Abstract
In recent years, a wealth of studies has identified various molecular species released by cardiac muscle under physiological and pathological conditions that exert local paracrine and/or remote endocrine effects. Conversely, humoral factors, principally produced by organs such as skeletal muscle, kidney, or adipose tissue, may affect the function and metabolism of normal and diseased hearts. Although this cross communication within cardiac tissue and between the heart and other organs is supported by mounting evidence, research on the role of molecular mediators carried by exosomes, microvesicles, and apoptotic bodies, collectively defined as extracellular vesicles (EVs), is at an early stage of investigation. Once released in the circulation, EVs can potentially reach any organ where they transfer their cargo of proteins, lipids, and nucleic acids that exert potent biological effects on recipient cells. Although there are a few cases where such signaling was clearly demonstrated, the results from many other studies can only be tentatively inferred based on indirect evidence obtained by infusing exogenous EVs in experimental animals or by adding them to cell cultures. This area of research is in rapid expansion and most mechanistic interpretations may change in the near future; hence, the present review on the role played by EV-carried mediators in the two-way communication between heart and skeletal muscle, kidneys, bone marrow, lungs, liver, adipose tissue, and brain is necessarily limited. Nonetheless, the available data are already unveiling new, intriguing, and ample scenarios in cardiac physiology and pathophysiology.
Keywords: exosomes, extracellular vesicles, heart, interorgan communication, microvesicles
INTRODUCTION
In a review article on cardiac endocrine function published in this journal 15 years ago, Clerico et al. (1) hypothesized the existence of a means of “cross-talk between cardiac endocrine function and other integrated systems of the body.” At that time, the role of cardiac muscle as a source of potent natriuretic factors was well defined (2); however, virtually nothing was known regarding possible two-way communication between heart and other organs. In more recent years, a wealth of studies identified a variety of peptides and proteins, collectively named myokines/cardiokines (3–5), as well as of lipids and nucleic acids released by cardiac muscle under physiological and pathological conditions, which exert local paracrine and/or remote endocrine effects (6, 7). For instance, an intriguing line of research is focusing on the modulation of systemic metabolism by cardiac factors, many of which have not been identified (8–10). On the other hand, humoral factors, principally released by organs such as skeletal muscle, kidney, or adipose tissue, may affect function and metabolism of normal and diseased hearts (6, 11). A typical example of myokine released by both skeletal and cardiac muscle is follistatin-like protein 1, a protective factor and possibly a mediator of metabolic function (12–15).
To add a further layer of complexity to this network, studies published over the past 10 years have uncovered a wealth of intraorgan and interorgan signals mediated by factors enclosed in vesicles known as extracellular vesicles (EVs) (Fig. 1). Virtually all cell types can release EVs, and cardiac tissue is no exception (16–20).
Figure 1.
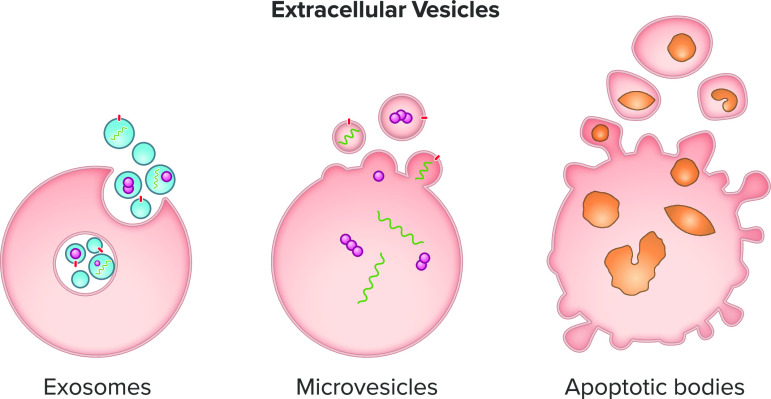
Schematic representation of extracellular vesicle production by viable (exosomes and microvesicles) and apoptotic cells (apoptotic bodies).
EV-mediated intracardiac cell-to-cell communication has been recently covered by extensive review articles (21, 22). Cardiomyocytes represent only ∼30% of cardiac cells (23, 24); hence, they need to communicate with neighboring fibroblasts, endothelial cells, macrophages, smooth muscle cells, and pericytes. Such constant communication ensures heart homeostasis (25). The flow of information inside cardiac tissue is a complex and highly regulated process, in which EVs play a substantial role. EVs can be released and taken up by cardiomyocytes (17), cardiac fibroblasts (16, 26–28), endothelial cells (18, 20, 29, 30), and vascular smooth muscle cells (19) under normal and pathological conditions. These vesicles function as vectors of cell-cell signaling. A large array of proteins and nucleic acids derived from cardiomyocyte mitochondria, cytosol, and plasma membrane have been identified in exosomes and may influence biological functions when delivered to other cells (31). Some examples are the Wnt-binding protein, a major player in the cardiac development signaling pathway (32), inflammatory factors such as IL-6 (33), IL-1β (34), and TNF-α (192), the metabolic transporters/enzymes GLUT1, GLUT4, and lactate dehydrogenase (29), and the heat shock proteins, such as Hsp20 (36) as well as a wide variety of miRNAs (26, 30, 37). The specific cargo carried by EVs is determined during their biogenesis and by stimuli affecting the parent cells (31). For instance, growth factor stimulation of cultured cardiomyocytes can induce a change in EV miRNA content (38).
Regarding the communication between heart and other organs, although many review articles, including a very recent position paper by the European Society of Cardiology Working Group (39), have covered the endocrine signals mediated by various free molecules, none of them has extensively discussed the current body of knowledge of interorgan signals carried by EVs. Here, we aimed at gathering representative literature on the role played by EV-carried mediators in the communication between the heart and other organs. In a few cases, such a role was clearly demonstrated however, in many others, it can be inferred based on indirect evidence obtained by infusing exogenous EVs in experimental animals or by adding them to cell cultures. The signaling described in the following paragraphs is schematically summarized in Fig. 2.
Figure 2.
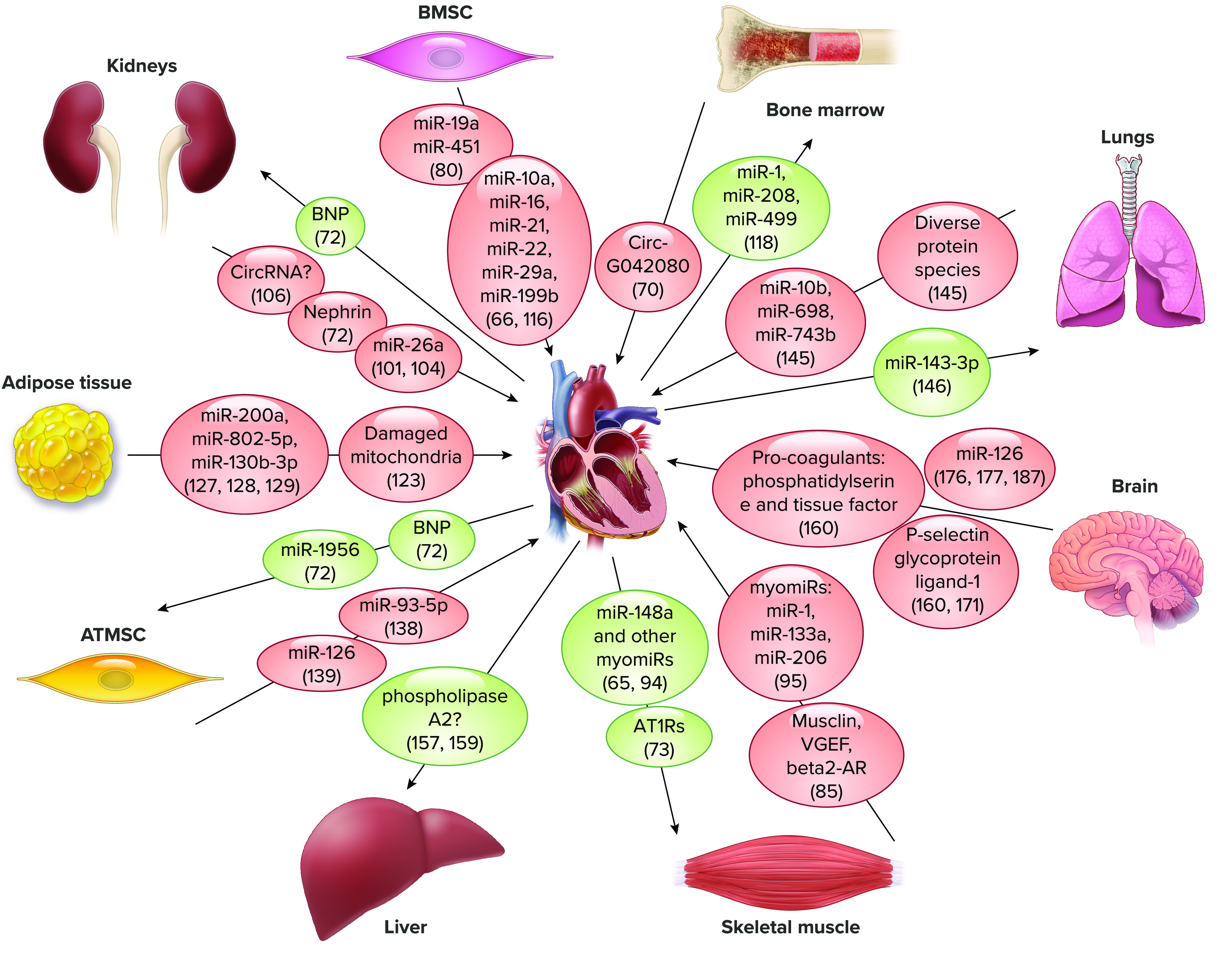
Extracellular vesicle-carried putative mediators of cell-cell communication between heart and other organs. ATIRa, angiotensin II type I receptors; ATMSC, adipose tissue mesenchymal stem cells; BMSC, bone marrow stem cells.
Extracellular Vesicles: Definition and Characteristics
EVs constitute a highly heterogeneous population of vesicles and are broadly classified as exosomes, microvesicles, and apoptotic bodies (5, 40) (Fig. 1). Despite attempts to further categorize EVs based on their size, origin, or function, major overlaps of these features do not allow a clear distinction among them (40, 41). Therefore, the International Society for Extracellular Vesicles (ISEV) consensus recommendation is to use “extracellular vesicle” as the “generic term for particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate (42),” which we will use in the present review; however, in some cases we will cite the same term used by the authors in their articles.
Dimensions and genesis of EVs vary considerably. Exosomes are 50–150-nm vesicles (43, 44), whereas microvesicles range between 50 and 1,000 nm (43, 45). Apoptotic bodies are typically larger in size and can reach 5,000 nm in diameter (46). The formation of exosomes starts in the endosomal system, where early endosomes mature into multivesicular bodies. This process involves double invagination of the plasma membrane (47, 48). The first invagination forms a cup-shaped structure composed of cell surface proteins and hydrophilic proteins. This results in the genesis of early endosomes (48, 49). The second, inward invagination of the endosomal limiting membrane produces multivesicular bodies containing intraluminal vesicles, i.e., the future exosomes (43, 50). The process ends with either multivesicular bodies content degradation by lysosomes or autophagosomes or with their fusion with the plasma membrane and release of intraluminal vesicles as exosomes (40, 51, 52).
Different from exosomes, microvesicles are directly shed from the cell membrane. A Ca2+-dependent enzymatic system, including aminophospholipid translocases, scramblases, and calpain, alters the organization of phospholipids in the cell membrane. Together with a rearrangement of the underlying actin cytoskeleton, the cell membrane is induced to bend outward (53–55). This favors membrane budding and formation of microvesicles (55).
Finally, apoptotic bodies are released by cells undergoing programmed cell death, which causes plasma membrane blebbing and nuclear fragmentation (56–58). Apoptotic bodies are then removed by phagocytic cells to prevent spillover and damage to the surrounding cells or tissues, hence their role in cell-cell interactions is poorly understood (59, 60).
Most EVs do not display a tropism for a specific cell type. One study demonstrated that fibroblast-derived exosomes uptake by cardiomyocytes depends on actin and temperature as cell treatment with cytochalasin D (an inhibitor of actin polymerization) or incubation of cells at 4°C decrease exosome uptake (16). Interestingly, three-dimensional (3-D) reconstruction of confocal image z-stacks demonstrated that exosomes were not simply attached to the cardiomyocyte surface but completely internalized by the cell (16). In other cases, exosomes can either bind to cell surface plasma membrane receptors and trigger signaling cascades (61, 62) or merge with the plasma membrane and release their cargo directly into the cytosol (63). Another mechanism of EV cargo release is the docking of Cx43-containing channels localized on both cell and exosome surfaces leading to the vesicle unloading into the target cells (64).
Exosomes and microvesicles convey messages encrypted in proteins (enzymes, biogenesis factors, chaperones, etc.), lipids, sugars, and nucleic acids (40). Over the last decade, EV proteins and nucleic acids have drawn remarkable interest as potential disease biomarkers or therapeutic targets (65–70), whereas EV lipids have not been widely studied (37, 71). The composition of EVs depends on the condition of the mother cells and may change depending on physiologic conditions such as normal aging, or in response to pathological processes like chronic or acute stress, inflammation, and fibrosis (72–74).
EV-MEDIATED COMMUNICATION BETWEEN HEART AND OTHER ORGANS
Mounting evidence supports a role for EVs in the communication between heart and other organs. Once released in the blood at a given site, EVs can virtually reach and target any organ (75) and transport a variety of different cargoes, including lipids, peptides, proteins, and nucleic acids (40). Interestingly, microvesicles and exosomes can cross also the blood-brain (76) and the maternal-fetal barrier (77). Bryl-Górecka et al. (78) found an inverse relationship between coronary flow rate and levels of circulating, protein loaded EVs in plasma, identified as endothelial cell microvesicles. Understanding the source of circulating EVs is a major challenge. For instance, tetraspanins CD9, CD63, and CD81, heat shock proteins HSP70 and HSP90, class I and II major histocompatibility complexes, and flotillin-1 are all particularly abundant in cardiac exosomes, yet they cannot be considered heart-specific (79, 80). Since EVs content changes depending on alterations of the parent cell, understanding their source may be less complicated under pathological than physiological conditions. A good example is represented by EVs carrying the cardiac hormone brain natriuretic peptide (BNP) during heart failure (72) or angiotensin II type I receptors during cardiac pressure overload (73). Nonetheless, identifying the EVs origin should be possible also under physiological conditions, based on the characterization of cell-specific protein cargos (7, 81).
EVs in Heart-Skeletal Muscle Cross Talk
Skeletal muscle is the most obvious organ to start with, as its cross-communication with cardiac muscle via myokines has been actively studied and might modulate their respective contractile functions (3, 10, 14, 82). Skeletal muscle is an important source of EVs (83) and, in fact, a rapid increase in circulating EVs has been observed during exercise (84) and following hindlimb ischemia (85). Fluorescently labeled muscle-derived exosomes injected in mice are taken up by the heart (86). Similarly, cardiosphere-derived exosomes are taken up by skeletal muscle (65).
The association between heart failure and abnormalities in skeletal muscle morphology and biochemistry is well known (87, 88). Possible mediators of the negative impact on skeletal muscle are myocardium-derived miRNAs released in the circulation during heart failure progression (89). The so called myomiRs, i.e., miR-1, miR-133, miR-208, and miR-499 (90–92), that strongly affect skeletal muscle, are abundantly represented in cardiac muscle and in the circulation after myocardial damage (93). As the EVs carry a significant amount of circulating myomiRs (94), they possibly play a role in heart-skeletal muscle communication. Aminzadeh et al. (65) found that cardiosphere-derived exosome treatment had a beneficial effect in mdx mice (a model of Duchenne muscular dystrophy) by increasing dystrophin expression, perhaps due to transfer of miR-148a in skeletal muscle cells. Conversely, the administration of circulating EVs isolated from the mdx mice decreased apoptosis in cultured C2C12 myocytes (95).
Pironti et al. (73) found that exosomes released from the heart of mice subjected to pressure overload are enriched with angiotensin II type 1 receptor and are transported to skeletal muscle, cardiomyocytes, and mesenteric resistance vessels. Importantly, trafficking and sorting of these receptors into exosomes required the multifunctional adaptor protein, β-arrestin (73).
Bei et al. (96) reported that intramyocardial injections of EVs collected from exercised mice had a better protective effect on the heart against acute ischemia/reperfusion injury compared with EVs from nonexercised controls. Correspondingly, exercise-associated EVs exerted an antiapoptotic effect in H2O2-treated H9C2 cardiomyocytes, mediated by the activation of ERK1/2 and HSP27 signaling (96). Homme et al. (85) recently reported that remote limb ischemic conditioning causes the release of exosomes from skeletal muscle that contain musclin, CD71, VEGF, and β2-adrenergic receptor (β2-AR) and alleviated heart failure in mice. Whitham et al. (97) also found that some myokine candidates could be enclosed in muscle-derived EVs; however, the validation of their function warrants further studies.
EVs in Heart-Kidney Cross Talk
Cardiac natriuretic peptides are long known to be secreted by degranulation (98). However, Gao et al. (72) found BNP and nephrin (a renal marker) enrichment in exosomes isolated from mouse serum after myocardial infarction, supposedly released by ischemic myocardium and by kidneys, respectively. Those authors suggested that exosomes may serve as circulating endocrine signals between the two organs (72). The BNP positive effects on kidneys are well known (99). Other organs may intervene in cardiac and renal EVs-mediated protection under pathological conditions. Recently, Lindoso et al. investigated the effects of adipose mesenchymal stromal cell-derived EVs in a rat model of deoxycorticosterone acetate-induced hypertension. They reported that these EVs can preserve renal filtration and prevent cardiac tissue fibrosis by maintaining blood pressure within normal levels (100). The authors hypothesized that both of these positive effects were due to vessel protection (100).
Circulating exosomal miRNAs have been recently profiled in patients with urinary albumin excretion, a marker of cardiovascular risk and renal damage in hypertension (101). In albuminuric subjects, one study identified a signature of 29 dysregulated circulating miRNAs, with miR-26a being the only one downregulated (101). Interestingly, human podocytes exposed to TGF-β1 in vitro did not show miR-26a downregulation at cellular level, but only in exosomes, suggesting a selective sorting of miRNAs (101). It has recently emerged that miR-26a is a regulator of podocyte homeostasis and plays an active role in the actin cytoskeleton maintenance (102). miR-26a is involved in cardiovascular repair mechanisms, such as cardiac angiogenesis and endothelial cell growth (103). Wang et al. (104) tested exosome-encapsulated miR-26a delivery in mice with uremic cardiopathy, a multifactorial complication of chronic kidney disease principally characterized by hemodynamic overload, circulating uremic toxins, metabolic acidosis, inflammation, increased oxidant stress, mineral bone disease, and insulin resistance (104, 105). Exogenous miR-26a in uremic mice diminished cardiac fibrosis and improved heart function, most probably by ameliorating insulin resistance (104).
Several circular RNAs are known to be altered during cardiovascular complications in chronic kidney disease (106). The circRNAs are believed to be principally transported by exosomes (107). Whether this kind of kidney-heart communication plays a pathogenic or rather a protective role remains an intriguing question.
EVs in Heart-Bone Marrow Cross Talk
The effects of the bone marrow-derived mesenchymal stem cells transplanted into infarcted myocardium have been the object of intensive investigations and represent a controversial topic. The initial hypothesis was that transplanted bone marrow-derived mesenchymal stem cells could induce cardiac repair by assuming a functional cardiomyocyte phenotype (108, 109). However, subsequent studies pointed to the paracrine release of bioactive molecules as an alternative reparative mechanism (110–112). These findings prompted research on exosomes released by bone marrow-derived mesenchymal stem cells and their cardioprotective/reparative effects. Yu et al. (113) reported diminished cardiac infarct size in rats after injection of bone marrow-derived mesenchymal stem cell-derived exosomes enriched with GATA-4 in the peri-infarct region. They also observed overexpression of miR-19a and miR-451 in the myocardium of treated rats (113). Recently, bone marrow-derived mesenchymal stem cell-derived exosomes have been reported to attenuate pressure overload-induced left ventricular hypertrophy and fibrosis in mice (66), as well as pulmonary pressure and right ventricular hypertrophy in rats with pulmonary artery hypertension (114). The same type of exosomes stimulates neovascularization and reduces the inflammatory response, thus improving heart function after ischemic injury (115). Mesenchymal stem cell-derived exosomes released after ischemic preconditioning were enriched with miR-22 and diminished apoptosis when delivered intramyocardially in infarcted mice (116). If added to fibroblasts isolated from patients with diabetes, these exosomes activate Akt, ERK, and STAT3 signaling pathways that are essential for wound healing and induce the expression of several growth factors, such as IL-6, hepatocyte growth factor, insulin-like growth factor-1, nerve growth factor, and stromal-derived growth factor-1 (117). These exosome-induced mechanisms of wound healing need deeper investigation to elucidate their possible role also in cardiac repair.
Interestingly, Sun et al. found a potential EV-mediated interorgan communication in patients with multiple myeloma. Exosomes extracted from the serum of these patients contained 10,106 differentially expressed circular RNAs compared with the control group (70). By correlation analysis they showed that the expression level of circ-G042080 was positively correlated with multiple myoloma-related myocardial damage and could interfere with myocardial damage processes through a downstream miRNA/TLR4 axis (70).
Possible EV-mediated signaling from heart to bone marrow is suggested by several studies. Cheng et al. (118) found that, soon after ischemic injury, myocardium releases a remarkable quantity of EVs loaded with cardiomyocyte-derived miRNAs, which travel with peripheral blood to target other tissues and preferentially bone marrow. MiR-1, miR-208, and miR-499 and, in part, miR-133 suppress chemokine receptor CXCR4 expression and mediate progenitor cell mobilization in bone marrow (118).
EVs in Heart-Adipose Tissue Cross Talk
Adipose tissue influences cardiovascular homeostasis by releasing adipocytokines (11). In obesity, overproduction of proinflammatory adipocytokines and decreased expression of anti-inflammatory adipocytokines are associated with cardiovascular diseases (119). Vice versa, proinflammatory and oxidative signals from stressed myocardium or vessels may modify the adipose tissue biological environment (11). Besides adipocytokines, adipose tissue is a major source of circulating miRNAs, a large fraction of which are carried by exosomes (120). Thomou et al. (120) proposed that the majority of circulating exosomes are derived from adipose tissue. However, another group found that under physiological conditions, putative adipose tissue exosomes were present at a low level in the blood stream (121). Aside from these conflicting reports, there is evidence that exosomes mediate the cross communication between heart and adipose tissue. This may even explain, at least in part, the “obesity paradox,” i.e., the better prognosis of cardiovascular diseases in patients with obesity compared with their slimmer matches (122). In fact, a recent major study by Crewe et al. (123) demonstrated that, in obesity, the stressed adipocytes release EVs transporting damaged mitochondrial particles, which enter the blood stream and are taken up by cardiomyocytes, where they induce the production of reactive oxygen species. This leads to the activation of an ischemic preconditioning-like adaptation mechanism possibly responsible for the attenuation of cardiac injury during ischemia (123).
Another study found that adiponectin, the most abundant circulating adipocytokine, stimulates exosome production from T cadherin-expressing cells in the heart, aorta, and muscle (124). This causes an impoverishment of ceramides in those cells, as ceramides are incorporated into exosomes that are then released in the circulation (125). Such a mechanism might be considered protective since excessive ceramide in cells is correlated with an elevated risk of atherosclerosis, cardiomyopathy, heart failure, and myocardial infarction (124–126). Fang et al. (127) proposed additional molecular players in the EV-mediated signaling between adipose tissue and cardiomyocytes. They found that peroxisome proliferator-activated receptor-gamma (PPARγ) activation in adipocytes causes the release of circulating miR-200a carried by exosomes that targets cardiomyocytes (127). MiR-200a downregulates TSC1, thus removing its inhibition of mammalian target of rapamycin (mTOR) signaling and consequently inducing cardiac hypertrophy (127). Other in vitro experiments demonstrated that hypertrophic adipocyte-derived exosomes are enriched with miR-802-5p that, if delivered to rat cardiomyocytes to target HSP60, causes unfolded protein response activation, oxidative stress, inflammation, and ultimately insulin resistance (128). Gan et al. (129) found significantly elevated levels of miR-130b-3p in EVs from diabetic rat adipocytes and from plasma of patients with diabetes compared with healthy controls. When the adipocyte EVs were delivered to the myocardium, detrimental effects after ischemia/reperfusion were observed. EV-treated hearts displayed significantly increased miR-130b-3p levels and AMPKα1/α2, Birc6, and Ucp3, all identified as miR-130b-3p direct downstream targets (129).
Recently, Guarnieri et al. (130) obtained data suggesting the involvement of exosome-mediated signaling in the development of cardiac fibrosis in mice with adipose-specific deletion of the human antigen R (Hur, an RNA binding protein). Adipo-HuR−/− mice displayed an interesting co-overexpression of genes related to exosomes formation/secretion and inflammation-related genes in subcutaneous white adipose tissue (130).
The link between atherosclerosis and adipose tissue-derived exosomes has been extensively investigated. One study demonstrated how these exosomes play a role in accelerating atherosclerosis by inducing M1 proinflammatory polarization of macrophages and so favoring the progression of atherosclerosis in APOE−/− mice (131). Another study reported that adipose tissue-derived mesenchymal stem cell-derived exosomes protects endothelial cells against atherosclerosis by restraining microRNA-342-5p expression (132).
Six weeks of intravenous administration of exosomes isolated from brown adipose tissue led to improved cardiac function and amelioration of metabolic syndrome in high-fat diet-fed mice (133). This beneficial effect can be attributed indirectly to the remodeled metabolism (reduced body weight, lowered blood glucose, and alleviated lipid accumulation); however, based on the fact that circulating exosomes can be internalized by cardiomyocytes (16, 17, 86), the authors hypothesized that there may be an additional direct effect on the heart (133). Further studies are warranted to test this hypothesis. On the other hand, exposure of cultured adipose tissue-derived mesenchymal stem cells to serum exosomes obtained from infarcted mice enhanced stem cell proliferation through the activation of ERK1/2 (72). Those exosomes, likely released by heart and kidney, were enriched in MiR-1956, identified as the possible messenger that stimulates angiogenesis and paracrine VEGF signaling in adipose tissue stem cells by downregulating Notch-1 (72).
Although exosomes from adipose tissue-derived mesenchymal stem cells have received less attention, several recent studies have documented their protective effects on cardiomyocytes during various types of stress (134, 135). Their administration to rats with myocardial ischemia/reperfusion injury significantly attenuated myocardial necrosis and apoptosis by 1) limiting Bcl-2 downregulation, 2) preventing Bax upregulation and caspase 3 activation, and 3) removing the inhibition of Wnt/β-catenin signaling (136). Other authors found that these exosomes attenuate cardiac damage after myocardial infarction by activating the S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization (137). Their positive effects during acute myocardial ischemic injury were enhanced by miRNA overexpression (miR-126 and miR-93-5p) (138, 139). Interestingly, adiponectin seems to be an effective stimulator of exosomes release by adipose tissue-derived mesenchymal stem cells (140). Additional adipocytokines such as omentin-1 have also been found enclosed in EVs released by adipose tissue (141) or other organs. For instance, lipocalin 2 has been found in neutrophil exosomes (142) and angiopoietin-like protein 2 in EVs released by vascular endothelial cells (143).
EVs in Heart-Lung Cross Talk
The lung is a major “consumer” of circulating exosomes (144). Even though heart and lung form a functional unit, EV-mediated communication among these two organs is relatively understudied. The published studies are mainly focused on EVs role in pulmonary arterial hypertension (114, 145–148). Circulating endothelial EVs were found elevated in adults with congenital atrial and ventricular septal defects, especially if complicated with pulmonary arterial hypertension (147). These EVs may enhance inflammatory processes leading to endothelial dysfunction and impaired vasodilatation via P38 MAPK-dependent pathways (147). Interestingly, EVs are not increased in children with congenital heart defects with or without associated pulmonary arterial hypertension (149). EVs isolated from blood and lungs of mice with monocrotaline-induced pulmonary arterial hypertension caused right ventricular hypertrophy and pulmonary vascular remodeling when injected into healthy mice (145). A recent study showed that pulmonary vascular endothelial cell-derived exosomes can prevent, through miR-107 transfer, the transdifferentiation of pericytes into a profibrotic phenotype (148). Mir-107 interfered with the HIF-1α/Notch1/PDGFRβ/YAP1/Twist1 signaling pathway (148). Deng et al. (146) found an upregulation of miR-143-3p in mouse lung and right ventricle after hypoxic stimuli causing pulmonary vasoconstriction, as well as in pulmonary artery smooth muscle cells of patients with pulmonary arterial hypertension. Mir-143-3p has also been detected in cardiac myocytes and fibroblasts. Furthermore, extracellular miR-143-3p was found to be transported by exosomes (146). Exosomal miRNA-143-3p activated migration and proliferation of pulmonary arterial endothelial cells (146).
EVs in Heart-Liver Cross Talk
The pathological condition known as cirrhotic cardiomyopathy attests the close relationship between heart and liver (150). Half of the patients with cirrhosis undergoing liver transplantation develop cardiac dysfunction, and 7%–21% of deaths after liver transplantation are caused by heart failure (150). Other associations between cardiac and liver diseases include: the increased risk of cardiac arrhythmias during nonalcoholic fatty liver disease (151, 152), the aberrant hepatic lipid metabolism accompanying hypertrophic cardiomyopathy (153), and the fatty liver as a cardiovascular disease risk factor (154–156). Nonetheless, virtually no information is available regarding EVs as part of the heart-liver communication under normal and pathological conditions. One study found that heart-liver communication involves phospholipase A2-mediated signaling (157) that modulates hepatic inflammation and lipid metabolism. Phospholipase A2 functions as a proinflammatory autocrine/paracrine factor (157, 158) and, in myocardium, it is stored in secretory granules (81, 158). Interestingly, there is evidence that lipid-associated proteins and bioactive lipids including the whole set of phospholipases A2, C, and D are enclosed in exosomes released by 2H3 mast cells (159). The comprehension of EVs role in heart-liver communication is certainly a very interesting topic for future research and might have important therapeutical implications.
EVs in Heart-Brain Cross Talk
Secondary cardiac abnormalities are common in neurological patients (160) and, conversely, cardiac dysfunction has been shown to elevate the risk of stroke (161). Heart-brain communication is an area of very intensive investigation, yet it is only recent that the role of EVs in heart-brain cross talk has come to the attention of the scientific community (162). Of note, 47% of postcardiac surgery patients display increased permeability of the blood-brain barrier (163), which is disrupted by stroke as well (164). EVs can bidirectionally cross the blood-brain barrier under physiological conditions (76) that is enhanced after barrier damage (35). In fact, the level of circulating EVs is elevated during ischemic or hemorrhagic stroke (165, 166) and is associated with endothelial dysfunction and coagulation activation, leading to thrombosis (160, 167). In the brain, EVs normally originate from neurons, astrocytes, microglia, and neural stem cells, and yet after stroke, their major source is represented by endothelial cells (168). Stroke-associated elevation of circulating microvesicles may mediate brain damage-induced cardiac dysfunction (160). These EVs cause endothelial dysfunction by decreasing NO synthesis as the result of endothelial NO synthase inhibition and by raising caveolin-1 levels (160). Moreover, microvesicles bind coagulation factors and activate them via two procoagulants, namely phosphatidylserine and tissue factor that are incorporated in the outer leaflet of their membrane (169, 170). Furthermore, microvesicles contain P-selectin glycoprotein ligand-1, which interacts with the P-selectin exposed by endothelial cells or platelets upon vascular damage. Such interactions activate tissue factor that triggers thrombosis (160, 171). These mechanisms may, at least in part, explain the results of the recently concluded clinical study PROSCIS-B, which reported an association between high levels of leukocyte- and endothelial cells-derived microvesicles after stroke and worse cardiovascular outcome within 3 years (172).
Several other miRNAs abundantly present in EVs have been shown to play a role both in cardiac and cerebral pathophysiology (173). Long et al. (174) found a significant downregulation of circulating levels of miR-126 and miR-30a in patients with stroke and proposed that they are transported by exosomes, which, in turn, would very likely target and influence other organs, including the heart. MiR-126 was later found to be concentrated in EVs (175), including neuronal EVs (176). Endothelial cells-derived miR-126 knockout mice showed more severe cardiac dysfunction and cardiomyocyte hypertrophy after ischemic stroke compared with control stroke mice (177). MiR-126 is also known to be downregulated in heart failure and atrial fibrillation (178).
Another important miRNA in this context is miR-210. It is transported by mesenchymal stem cell-derived EVs and is a relevant player in angiogenesis within the brain (179) and heart (180). MiR-210 enhances the expression of hepatocyte growth factor, a known stimulator of angiogenesis, neurogenesis, and synaptogenesis (181).
The miR-17–92 cluster, carried also by mesenchymal stem cell-derived EVs (182), is a critical proliferation regulator of cardiomyocytes in neonatal and adult hearts (183) and of neural progenitor cells after stroke (184). Moreover, miR-17–92 has a direct protective effect in the ischemic heart and brain by targeting the PI3K/AKT and the MAPK/ERK pathways (173, 185).
MiR-124, primarily expressed in the brain and involved in the activation of the protective PI3K/AKT and MAPK/ERK pathways (186), was significantly decreased within 24 h after stroke and negatively correlated with the infarct volume (187). Consistent with these findings, the level of circulating EV-carried miR-124-3p declined during acute ischemic stroke (186). As for the heart, miR-124 is significantly upregulated during myocardial infarction and its inhibition reduces cardiomyocyte apoptosis (188).
Zhao et al. (189) investigated alterations of circular RNA expression in exosomes isolated from the brain extracellular space of mice with traumatic brain injury. Cardiac muscle contraction and calcium signaling were two of the 10 most upregulated functions affected by circular RNA, as predicted by KEGG pathway analysis (189). These findings are still very preliminary, nonetheless they can provide the reference for future research on the role of exosome carried circular RNA in the brain-heart communication axis.
CONCLUSIONS AND PERSPECTIVES
Research on EVs-mediated communication between heart and other organs is still at its early stage and the related literature is speedily growing. Therefore, the present review is necessarily limited and most of the mechanistic interpretations we reported here are obviously destined to obsolescence. For instance, other subcellular components like mitochondrial damage-associated molecular patterns (DAMS) (190), lysosomes, peroxisomes, cytoskeletal proteins, etc. might function as messengers carried by EVs. In the light of the available body of knowledge described in the aforementioned paragraphs and summarized in Fig. 2 and Table 1, it can be concluded that at least two formidable challenges await investigators: 1) the precise identification of organs and, in particular, cell types that produce or take up specific circulating EVs, in vivo, and 2) the comprehension of the real impact of EV-carried signals on organ functions, in vivo, relative to nervous and other paracrine/endocrine signals. Addressing the first challenge will require well-designed strategies in proper animal models and novel techniques to label organ-specific EVs. The second challenge will perhaps require the generation of genetic animal models with organ-specific impairment of EVs production. Another important open question is whether EVs produced in vitro may function as a new generation of vectors, alternative to viral or synthetic nanovectors, for organ-directed biological therapies (67–69, 191). Similarly, a major challenge is posed by isolation and purity of EVs from circulation, which warrants more accurate and robust methods for measuring EV number and biodistribution and to separate functionally and morphologically distinct subpopulations.
Table 1.
Extracellular vesicle-mediated communication between heart and other organs
| EV Content | Source Organ | Recipient Organ | Effect and Proposed Mechanisms of Action | Trigger for EV Release | Ref. |
|---|---|---|---|---|---|
| Group 1 | |||||
| Musclin | Skeletal muscle | Heart | Cardioprotection, adrenergic signaling, decrease in oxidative stress, mitigated endothelial dysfunction and fibrosis, and subsequently better cardiac performance. | Remote limb ischemic conditioning | 85 |
| VEGF | |||||
| β2-AR | |||||
| MiR-1 | Decreased apoptosis; caspase-9, possible target of miR-133a. | Duchenne muscular dystrophy | 95* | ||
| MiR-133a | |||||
| MiR-206 | |||||
| Numerous myokines: | Unknown | Exercise | 97** | ||
| Cathepsin D | |||||
| Calcium and integrin-binding protein | |||||
| α2-Antiplasmin etc. | |||||
| Unknown | Reduced myocardial I/R injury, decreased cardiomyocyte apoptosis by activation of ERK1/2 and HSP27 signaling. | Exercise | 96 | ||
| Group 2 | |||||
| Angiotensin II type 1 receptor | Heart | Skeletal muscle | Blood pressure response reconstitution by modulation of vascular responses and cardiac ERK signaling. (ERK phosphorylation). | Pressure overload | 73 |
| MiR-148a | Restored expression of dystrophin in mdx hearts. | (In vivo administration) | 65* | ||
| MiR-1 | Unknown | Myocardial damage | 93** | ||
| MiR-133a | |||||
| MiR-133b | |||||
| MiR-206 | |||||
| Group 3 | |||||
| MiR-26a | Kidney | Heart | Cardiac function improvement, diminished fibrosis, and increased insulin sensitivity by inactivation of multiple targets such as GSK-3β, PTEN, CTGF and collagen I, and most importantly FoxO1. miR-26a intervention reduces CTGF abundance and restricts collagen deposition in the heart. | Exosomal MiR-26a levels are decreased in uremic cardiomyopathy and albuminuria | 104* |
| Circular RNAs | Unknown | Chronic kidney disease | 106** | ||
| Nephrin | Unknown | MI | 72** | ||
| BNP | Heart | Kidney | Unknown | ||
| Group 4 | |||||
| Damaged mitochondria | Adipose tissue | Heart | Mediation of the ROS-signal, attenuation of cardiac injury during ischemia by ischemic preconditioning-like adaptation mechanism. | Mitochondrial stress | 123 |
| MiR-802-5p | Insulin resistance, increased oxidative stress by targeting HSP60. | Adipocyte hypertrophy | 128* | ||
| MiR-130b-3p | Worsened myocardial infarction/reperfusion effects by targeting AMPKα1/α2, Birc6, and Ucp3. | Diabetes | 129* | ||
| Mir-200a | Cardiac hypertrophy by removing TSC1 inhibition of mTOR signaling. | PPARγ activation in adipocytes | 127 | ||
| Numerous proteins (genes: Cav1, Gpd, Ugp2, Pgd, Etfa, Cs, etc.) | Brown adipose tissue | Amelioration of the metabolism leading to hepatic and cardiac function restoration, decreased expression of the two inflammatory genes in the liver, (TNFα and IL-1β). reduced cardiomyocyte hypertrophy. | - | 133* | |
| Unknown | ATMSC | Endothelial cell protection against atherosclerosis by restraining miR-324-5p expression. | - | 132* | |
| Mir-126 | Reduced cardiac fibrosis, cell apoptosis, suppressed inflammatory cytokine expression and promoted angiogenesìs possibly by targeting Spred1 and PI3KR2 and enhancing the VEGF signaling pathway. | ATMSC have been transfected with miR-126 | 138* | ||
| MiR-93-5p | Suppressed autophagy and inflammation after MI, infarction-induced TLR4 expression and NF-κB p65 phosphorylation, decreased inflammatory factors: IL-6, IL-1β, and TNF-α in MI patients. | ATMSC have been transfected with miR-93-5p | 139* | ||
| Unknown | Reduction of apoptosis, induced by oxidative stress | - | 134* | ||
| Unknown | Protection against I/R-induced myocardial injury by limiting Bcl-2 downregulation, preventing Bax upregulation and caspase 3 activation, and removing the inhibition of Wnt/β-catenin signaling | - | 136* | ||
| Unknown | Heart | ATMSC | Proliferation of ATMSC through the activation of ERK1/2 | MI | 72* |
| BNP | Unknown | ||||
| MiR-1956 | Angiogenesis stimulation and paracrine VEGF signaling in ATMSC by downregulating Notch-1 | ||||
| Group 5 | |||||
| MiR‐19a | BMSC | Heart | Downregulation of two genes: PTEN and BIM, followed by increased ERK and Akt phosphorylation and subsequently cardiomyocyte survival and reduced apoptosis. | BMSC have been transduced by GATA4 | 113* |
| MiR‐451 | |||||
| MiR-10a | Cardiac hypertrophy prevention, apoptosis attenuation, fibrosis, and cardiac function preservation during pressure overload. (Proposed mechanism: increase in the Bcl-2/Bax ratio and decrease of cleaved caspase-3 expression). | - | 66* | ||
| MiR-16 | |||||
| MiR-21 | |||||
| MiR-29a | |||||
| MiR-199b | |||||
| MiR-22 | Apoptosis reduction by direct targeting of methyl CpG binding protein 2, diminished cardiac fibrosis | Ischemic preconditioning | 116* | ||
| Unknown | The mean pulmonary artery pressure and mean right ventricle pressure amelioration, decrease the right ventricle hypertrophy | - | 114* | ||
| Unknown | Cardioprotection through angiogenesis and anti-inflammation, (promoted tube formation of HUVECs and decreased the proliferation of spleen lymphocytes). Improvement in cardiac function and amelioration of fibrosis after myocardial infarction. | - | 115* | ||
| Circ-G042080 | Bone marrow | Myocardial damage by the circ-G042080/hsa-miR-4268/TLR4 ceRNA axis. increased LC3 and Beclin1 levels and a decreased P62, increased number of autophagic vesicles leading to autophagic death in cardiomyocytes | Multiple myeloma | 70 | |
| MiR-1 | Heart | Bone marrow | Chemokine receptor CXCR4 expression suppression and progenitor cell mobilization in bone marrow | MI | 118 |
| Mir-208 | |||||
| Mir-499 | |||||
| Group 6 | |||||
| Phospholipase A2 | Heart | Liver | Regulation of inflammation and lipid metabolism in the liver | - | 157, 159** |
| Group 7 | |||||
| miR-143-3p | Heart | Lung | Possibly take part in pulmonary artery hypertension pathogenesis | Hypoxia | 146** |
| miR-10b | Lung | Heart | Bone marrow progenitor cell differentiation to endothelial progenitor cells that contribute to pulmonary hypertensive changes and RV hypertrophy in normal mice. | Monocrotaline treatment | 145* |
| miR-698 | |||||
| miR-743b | |||||
| Diverse protein species: low-density lipoprotein receptor-related protein 2, alanyl-tRNA synthetase, myeloperoxidase, etc. | |||||
| Group 8 | |||||
| Phosphatidylserine and tissue factor | Brain | Heart | Initiation of the extrinsic coagulation pathways and promotion of the assembly of clotting enzymes leading to thrombin generation | Ischemic stroke | 160, 172 |
| P-selectin glycoprotein ligand-1 | Thrombosis, by the concentration of the tissue factor activity at the thrombus | ||||
| MiR-126 | Unknown | 174–177** |
ATIRa, angiotensin II type I receptors; ATMSC, adipose tissue mesenchymal stem cells; BIM, B-cell lymphoma-2-like 11 gene; BMSC, bone marrow stem cells; CTGF, connective tissue growth factor; EV, extracellular vesicle; GSK-3β, glycogen synthase kinase 3β; HUVECs, human umbilical vein endothelial cells; MI, myocardial infarction; mTOR, mammalian target of rapamycin; PPAR, peroxisome proliferator-activated receptor; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species.
*Indirect evidence obtained by exogenous EV infusion in experimental animals or exposure of cultured cells; **presumed mechanism, requires further studies.
The identification and mapping of a new bidirectional communication network between the heart as a hub and other organs, mediated by circulating EV, might open at least two intriguing perspectives. One is the addition of a major chapter of integrative physiology to the classical knowledge of communications mediated by nervous systems, hormones, and hormone-like mediators, which may inspire similar research on other “hubs.” The second perspective is the possible exploitation of EV cardioprotective messengers for therapeutic use: molecules indicated in Table 1 and represented in Fig. 2 might be potentially used as curative biological agents if properly purified and delivered through effective vectors.
The new scenarios that are opening before us are ample and intriguing and are calling for intensive investigation that will keep physiologists and pathophysiologists busy for several more years.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL151345 (to F. A. Recchia) and HL135177 (to M. Khan) and American Heart Association Transformational Project Award 20TPA35490355 (to M. Khan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.G. prepared figures; K.G. drafted manuscript; K.G., M.K., and F.A.R. edited and revised manuscript; K.G., M.K., and F.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Victor Rizzo for valuable input.
REFERENCES
- 1.Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol 290: H17–H29, 2006. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 2.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Chiba A, Mochizuki N. Heart Hormones. In: Hormonal Signaling in Biology and Medicine. Academic Press, ch.14, p. 327–340, 2020. [Google Scholar]
- 4.Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med 17: 207–214, 2011. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y, Anderson JD, Rahnama LMA, Gu SV, Knowlton AA. Exosomes in disease and regeneration: biological functions, diagnostics, and beneficial effects. Am J Physiol Heart Circ Physiol 319: H1162–H1180, 2020. doi: 10.1152/ajpheart.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccarelli M, Dawson D, Falcao-Pires I, Giacca M, Hamdani N, Heymans S, Hooghiemstra A, Leeuwis A, Hermkens D, Tocchetti CG, van der Velden J, Zacchigna S, Thum T. Reciprocal organ interactions during heart failure: a position paper from the ESC working group on myocardial function. Cardiovasc Res 117: 2416–2433, 2021. doi: 10.1093/cvr/cvab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy-Rando E, Fernandez-Patron C. Emerging pathways of communication between the heart and non-cardiac organs. J Biomed Res 33: 145–155, 2019. doi: 10.7555/JBR.32.20170137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskin KK, Grueter CE, Kusminski CM, Holland WL, Bookout AL, Satapati S, Kong YM, Burgess SC, Malloy CR, Scherer PE, Newgard CB, Bassel-Duby R, Olson EN. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol Med 6: 1610–1621, 2014. doi: 10.15252/emmm.201404218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 149: 671–683, 2012. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell 164: 1248–1256, 2016. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 16: 83–99, 2019. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 12.El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittköpper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in chronic systolic heart failure: a marker of left ventricular remodeling. Circ Heart Fail 4: 621–627, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117: 3099–3108, 2008. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki M, Powers JC, Maruyama S, Zuriaga MA, Wu C-L, Kurishima C, Kim L, Johnson J, Poidomani A, Wang T, Muñoz E, Rajan S, Park JY, Walsh K, Recchia FA. Acute and chronic increases of circulating FSTL1 normalize energy substrate metabolism in pacing-induced heart failure. Circ Heart Fail 11: e004486, 2018. doi: 10.1161/CIRCHEARTFAILURE.117.004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJB, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci USA 108: E899–E906, 2011. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292: H3052–H3056, 2007. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256, 2012. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 19.Kapustin AN, Chatrou MLL, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RTM, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 116: 1312–1323, 2015. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S. Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Sci Rep 4: 7583, 2014. doi: 10.1038/srep07583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen BY, Azam T, Wang X. Cellular signaling cross-talk between different cardiac cell populations: an insight into the role of exosomes in the heart diseases and therapy. Am J Physiol Heart Circ Physiol 320: H1213–H1234, 2021. doi: 10.1152/ajpheart.00718.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saheera S, Jani VP, Witwer KW, Kutty S. Extracellular vesicle interplay in cardiovascular pathophysiology. Am J Physiol Heart Circ Physiol 320: H1749–H1761, 2021. doi: 10.1152/ajpheart.00925.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293: H1883–H1891, 2007. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 24.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28: 41–61, 1980. [PubMed] [Google Scholar]
- 25.Perbellini F, Watson SA, Bardi I, Terracciano CM. Heterocellularity and cellular cross-talk in the cardiovascular system. Front Cardiovasc Med 5: 143, 2018. doi: 10.3389/fcvm.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri A, Yang Xiao C, Meechoovet B, Alsop E, Rodosthenous RS, Kundu P, Huan T, Levy D, Tigges J, Pico AR, Ghiran I, Silverman MG, Meng X, Kitchen R, Xu J, Van Keuren-Jensen K, Shah R, Xiao J, Das S. Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res 128: e1–e23, 2021. doi: 10.1161/CIRCRESAHA.120.317244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 89: 268–279, 2015. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res 10: 4350–4366, 2018. [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res 109: 397–408, 2016. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan G-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74: 139–150, 2014. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol 304: H954–H965, 2013. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139: 393–404, 2009. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta R, Bansal T, Rana S, Datta K, Datta Chaudhuri R, Chawla-Sarkar M, Sarkar S. Myocyte-derived Hsp90 modulates collagen upregulation via biphasic activation of STAT-3 in fibroblasts during cardiac hypertrophy. Mol Cell Biol 37: e00611-16, 2017. doi: 10.1128/MCB.00611-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D-W, Ge P-P, Liu A-L, Yu X-Y, Liu T-T. HSP20-mediated cardiomyocyte exosomes improve cardiac function in mice with myocardial infarction by activating Akt signaling pathway. Eur Rev Med Pharmacol Sci 23: 4873–4881, 2019. doi: 10.26355/eurrev_201906_18075. [DOI] [PubMed] [Google Scholar]
- 35.Dickens AM, Tovar-Y-Romo LB, Yoo S-W, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal 10: eaai7696, 2017. doi: 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan G-C. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One 7: e32765, 2012. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7: e34653, 2012. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gennebäck N, Hellman U, Malm L, Larsson G, Ronquist G, Waldenström A, Mörner S. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. J Extracell Vesicles 2: 20167, 2013. doi: 10.3402/jev.v2i0.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 40.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 42.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8: 1648167, 2019. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94: 3791–3799, 1999. [PubMed] [Google Scholar]
- 44.Logozzi M, Mizzoni D, Di Raimo R, Giuliani A, Maggi M, Sciarra A, Fais S. Plasmatic exosome number and size distinguish prostate cancer patients from healthy individuals: a prospective clinical study. Front Oncol 11: 727317, 2021. doi: 10.3389/fonc.2021.727317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476, 2008. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IKH. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 6: 7439, 2015. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch JG, Fedorko ME, Cohn ZA. Vesicle fusion and formation at the surface of pinocytic vacuoles in macrophages. J Cell Biol 38: 629–632, 1968. doi: 10.1083/jcb.38.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749, 2005. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108: 261–269, 2002. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 50.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33: 967–978, 1983. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 51.Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol 132: 1011–1023, 1996. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O, Baldanta S, Enrich C, Guerra S, Sánchez-Madrid F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun 7: 13588, 2016. doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Comfurius P, Senden JM, Tilly RH, Schroit AJ, Bevers EM, Zwaal RF. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim Biophys Acta 1026: 153–160, 1990. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 54.Kelton JG, Warkentin TE, Hayward CP, Murphy WG, Moore JC. Calpain activity in patients with thrombotic thrombocytopenic purpura is associated with platelet microparticles. Blood 80: 2246–2251, 1992. [PubMed] [Google Scholar]
- 55.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21: 157–171, 2007. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Häcker G. The morphology of apoptosis. Cell Tissue Res 301: 5–17, 2000. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 57.Oberhammer FA, Hochegger K, Fröschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol 126: 827–837, 1994. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, Bienvenut WV, Olson MF. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 20: 1293–1305, 2013. doi: 10.1038/cdd.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel) 9: 21, 2020. doi: 10.3390/biology9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239–257, 1972. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch R, Demant M, Aung T, Diering N, Cicholas A, Chapuy B, Wenzel D, Lahmann M, Güntsch A, Kiecke C, Becker S, Hupfeld T, Venkataramani V, Ziepert M, Opitz L, Klapper W, Trümper L, Wulf GG. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood 123: 2189–2198, 2014. doi: 10.1182/blood-2013-08-523886. [DOI] [PubMed] [Google Scholar]
- 62.Prada I, Amin L, Furlan R, Legname G, Verderio C, Cojoc D. A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. Biotechniques 60: 35–41, 2016. doi: 10.2144/000114371. [DOI] [PubMed] [Google Scholar]
- 63.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766, 2012. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M, Isabel Anjo S, Manadas B, P G Sluijter J, Pereira P, Girao H. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep 5: 13243, 2015. [Erratum in Sci Rep 5: 14888, 2015]. doi: 10.1038/srep13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aminzadeh MA, Rogers RG, Fournier M, Tobin RE, Guan X, Childers MK, Andres AM, Taylor DJ, Ibrahim A, Ding X, Torrente A, Goldhaber JM, Lewis M, Gottlieb RA, Victor RA, Marbán E. Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Reports 10: 942–955, 2018. doi: 10.1016/j.stemcr.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen F, Li X, Zhao J, Geng J, Xie J, Xu B. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell Dev Biol Anim 56: 567–576, 2020. doi: 10.1007/s11626-020-00481-2. [DOI] [PubMed] [Google Scholar]
- 67.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38: 201–211, 2017. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schena GJ, Murray EK, Hildebrand AN, Headrick AL, Yang Y, Koch KA, Kubo H, Eaton D, Johnson J, Berretta R, Mohsin S, Kishore R, McKinsey TA, Elrod JW, Houser SR. Cortical bone stem cell-derived exosomes’ therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling. Am J Physiol Heart Circ Physiol 321: H1014–H1029, 2021. doi: 10.1152/ajpheart.00197.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun R, Liu W, Zhao Y, Chen H, Wang Z, Zhang Y, Sun X, Cui X. Exosomal circRNA as a novel potential therapeutic target for multiple myeloma-related myocardial damage. Cancer Cell Int 21: 311, 2021. doi: 10.1186/s12935-021-02011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bank IEM, Timmers L, Gijsberts CM, Zhang Y-N, Mosterd A, Wang J-W, Chan MY, De Hoog V, Lim SK, Sze SK, Lam CSP, De Kleijn DV. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn 15: 1577–1588, 2015. doi: 10.1586/14737159.2015.1109450. [DOI] [PubMed] [Google Scholar]
- 72.Gao L, Mei S, Zhang S, Qin Q, Li H, Liao Y, Fan H, Liu Z, Zhu H. Cardio-renal exosomes in myocardial infarction serum regulate proangiogenic paracrine signaling in adipose mesenchymal stem cells. Theranostics 10: 1060–1073, 2020. doi: 10.7150/thno.37678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131: 2120–2130, 2015. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 75.Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol 17: 879–887, 2005. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 76.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci 21: 4407, 2020. doi: 10.3390/ijms21124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol 221: 502.e1–502.e12, 2019. doi: 10.1016/j.ajog.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Bryl-Górecka P, James K, Torngren K, Haraldsson I, Gan L-M, Svedlund S, Olde B, Laurell T, Omerovic E, Erlinge D. Microvesicles in plasma reflect coronary flow reserve in patients with cardiovascular disease. Am J Physiol Heart Circ Physiol 320: H2147–H2160, 2021. doi: 10.1152/ajpheart.00869.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113: E968–E977, 2016. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res 114: 325–332, 2014. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 81.Chock SP, Schmauder-Chock EA, Cordella-Miele E, Miele L, Mukherjee AB. The localization of phospholipase A2 in the secretory granule. Biochem J 300: 619–622, 1994. doi: 10.1042/bj3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W, Wang L, You W, Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. J Cell Physiol 236: 2393–2412, 2021. doi: 10.1002/jcp.30033. [DOI] [PubMed] [Google Scholar]
- 83.Aoi W, Tanimura Y. Roles of skeletal muscle-derived exosomes in organ metabolic and immunological communication. Front Endocrinol (Lausanne) 12: 697204, 2021. doi: 10.3389/fendo.2021.697204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers E-M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles 4: 28239, 2015. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Homme RP, Zheng Y, Smolenkova I, Singh M, Tyagi SC. Remote hind-limb ischemia mechanism of preserved ejection fraction during heart failure. Front Physiol 12: 745328, 2021. doi: 10.3389/fphys.2021.745328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aswad H, Forterre A, Wiklander OPB, Vial G, Danty-Berger E, Jalabert A, Lamazière A, Meugnier E, Pesenti S, Ott C, Chikh K, El-Andaloussi S, Vidal H, Lefai E, Rieusset J, Rome S. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 57: 2155–2164, 2014. doi: 10.1007/s00125-014-3337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol 18: 187–195, 1988. [Erratum in Int J Cardiol 20: 161, 1988 and in Int J Cardiol 19: 396, 1988]. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 88.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 89.Murach KA, McCarthy JJ. MicroRNAs, heart failure, and aging: potential interactions with skeletal muscle. Heart Fail Rev 22: 209–218, 2017. doi: 10.1007/s10741-016-9572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics 39: 219–226, 2009. doi: 10.1152/physiolgenomics.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Rooij E, Liu N, Olson EN. MicroRNAs flex their muscles. Trends Genet 24: 159–166, 2008. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 92.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJJ, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 4: 446–454, 2011. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 94.Mytidou C, Koutsoulidou A, Katsioloudi A, Prokopi M, Kapnisis K, Michailidou K, Anayiotos A, Phylactou LA. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. FASEB J 35: e21279, 2021. doi: 10.1096/fj.201902468RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsuzaka Y, Tanihata J, Komaki H, Ishiyama A, Oya Y, Rüegg U, Takeda S-I, Hashido K. Characterization and functional analysis of extracellular vesicles and muscle-abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 myocytes and mdx mice. PLoS One 11: e0167811, 2016. doi: 10.1371/journal.pone.0167811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol 112: 38, 2017. [Erratum in Basic Res Cardiol 114: 44, 2019]. doi: 10.1007/s00395-017-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura S, Naruse M, Naruse K, Kawana M, Nishikawa T, Hosoda S, Tanaka I, Yoshimi T, Yoshihara I, Inagami T. Atrial natriuretic peptide and brain natriuretic peptide coexist in the secretory granules of human cardiac myocytes. Am J Hypertens 4: 909–912, 1991. doi: 10.1093/ajh/4.11.909. [DOI] [PubMed] [Google Scholar]
- 99.Okamoto R, Ali Y, Hashizume R, Suzuki N, Ito M. BNP as a major player in the heart-kidney connection. Int J Mol Sci 20: 3581, 2019. doi: 10.3390/ijms20143581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindoso RS, Lopes JA, Binato R, Abdelhay E, Takiya CM, Miranda KD, Lara LS, Viola A, Bussolati B, Vieyra A, Collino F. Adipose mesenchymal cells-derived EVs alleviate DOCA-salt-induced hypertension by promoting cardio-renal protection. Mol Ther Methods Clin Dev 16: 63–77, 2020. doi: 10.1016/j.omtm.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perez-Hernandez J, Riffo-Campos AL, Ortega A, Martinez-Arroyo O, Perez-Gil D, Olivares D, Solaz E, Martinez F, Martínez-Hervás S, Chaves FJ, Redon J, Cortes R. Urinary- and plasma-derived exosomes reveal a distinct microRNA signature associated with albuminuria in hypertension. Hypertension 77: 960–971, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16598. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Pan X, Fu X, Yang Y, Chen J, Lin W. MicroRNA-26a: an emerging regulator of renal biology and disease. Kidney Blood Press Res 44: 287–297, 2019. doi: 10.1159/000499646. [DOI] [PubMed] [Google Scholar]
- 103.Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med 24: 241–248, 2014. doi: 10.1016/j.tcm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B, Zhang A, Wang H, Klein JD, Tan L, Wang Z-M, Du J, Naqvi N, Liu B-C, Wang XH. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics 9: 1864–1877, 2019. doi: 10.7150/thno.29579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garikapati K, Goh D, Khanna S, Echampati K. Uraemic cardiomyopathy: a review of current literature. Clin Med Insights Cardiol 15: 1179546821998347, 2021. doi: 10.1177/1179546821998347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Zonneveld AJ, Kölling M, Bijkerk R, Lorenzen JM. Circular RNAs in kidney disease and cancer. Nat Rev Nephrol 17: 814–826, 2021. doi: 10.1038/s41581-021-00465-9. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 25: 981–984, 2015. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bittner RE, Schöfer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Höger H, Elbe-Bürger A, Wachtler F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 199: 391–396, 1999. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- 109.Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L, Gómez-Bueno M, Cantalapiedra A, Fernández J, Gutierrez O, Sánchez PL, Hernández C, Sanz R, García-Sancho J, Sánchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res 95: 742–748, 2004. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 110.Haider KH, Aramini B. Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy. Stem Cell Res Ther 11: 23, 2020. doi: 10.1186/s13287-019-1548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kelkar AA, Butler J, Schelbert EB, Greene SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun W, Luger D, Epstein SE. Mechanisms contributing to the progression of ischemic and nonischemic dilated cardiomyopathy: possible modulating effects of paracrine activities of stem cells. J Am Coll Cardiol 66: 2038–2047, 2015. doi: 10.1016/j.jacc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki G, Weil BR, Young RF, Fallavollita JA, Canty JM Jr.. Nonocclusive multivessel intracoronary infusion of allogeneic cardiosphere-derived cells early after reperfusion prevents remote zone myocyte loss and improves global left ventricular function in swine with myocardial infarction. Am J Physiol Heart Circ Physiol 317: H345–H356, 2019. doi: 10.1152/ajpheart.00124.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182: 349–360, 2015. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen J, An R, Liu Z, Wang J, Chen S, Hong M, Liu J, Xiao M, Chen Y. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin 35: 1121–1128, 2014. doi: 10.1038/aps.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37: 2415–2424, 2015. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 116.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 9: e88685, 2014. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24: 1635–1647, 2015. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y, Yang L, Wu B, Yi G, Mao X, Huang K, Dong N, Xie M, Limdi NA, Prabhu SD, Zhang J, Qin G. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun 10: 959, 2019. doi: 10.1038/s41467-019-08895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feijóo-Bandín S, Aragón-Herrera A, Moraña-Fernández S, Anido-Varela L, Tarazón E, Roselló-Lletí E, Portolés M, Moscoso I, Gualillo O, González-Juanatey JR, Lago F. Adipokines and inflammation: focus on cardiovascular diseases. Int J Mol Sci 21: 7711, 2020. doi: 10.3390/ijms21207711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542: 450–455, 2017. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Flaherty SE 3rd, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AWJ. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363: 989–993, 2019. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol 63: 1345–1354, 2014. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 123.Crewe C, Funcke J-B, Li S, Joffin N, Gliniak CM, Ghaben AL, An YA, Sadek HA, Gordillo R, Akgul Y, Chen S, Samovski D, Fischer-Posovszky P, Kusminski CM, Klein S, Scherer PE. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab 33: 1853–1868.e11, 2021. doi: 10.1016/j.cmet.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest 129: 4041–4049, 2019. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, Fujishima Y, Nagao H, Masuda S, Tanaka Y, Nakamura Y, Nishizawa H, Funahashi T, Ranscht B, Izumi Y, Bamba T, Fukusaki E, Hanayama R, Shimada S, Maeda N, Shimomura I. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3: e99680, 2018. doi: 10.1172/jci.insight.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Field BC, Gordillo R, Scherer PE. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front Endocrinol (Lausanne) 11: 569250, 2020. doi: 10.3389/fendo.2020.569250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND, Gu Y, Wu T, Peterson KL, Huang H-D, Chen J, Wang N. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 1: e89908, 2016. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wen Z, Li J, Fu Y, Zheng Y, Ma M, Wang C. Hypertrophic adipocyte-derived exosomal miR-802-5p contributes to insulin resistance in cardiac myocytes through targeting HSP60. Obesity (Silver Spring) 28: 1932–1940, 2020. doi: 10.1002/oby.22932. [DOI] [PubMed] [Google Scholar]
- 129.Gan L, Xie D, Liu J, Bond Lau W, Christopher TA, Lopez B, Zhang L, Gao E, Koch W, Ma X-L, Wang Y. Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation 141: 968–983, 2020. doi: 10.1161/CIRCULATIONAHA.119.042640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guarnieri AR, Anthony SR, Gozdiff A, Green LC, Fleifil SM, Slone S, Nieman ML, Alam P, Benoit JB, Owens AP, Kanisicak O, Tranter M. Adipocyte-specific deletion of HuR induces spontaneous cardiac hypertrophy and fibrosis. Am J Physiol Heart Circ Physiol 321: H228–H241, 2021. doi: 10.1152/ajpheart.00957.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, Tian J, Gu X, Wang H, Tian J, Yu B. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc 7: e007442, 2018. doi: 10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xing X, Li Z, Yang X, Li M, Liu C, Pang Y, Zhang L, Li X, Liu G, Xiao Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY) 12: 3880–3898, 2020. doi: 10.18632/aging.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G, Yuan L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics 10: 8197–8210, 2020. doi: 10.7150/thno.43968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov 5: 79, 2019. doi: 10.1038/s41420-019-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem 121: 2089–2102, 2020. doi: 10.1002/jcb.27399. [DOI] [PubMed] [Google Scholar]
- 136.Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol 70: 225–231, 2017. doi: 10.1097/FJC.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol 114: 105564, 2019. doi: 10.1016/j.biocel.2019.105564. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, Wang H. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids 11: 103–115, 2018. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem Int Biochem 44: 2105–2116, 2017. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 140.Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, Nishida H, Takahashi Y, Kawachi Y, Tsugawa-Shimizu Y, Fujishima Y, Nishizawa H, Takakura Y, Miyagawa S, Sawa Y, Maeda N, Shimomura I. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther 28: 2203–2219, 2020. doi: 10.1016/j.ymthe.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dracheva KV, Pobozheva IA, Anisimova KA, Balandov SG, Hamid ZM, Panteleeva AA, Vasilevsky DI, Pchelina SN, Miroshnikova VV. Omentin-1 and adiponectin secretion via adipose tissue extracellular vesicles. Atherosclerosis 331: e146, 2021. doi: 10.1016/j.atherosclerosis.2021.06.436. [DOI] [Google Scholar]
- 142.Shao S, Fang H, Zhang J, Jiang M, Xue K, Ma J, Zhang J, Lei J, Zhang Y, Li B, Yuan X, Dang E, Wang G. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J 33: 6813–6828, 2019. doi: 10.1096/fj.201802090RR. [DOI] [PubMed] [Google Scholar]
- 143.Huang D, Sun G, Hao X, He X, Zheng Z, Chen C, Yu Z, Xie L, Ma S, Liu L, Zhou BO, Cheng H, Zheng J, Cheng T. ANGPTL2-containing small extracellular vesicles from vascular endothelial cells accelerate leukemia progression. J Clin Invest 131: e138986, 2021. doi: 10.1172/JCI138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 165: 77–84, 2013. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 145.Aliotta JM, Pereira M, Amaral A, Sorokina A, Igbinoba Z, Hasslinger A, El-Bizri R, Rounds SI, Quesenberry PJ, Klinger JR. Induction of pulmonary hypertensive changes by extracellular vesicles from monocrotaline-treated mice. Cardiovasc Res 100: 354–362, 2013. doi: 10.1093/cvr/cvt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res 117: 870–883, 2015. [Erratum in Circ Res 120: e6, 2017]. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin Z-B, Ci H-B, Li Y, Cheng T-P, Liu D-H, Wang Y-S, Xu J, Yuan H-X, Li H-M, Chen J, Zhou L, Wang Z-P, Zhang X, Ou Z-J, Ou J-S. Endothelial microparticles are increased in congenital heart diseases and contribute to endothelial dysfunction. J Transl Med 15: 4, 2017. doi: 10.1186/s12967-016-1087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y-C, Xie H, Zhang Y-C, Meng Q-H, Xiong M-M, Jia M-W, Peng F, Tang D-L. Exosomal miR-107 antagonizes profibrotic phenotypes of pericytes by targeting a pathway involving HIF-1α/Notch1/PDGFRβ/YAP1/Twist1 axis in vitro. Am J Physiol Heart Circ Physiol 320: H520–H534, 2021. doi: 10.1152/ajpheart.00373.2020. [DOI] [PubMed] [Google Scholar]
- 149.Smadja DM, Gaussem P, Mauge L, Lacroix R, Gandrille S, Remones V, Peyrard S, Sabatier F, Bonnet D, Lévy M. Comparison of endothelial biomarkers according to reversibility of pulmonary hypertension secondary to congenital heart disease. Pediatr Cardiol 31: 657–662, 2010. doi: 10.1007/s00246-010-9674-0. [DOI] [PubMed] [Google Scholar]
- 150.Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, Van Tassel BW, Afeltra A, Sanyal AJ. Cirrhotic cardiomyopathy. J Am Coll Cardiol 56: 539–549, 2010. [Erratum in J Am Coll Cardiol 56: 1000, 2010]. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 151.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 15: 425–439, 2018. doi: 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 152.Mantovani A. Nonalcoholic fatty liver disease (NAFLD) and risk of cardiac arrhythmias: a new aspect of the liver-heart axis. J Clin Transl Hepatol 5: 134–141, 2017. doi: 10.14218/JCTH.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baskin KK, Bookout AL, Olson EN. The heart-liver metabolic axis: defective communication exacerbates disease. EMBO Mol Med 6: 436–438, 2014. doi: 10.1002/emmm.201303800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61: 1547–1554, 2015. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 155.Ismaiel A, Dumitraşcu DL. Cardiovascular risk in fatty liver disease: the liver-heart axis—literature review. Front Med (Lausanne) 6: 202, 2019. doi: 10.3389/fmed.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51: 595–602, 2010. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 157.Hernandez-Anzaldo S, Berry E, Brglez V, Leung D, Yun TJ, Lee JS, Filep JG, Kassiri Z, Cheong C, Lambeau G, Lehner R, Fernandez-Patron C. Identification of a novel heart-liver axis: matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate lipid metabolism and inflammation in the liver. J Am Heart Assoc 4: e002553, 2015. doi: 10.1161/JAHA.115.002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berry E, Hernandez-Anzaldo S, Ghomashchi F, Lehner R, Murakami M, Gelb MH, Kassiri Z, Wang X, Fernandez-Patron C. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc 4: e001868, 2015. doi: 10.1161/JAHA.115.001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, De Medina P, Monsarrat B, Perret B, Silvente-Poirot S, Poirot M, Record M. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res 51: 2105–2120, 2010. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res 121: 451–468, 2017. doi: 10.1161/CIRCRESAHA.117.311170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Doehner W, Ural D, Haeusler KG, Čelutkienė J, Bestetti R, Cavusoglu Y, Peña-Duque MA, Glavas D, Iacoviello M, Laufs U, Alvear RM, Mbakwem A, Piepoli MF, Rosen SD, Tsivgoulis G, Vitale C, Yilmaz MB, Anker SD, Filippatos G, Seferovic P, Coats AJS, Ruschitzka F. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 20: 199–215, 2018. doi: 10.1002/ejhf.1100. [DOI] [PubMed] [Google Scholar]
- 162.Venkat P, Chen J, Chopp M. Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow 38: 2165–2178, 2018. doi: 10.1177/0271678X18782789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, Lazar RM, Horvath KA, Corso PJ, Warach S. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol 34: 518–523, 2013. doi: 10.3174/ajnr.A3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42: 3323–3328, 2011. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang M, Hu Y-Y, Dong X-Q. High concentrations of procoagulant microparticles in the cerebrospinal fluid and peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Surg Neurol 72: 481–489, 2009. doi: 10.1016/j.surneu.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 166.Lackner P, Dietmann A, Beer R, Fischer M, Broessner G, Helbok R, Marxgut J, Pfausler B, Schmutzhard E. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke 41: 2353–2357, 2010. doi: 10.1161/STROKEAHA.110.584995. [DOI] [PubMed] [Google Scholar]
- 167.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 97: 425–434, 2007. [PubMed] [Google Scholar]
- 168.Brenna S, Krisp C, Altmeppen HC, Magnus T, Puig B. Brain-derived extracellular vesicles in health and disease: a methodological perspective. Int J Mol Sci 22: 1365, 2021. doi: 10.3390/ijms22031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost 3: 44–48, 2019. doi: 10.1002/rth2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei X, Liu C, Wang H, Wang L, Xiao F, Guo Z, Zhang H. Surface phosphatidylserine is responsible for the internalization on microvesicles derived from hypoxia-induced human bone marrow mesenchymal stem cells into human endothelial cells. PLoS One 11: e0147360, 2016. doi: 10.1371/journal.pone.0147360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu M-L, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes 19: 121–127, 2012. doi: 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huo S, Kränkel N, Nave AH, Sperber PS, Rohmann JL, Piper SK, Heuschmann PU, Landmesser U, Endres M, Siegerink B, Liman TG. Endothelial and leukocyte-derived microvesicles and cardiovascular risk after stroke: PROSCIS-B. Neurology 96: e937–e946, 2021. doi: 10.1212/WNL.0000000000011223. [DOI] [PubMed] [Google Scholar]
- 173.Zheng X, Hermann DM, Bähr M, Doeppner TR. The role of small extracellular vesicles in cerebral and myocardial ischemia—molecular signals, treatment targets, and future clinical translation. Stem Cells 39: 403–413, 2021. doi: 10.1002/stem.3329. [DOI] [PubMed] [Google Scholar]
- 174.Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol 13: 178, 2013. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grimolizzi F, Monaco F, Leoni F, Bracci M, Staffolani S, Bersaglieri C, Gaetani S, Valentino M, Amati M, Rubini C, Saccucci F, Neuzil J, Tomasetti M, Santarelli L. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep 7: 15277, 2017. doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H, Chen F-S, Zhang Z-L, Zhou H-X, Ma H, Li X-Q. MiR-126-3p-enriched extracellular vesicles from hypoxia-preconditioned VSC 4.1 neurons attenuate ischaemia-reperfusion-induced pain hypersensitivity by regulating the PIK3R2-mediated pathway. Mol Neurobiol 58: 821–834, 2021. doi: 10.1007/s12035-020-02159-y. [DOI] [PubMed] [Google Scholar]
- 177.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res 8: 374–385, 2017. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wei XJ, Han M, Yang FY, Wei GC, Liang ZG, Yao H, Ji CW, Xie RS, Gong CL, Tian Y. Biological significance of miR-126 expression in atrial fibrillation and heart failure. Braz J Med Biol Res 48: 983–989, 2015. doi: 10.1590/1414-431X20154590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Wang Y, Fu Y, Yang G-Y. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther 21: 37–43, 2014. doi: 10.1038/gt.2013.55. [DOI] [PubMed] [Google Scholar]
- 180.Fan Z-G, Qu X-L, Chu P, Gao Y-L, Gao X-F, Chen S-L, Tian N-L. MicroRNA-210 promotes angiogenesis in acute myocardial infarction. Mol Med Rep 17: 5658–5665, 2018. doi: 10.3892/mmr.2018.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shang J, Deguchi K, Ohta Y, Liu N, Zhang X, Tian F, Yamashita T, Ikeda Y, Matsuura T, Funakoshi H, Nakamura T, Abe K. Strong neurogenesis, angiogenesis, synaptogenesis, and antifibrosis of hepatocyte growth factor in rats brain after transient middle cerebral artery occlusion. J Neurosci Res 89: 86–95, 2011. doi: 10.1002/jnr.22524. [DOI] [PubMed] [Google Scholar]
- 182.Zhang Y, Zhang Y, Chopp M, Pang H, Zhang ZG, Mahmood A, Xiong Y. MiR-17–92 cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma 38: 1535–1550, 2021. doi: 10.1089/neu.2020.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen J, Huang Z-P, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang D-Z. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res 112: 1557–1566, 2013. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem 288: 12478–12488, 2013. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou M, Cai J, Tang Y, Zhao Q. MiR-17-92 cluster is a novel regulatory gene of cardiac ischemic/reperfusion injury. Med Hypotheses 81: 108–110, 2013. doi: 10.1016/j.mehy.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 186.Qi Z, Zhao Y, Su Y, Cao B, Yang J-J, Xing Q. Serum extracellular vesicle-derived miR-124-3p as a diagnostic and predictive marker for early-stage acute ischemic stroke. Front Mol Biosci 8: 685088, 2021. doi: 10.3389/fmolb.2021.685088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Y, Zhang J, Han R, Liu H, Sun D, Liu X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J Clin Neurosci 22: 291–295, 2015. doi: 10.1016/j.jocn.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 188.He F, Liu H, Guo J, Yang D, Yu Y, Yu J, Yan X, Hu J, Du Z. Inhibition of microRNA-124 reduces cardiomyocyte apoptosis following myocardial infarction via targeting STAT3. Cell Physiol Biochem Int Biochem 51: 186–200, 2018. doi: 10.1159/000495173. [DOI] [PubMed] [Google Scholar]
- 189.Zhao R-T, Zhou J, Dong X-L, Bi C-W, Jiang R-C, Dong J-F, Tian Y, Yuan H-J, Zhang J-N. Circular ribonucleic acid expression alteration in exosomes from the brain extracellular space after traumatic brain injury in mice. J Neurotrauma 35: 2056–2066, 2018. doi: 10.1089/neu.2017.5502. [DOI] [PubMed] [Google Scholar]
- 190.Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol 38: 768–776, 2017. doi: 10.1016/j.it.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kwon J-S, Schumacher SM, Gao E, Chuprun JK, Ibetti J, Roy R, Khan M, Kishore R, Koch WJ. Characterization of βARKct engineered cellular extracellular vesicles and model specific cardioprotection. Am J Physiol Heart Circ Physiol 320: H1276–H1289, 2021. doi: 10.1152/ajpheart.00571.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. J Mol Cell Cardiol 53: 848–857, 2012. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]