | 16 4 | 16 0 |
| Prone (days) | 55 h | 0 | 9 | 6 |
| Other systems involvement | myocarditis, hypertension, AKI | no | Neumopericardium, neumomediastin, AKI | type 2 DM,SIRS |
| Antibiotics, steroids, vasoactive drugs | ceftriaxone, cloxacilin, vancomincin, amikacin, dexametasone | ceftriaxone, ampicillin/ sulbactam, dexametasone | ampicillin/ sulbacatam ceftriaxone, dexamethasone, epinefrin, milrinone | ampicillin/ sulbactam, linezolid, piperaziline/ tazobactam, meropenem, cotrimoxasol, dexamethasone, methylprednisolone |
| Vecuronium (days) | 7 | 0 | 9 | 10 |
| ICU (days) | 45 | 11 | 21 | 21 |
Adm: admission, ALC: absolute lymphocyte count, ANC: absolute neutrophil count, BMI: body mass index, CRP: C reactive protein, IMV: invasive mechanical ventilation, NIMV: non-invasive mechanical ventilation, P: patient, WBC: white blood cell count.
Table 3.
Neuromuscular complications in four paediatric patients with severe COVID-19: neuromuscular characterisation.
| P | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1st symptoms | Generalised weakness | Left hand outer border hypostesia, inability to take objects with the left hand. Daily living activities with support | Generalised weakness | Left leg and foot hypostesia and pain, left foot drop |
| 1st NM evaluation: MRC/MRC Sumscore (days after admission/days after paralysing drugs suspension) | MRC Sumscore: 6/60 (21/7) | MRC: left hand: 4/5 ulnar finger flexors/extensors (31/NP) | MRC Sumscore 28/60 (19/7) | MRC: left lower limb: knee flexors 4/5, plantar flexion 0/5, dorsiflexion 0/5 (23/10) |
| 1st EMG (days after admission) | Sensorimotor axonal neuropathy (day 21) | Left cubital neuropathy (day 148) | Generalised myopathic signs (day 19) | Left sciatic neuropathy (day 94) |
| Final NM diagnosis | Critical patient neuropathy, left brachial plexopathy | Left ulnar neuropathy | Critical patient myopathy | Left sciatic neuropathy |
| Follow-up | Day 55: left upper limb: deltoid 1/5 deltoid, 2/5 biceps. Sumscore 38/60. 2nd EMG: left brachial plexopathy 5 months: Walks two steps with support. Left deltoid. Left biceps 2/5. MRC Sumscore 51/60 |
4 months: able to take objects with the left hand. Daily living activities without support. Left hand hypostesia and weakness. left hand: 4/5 ulnar finger flexors/extensors. | 4 months: walks with support. Sumscore 60/60 | 4 months: no recovery. Walks with left steppage |
MRC: Medical Research Council, NM: neuromuscular, NP: no paralysing drugs, P: patie.
Three of the four patients with NM complications were ventilated in prone position for a mean period of 80 h. In contrast, only two of the 32 patients who did not have NM complications were ventilated in a prone position for a mean period of 54 h. Additionally, all the patients with NM complications were obese, with a mean BMI of 35, but only 15 of the 32 patients without NM complications were obese, having a mean BMI of 31.
2.1. Case 1
A 17-year-old boy with type III obesity was admitted to the ICU, intubated, and placed in the prone position. On the second day after admission, an acute myocardial infarction was suspected, because ultrasensitive troponine I increased up to 1005 pg/mL (normal value<34 pg/mL). Therefore, salicylic acetyl acid and atorvastatin were initiated, and cardiac ultrasound was performed and showed normal findings. Posteriorly, troponin declined to 10 pg/mL, on day eleven after admission, while a progressive elevation of CK was observed during atorvastatin treatment, reaching up to 3.400 UI/L (normal value 30–200 UI/L) on day 10 after admission. We did not measure myoglobin, as it was not available at our laboratory at that moment. Atorvastatin-induced myositis was then suspected. Hence, we decided to stop this medication, and CK normalized posteriorly. A final diagnosis of myocarditis was made, but the patient did not meet other MIS-C criteria.
He required invasive MV (IMV) for 17 days, was prone for 55 h, and paralysed for 7 days. On the 21st day post-admission, after extubation and several days of suspended sedation and paralysis, the patient presented with tetraplegia. On examination, he was awake – with preserved occulomotility and facial mimicry – but also had tetraplegia and Achilles areflexia, with a Medical Research Council (MRC) sum score [9] of 6/60. An electrophysiology study showed a decrease in the amplitude of CMAPs (compound muscle action potentials) and SNAPs (sensitive nerve action potentials), with signs of active denervation without voluntary muscle activation, which is suggestive of CIP. His-mobility recovered over time, but on admission day 55, as he regained muscle strength, asymmetry was observed in the mobility of the upper limbs with paresis of the left upper limb, for which brain CT and angio-CT were requested. No abnormalities were noted on the images. On re-examination, hypotonia of the left upper limb was observed, with the upper limb adducted and extended. Thus, left brachial plexopathy was suspected. The electrophysiology study was repeated and the clinical diagnosis was confirmed, suggesting a compromise of the upper trunk. At the last follow-up, 5 months after ICU admission, the patient could stand independently for 30 s and had recovered partial mobility of the left upper limb, with a strength score of 2/5 on the MRC scale in the most affected muscles. The electrophysiological study was repeated, and signs of re-innervation in the affected muscles were observed.
2.2. Case 2
A 15-year-old girl with a history of obesity was admitted to the ICU, requiring non-invasive MV (NIMV) for 6 days. During her ICU stay, she noticed hypaesthesia of the ulnar surface of the hand, and difficulty picking up objects. She was evaluated by a neurologist on day 31 of her ICU admission. Examination revealed the hypaesthesia reported by the patient and weakness in her left hand, with MRC 4/5 in flexion and extension of the fourth and fifth fingers, which limited her activities of daily living. Electroneuromyography performed 4 months after her ICU admission showed ulnar neuropathy at the elbow level. At the last follow-up, the patient still experienced persistent weakness and hypaesthesia but was able to perform her daily living activities.
2.3. Case 3
An obese 10-month-old female infant was admitted to the ICU, requiring IMV for 16 days, nine of which she was ventilated in the prone position. On day 19 of the disease – after extubation and suspension of sedation and paralysis, while she was with NIMV – weakness of the four extremities was observed and an MRC sum score of 28/60 was observed on neurological examination. A myopathic process was suspected and subsequently confirmed by an electrophysiological study. She progressively improved; at 3 months after ICU admission, the patient had returned to her neurological baseline. At the last follow-up, 4 months after admission, neurological examination showed a normal strength MRC sum score of 60/60, and she was able to walk with assistance.
2.4. Case 4
A 15-year-old girl with a history of type III obesity was admitted to the ICU and was intubated and pronated for 7 days. After sedation was suspended, pain was evident in the posterior region of the left leg and in the left foot with foot drop. Neurological examination on day 23 of ICU admission revealed left Achilles areflexia, as well as knee flexion 4/5, plantar flexion 0/5, and dorsiflexion 0/5 according to the MRC scale. Pain management was provided using pregabalin (75 mg a day) – with a good response – and physical therapy. She was pain-free in the pregabalin suspension phase, and could walk 4 months after ICU admission; unfortunately, the foot drop remained and motor examination revealed no clinical improvement.
3. Discussion
Here, we presented four cases of paediatric patients with severe respiratory distress syndrome due to COVID-19 and serious complications of the peripheral nervous system. Three cases were adolescents with focal lesions of the peripheral nerves and plexuses, and one patient also had a CIP. One patient was an infant with CIM. Notably, three of the four cases were associated with the gamma genetic variant, required IMV, and experienced more severe motor impairment.
COVID-19 is associated with different neurological manifestations and complications in adults and children [10,11]. Amongst them, milder NM manifestations and complications include anosmia, ageusia, and myalgia. [2,12]. Patients with severe COVID-19 generally present with more severe NM sequelae – mainly CIP and CIM [1,13], focal neuropathies, and plexopathies [4,5,14].
In adults, the NM complications of COVID-19 have been better described and appear to be more frequent than in children. CIP and CIM have been observed in 10–40% of ICU patients with COVID-19 [1,3,13]. One study identified that CIP was more frequent than CIM in patients with severe COVID-19, and an early elevation of neuronal damage markers, neurofilament light chain and fibrillary acidic protein, suggested that there was an association between COVID-19 and nerve damage [3]. Additionally, there are several case reports of adult patients over 40 years of age who developed focal polyneuropathies and plexopathies [4,5,14]. In these patients, ulnar, sciatic, and brachial plexus nerve involvement were more frequently observed, similar to our paediatric patients.
After several months of the pandemic, we are observing the consequences of COVID-19 at the muscular level [15], [16], [17]. A longitudinal study of 1655 adult patients reported that at 6-month follow-up, the most frequent symptom post COVID-19 was muscle weakness (63% of cases) [18]. Patients with the highest risk of presenting this motor sequela were those with more severe respiratory compromise during hospitalisation.
Several case series have detailed paediatric patients presenting with neurological complications associated with COVID-19, including NM ones. One study reported that 13% [7] of paediatric patients hospitalised for COVID-19 presented with neurological complications and of those, 60% experienced complications of the peripheral nervous system – mainly Guillain Barré syndrome and flaccid tetraparesis [7]. However, pure muscle involvement in children with severe COVID-19 appears to be rare. We found only one case report of a child with myositis after hospitalisation requiring IMV [19]. In our group of patients, only patient 3 presented with a myopathic condition, which was classified as CIM and associated with a moderate elevation of creatine kinase.
All patients who had serious NM complications were obese, and the most severe complications were observed in the two children who had type III obesity and were also carriers of the gamma variant. Further, there was a temporal association between the predominance of gamma variants in SARS-CoV-2 in our country (Chile) and the described cases. These cases were registered in May and June 2021, during the largest wave so far in Chile, with the highest incidence paediatric ICU admission due to COVID-19. A Brazilian study also reported that there was a higher proportion of young adults with severe COVID-19, associated with the circulation of the gamma variant [20]. Due to the greater severity of COVID-19 in younger people, a greater risk of NM complications that seem to be related to the severity of respiratory condition can be expected. The greater severity of gamma variant infection in these patients could be explained by a reduced neutralising reaction to SARS-CoV-2 [21].
The underlying mechanisms of focal neuropathies in patients with severe COVID-19 have been attributed to prone ventilation, including compression and traction, although some authors have proposed that inflammatory mechanisms may also be contributing factors [4,5]. An inflammatory mechanism is postulated since neuropathies associated with mild COVID-19, whose parainfectious origin is known, such as Parsonage Turner Syndrome, have been observed [22]. Moreover, a clinical series observed electrophysiological signs suggestive of multiple mononeuritis in patients with COVID-19 [4]. In patients with COVID-19 and CIP/CIM risk factors, such as a longer stay in the ICU, more thromboembolic events, and IMV for more than 2 weeks, have been recognised [18].
We recommend conducting comprehensive clinical examinations in paediatric patients with motor deficits associated with COVID-19 and a high likelihood of peripheral and focal involvement. Patients who present an initial generalised compromise should be followed-up over time since focal neuropathies may manifest later. Evaluating children with suspected NM complications, using the MRC and MRC sum score scale, and routine electrophysiological studies would be most appropriate.
The focal complications in the two patients with type III obesity are attributable to being in a prone position for an extended period and the use of sedation and paralysis. In one of the patients who presented with ulnar neuropathy, but did not require IMV, sedation and paralysis or being in a prone position – focal complications were probably mediated by inflammatory mechanisms. In patients with CIP/CIM, we attribute the clinical symptoms to risk factors such as corticoids, sedation and paralysis multi-organ failure, and prolonged IMV.
The main limitations of this report are the low number and heterogeneity of the patients and the retrospective nature of the investigation. However, the concomitance of the four cases reported here with COVID-19 and their similarity to the series previously reported in adult patients, suggests that there is an association between the observed NM complications and COVID-19.
In the future, prospective and multicentre studies should examine a larger number of patients to help clarify the mechanisms underlying NM complications. It remains unclear whether these complications are only a consequence of the severity of the disease or whether an inflammatory mechanism associated with COVID-19 is also involved; obesity seems to be a predominant comorbidity in this series. Similarly, it is crucial to understand the long-term prognosis of neuropathies, since in our series, the prognosis was unfavourable.
This is the first report to include neuropathies and plexopathies as NM complications in paediatric patients with severe COVID-19, which is why we believe it is important that we provide detailed descriptions of the clinical presentation and medium-term outcomes of these cases. We recommend being aware of these possibly serious complications in paediatric patients and observing their disease course and possible sequelae accordingly.
4. Conclusion
We conclude that paediatric patients with severe COVID-19 may present neuromuscular complications similar to adults and that their medium-term prognosis seems unfavourable.
Author contributions
Pamela Céspedes, Agustín Cavagnaro, and Adriana Wegner were responsible for the intensive care management of the patients. Jenniffer Angulo and Felipe Reyes we responsible for the analysis of SARS-CoV-2 variants. Daniela Avila-Smirnow was responsible of the neurological and electrophysiological assessment of the patients and data analysis. All authors contributed to the writing of the manuscript and have approved the final version.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgements
The authors want to thank the clinicians who referred the four patients reported here.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Van Aerde N., Van den Berghe G., Wilmer A., Gosselink R., Hermans G., Meersseman P., et al. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. 2020;26:10–12. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frithiof R., Rostami E., Kumlien E., Virhammar J., Fällmar D., Hultström M., et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: a prospective study. Clin Neurophysiol. 2021;132:1733–1740. doi: 10.1016/j.clinph.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needham E., Newcombe V., Michell A., Thornton R., Grainger A., Anwar F., et al. Mononeuritis multiplex: an unexpectedly frequent feature of severe COVID-19. J Neurol. 2021;268:2685–2689. doi: 10.1007/s00415-020-10321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugliera L., Filippi M., Del Carro U., Butera C., Bianchi F., Castellazzi P., et al. Nerve compression injuries after prolonged prone position ventilation in patients with SARS-CoV-2: a case series. Arch Phys Med Rehabil. 2021;102:359–362. doi: 10.1016/j.apmr.2020.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacohen Y., Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval F., Julio K., Méndez G., Valderas C., Echeverría A.C., Perinetti M.J., et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol. 2021;36:853–866. doi: 10.1177/0883073821989164. [DOI] [PubMed] [Google Scholar]
- 8.Singer T.G., Evankovich K.D., Fisher K., Demmler-Harrison G.J., Risen S.R. Coronavirus infections in the nervous system of children: a scoping review making the case for long-term neurodevelopmental surveillance. Pediatr Neurol. 2021;117:47–63. doi: 10.1016/j.pediatrneurol.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan Z., Topaloglu M., Ozyemisci Taskiran O. Medical research council-sumscore: a tool for evaluating muscle weakness in patients with post-intensive care syndrome. Crit Care. 2020;24:4–5. doi: 10.1186/s13054-020-03282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munhoz R.P., Pedroso J.L., Nascimento F.A., De Almeida S.M., Barsottini O.G.P., Cardoso F.E.C., et al. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq Neuropsiquiatr. 2020;78:290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- 11.Panda P.K., Sharawat I.K., Panda P., Natarajan V., Bhakat R., Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr. 2021;67:1–11. doi: 10.1093/tropej/fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero J., Barragán L., Martínez J., Montoya J., Peña A., Sobrino F., et al. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis. 2021;21:515. doi: 10.1186/s12879-021-06185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bax F., Lettieri C., Marini A., Pellitteri G., Surcinelli A., Valente M., et al. Clinical and neurophysiological characterization of muscular weakness in severe COVID-19. Neurol Sci. 2021;42:2173–2178. doi: 10.1007/s10072-021-05110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C., O'Sullivan J., Jeffrey J., Power D. Brachial plexus neuropathies during the COVID-19 pandemic: a retrospective case series of 15 patients in critical care. Phys Ther. 2021;101:1–8. doi: 10.1093/ptj/pzaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medrinal C., Prieur G., Bonnevie T., Gravier F.E., Mayard D., Desmalles E., et al. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021;21:1–5. doi: 10.1186/s12871-021-01274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan K., Antognini D., Combes A., Paden M., Zakhary B., Ogino M., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2020;397:19–21. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [Google Scholar]
- 19.De Azevedo Z.M.A., Camacho K.G., Caixeta D.M.D.L., Lima-Setta F., Salles T.R.D.S., De Góes F.V., et al. Children's multisystem inflammatory syndrome with myopathy. Rev Soc Bras Med Trop. 2021;54:1–4. doi: 10.1590/0037-8682-0865-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonaka C.K.V., Gräf T., Barcia CA de L., Costa V.F., de Oliveira J.L., Passos R da H., et al. SARS-CoV-2 variant of concern P.1 (Gamma) infection in young and middle-aged patients admitted to the intensive care units of a single hospital in Salvador, Northeast Brazil, February 2021. Int J Infect Dis. 2021;111:47–54. doi: 10.1016/j.ijid.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22:757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitry M.A., Collins L.K., Kazam J.J., Kaicker S., Kovanlikaya A. Parsonage-turner syndrome associated with SARS-CoV2 (COVID-19) infection. Clin Imaging. 2021;72:8–10. doi: 10.1016/j.clinimag.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]