Abstract
Histamine is an important immunomodulator, as well as a regulator of allergic inflammation, gastric acid secretion, and neurotransmission. Although substantial histamine level has been reported in the kidney, renal pathological and physiological effects of this compound have not been clearly defined. The goal of this study was to provide insight into the role of histamine-related pathways in the kidney, with emphasis on the collecting duct (CD), a distal part of the nephron important for the regulation of blood pressure. We report that all four histamine receptors (HRs) as well as enzymes responsible for histamine metabolism and synthesis are expressed in cultured mouse mpkCCDcl4 cells, and histamine evokes a dose-dependent transient increase in intracellular Ca2+ in these cells. Furthermore, we observed a dose-dependent increase in cAMP in the CD cells in response to histamine. Short-circuit current studies aimed at measuring Na+ reabsorption via ENaC (epithelial Na+ channel) demonstrated inhibition of ENaC-mediated currents by histamine after a 4-h incubation, and single-channel patch-clamp analysis revealed similar ENaC open probability before and after acute histamine application. The long-term (4 h) effect on ENaC was corroborated in immunocytochemistry and qPCR, which showed a decrease in protein and gene expression for αENaC upon histamine treatment. In summary, our data highlight the functional importance of HRs in the CD cells and suggest potential implications of histamine in inflammation-related renal conditions. Further research is required to discern the molecular pathways downstream of HRs and assess the role of specific receptors in renal pathophysiology.
Keywords: histamine, kidney, collecting duct, histamine receptors, epithelial Na+ channels
INTRODUCTION
The main functions of the kidney are maintenance of normal body fluid volume, electrolyte balance, and blood pressure (1, 2), which are achieved by tightly regulated successive reabsorption of electrolytes and water along the nephron. The distal nephron segment, the collecting duct (CD), reabsorbs Na+, Cl−, K+, H+, water, and urea (3). Although only 1% to 3% of the filtered load remains in the filtrate by the time reaches the CD, Na+ transport in this segment is highly variable, susceptible to regulation by hormones, and its dysregulation is sufficient to cause blood pressure disorders (4–6). Na+ reabsorption via the principal cells of the CD is described by the two-membrane model of Na+ transport. The electrochemical gradient produced by the basolateral Na+/K+ ATPase allows Na+ to be reabsorbed down its gradient via apical epithelial Na+ channels (ENaCs) (6–8). ENaC, the rate limiting factor of the entire Na+ reabsorption process in the cortical CD (CCD), is a constitutively active amiloride-sensitive channel that is a member of the ENaC/Degenerin channel superfamily (7, 9). The channel consists of three subunits, α, β, and γ (9, 10). Together, these subunits form a tripartite funnel with rotational symmetry and the ability to dynamically constrict or expand to regulate ion flow (11). ENaC gain-of-function mutations occur in Liddle’s syndrome and lead to volume expansion, hypertension, hypokalemia, and metabolic alkalosis, whereas loss-of-function mutations in pseudohypoaldosteronism type I (PHAI) result in volume depletion, hypotension, and hyperkalemia (11, 12). ENaC subunit abundance has been found to be increased in diabetes (13, 14), and ENaC blockers have been suggested as possible treatment for some renal diseases (15, 16). The activity and expression of ENaC is regulated by numerous factors, including hormones such as aldosterone, vasopressin (AVP), atrial natriuretic peptide, and insulin (6, 17, 18); serine and cysteine proteases (9, 19); serum/glucocorticoid-regulated kinase (SGK), protein kinase A (PKA), casein kinase 2 (CK2), G-protein-coupled receptor kinase 2 (GRK2), protein kinase D1 (PKD1), extracellular signal-regulated kinase (ERK), and 5' adenosine monophosphate-activated protein kinase (AMPK) (20–22); as well as Ca2+ (23, 24).
The inflammatory response is crucial for progression and initiation of renal disease. It is usually triggered by various stressors, for instance, high salt diet or increased glucose levels (25–27). These stressors generally lead to the activation of the immune response, subsequent infiltration of immune cells, and damage to renal tissues as a result of cytokine release, reactive oxygen species (ROS) production, and other factors (28). Among known ENaC regulators, many are associated with inflammatory pathways, such as ROS. For instance, angiotensin II (Ang II) was shown to increase ENaC activity in distal nephron via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent signaling pathway (29). Furthermore, it has been suggested that insulin- or epidermal growth factor (EGF)-induced ENaC-mediated Na+ transport in cultured mouse CCD cells is mediated by ROS-production (30). NADPH oxidase 4 (NOX4), the most abundant NOX isoform in the kidney, plays a critical role in increasing H2O2 production and subsequent ENaC upregulation seen in salt sensitive (SS) hypertension and diabetic kidney disease (13).
One potent proinflammatory mediator is histamine, which has the capacity to promote vascular and tissue changes (although in some tissues it has been shown to exhibit anti-inflammatory regulatory properties) (31). Histamine produces physiological effects by binding to its receptors 1–4 (HR1-4), all of which are constitutively activated G-protein coupled receptors with similar basic topology (32). The receptors differ in their affinity for histamine, intracellular signaling, immunological activity, tissue expression, and physiological function (27, 31–33). The main enzymes of the histaminergic system that mediate histamine synthesis and degradation in the tissues are histamine N-methyltransferase (HNMT), histidine decarboxylase (HDC), and diamine oxidase (DAO) (34). Histamine is synthesized from l-histidine by HDC and metabolized by DAO or HNMT. There is a gap in knowledge regarding the role of HRs and overall histaminergic system in kidney physiology, particularly in conditions associated with altered Na+ balance. Very few studies have examined the role of histamine and its signaling in renal function and/or progression of kidney diseases. However, histamine levels have been found to be increased in nephrotic syndrome, diabetic nephropathy, ischemia-induced acute renal failure, and end stage kidney disease, suggesting a role for histamine in the development of these renal pathologies (35, 36). Therefore, underlying kidney disease may be an important factor to consider when taking antihistamines. More information about the role of histamine in renal pathophysiology can be found in two most recent reviews by Grange et al. (36) and Sudarikova et al. (27). In this study, we hypothesized that ENaC in the CCD can be regulated via histamine-mediated signaling, and this mechanism could play a role in renal physiology and pathophysiology.
MATERIALS AND METHODS
Cell Culture
Mouse principal kidney cortical collecting duct cell line (mpkCCDcl4; male mouse) cells were kindly provided by Dr. Abdel Alli (University of Florida) and have been described previously (37, 38). Cells were maintained with DMEM/F-12 medium from Thermo Fisher Scientific, 2% FBS and supplements (Insulin, Na+ selenite, triiodothyronine, dexamethasone, EGF, Apo-Transferrin, and penicillin/streptomycin) until they polarized and formed monolayers with high resistance and avid Na+ transport (38). Histamine concentrations tested in these studies were chosen based on published literature that showed that high doses of histamine elicit an antidiuretic response (39). Furthermore, acid-sensing ion channels (ASIC, structurally similar to ENaC) are known to be functionally responsive to 500 μM to 1 mM of histamine (40). Van Unen et al. (41) showed a stimulatory action of 100 μM histamine on calcium level in renal HEK 293 cells using FRET biosensors as well.
Transepithelial Short Circuit Current Measurements
Cells were seeded onto permeable supports (Costar Transwells, 0.4-m pore, 24-mm diameter; Corning Life Sciences, Corning, NY). To determine the net Na+ transport through ENaC, 10 μM amiloride was added to the apical cell surface at the end of each experiment. Transepithelial Na+ current across the mpkCCDcl4 cell monolayer was calculated, using Ohm’s law, as the quotient of transepithelial voltage to transepithelial resistance under open-circuit conditions, using a EVOM (WPI) voltmeter with AgCl electrodes to measure voltage and resistance, as described previously (38, 42, 43).
Cell-Attached Patch-Clamp Recordings of ENaC Activity
For electrophysiological experiments, mpkCCDcl4 cells were passaged on sterile cover glass chips in 35-mm Petri dishes. Cells were incubated with aldosterone (10 nM) for 24 h to increase the density of the functional ENaC channels in the membrane of the nonpolarized mpkCCD cells. Electrophysiological recordings were performed using the cell-attached patch-clamp technique in a voltage-clamp configuration. Gap-free single channel current data were acquired with Axopatch 200B or 700B amplifiers (Axon Instruments) interfaced via a Digidata 1550 A to a PC running the pClamp 11.2 suite of software (Axon Instruments). After a formation of high resistance gigaOhm seal, the recordings were started immediately. Background control currents were recorded for at least 2 min. Histamine was added to the bath solution at the final concentration of 100 μM. Signals were sampled at 5 kHz and low-pass filtered at 300 Hz with an eight-pole Bessel filter (Warner Instruments). Resistances of borosilicate micropipettes (WPI, 1B150F-4) ranged from 5 to 10 MΩ. Typical bath solution contained (in mM): 150 NaCl, 1 CaCl2, 2 MgCl2, and 10 HEPES (pH 7.4). Pipette solution was (in mM): 140 LiCl, 1 CaCl2, 2 MgCl2, and 10 HEPES (pH 7.4). NPo, the product of the total number of functional channels in the patch (N) and the open probability (Po), or Po itself, were used to measure the channel activity within a patch. The NPo parameter was calculated as NPo = I/i, where I is total current recorded in a patch, and i is unitary current at this voltage. Data are presented as means ± SE.
Western Blotting on Cultured Cells
mpkCCDcl4 cells were washed twice with PBS and homogenized in a lysis buffer as described previously (44). Equal amounts of proteins were separated by using a pre-cast 4%–20% SDS-PAGE gel, transferred onto nitrocellulose membrane (Bio-Rad), immunoblotted with the appropriate antibody, and visualized by enhanced chemiluminescence (Li-COR Odyssey FC) (42). For the catalogue numbers and dilutions of the antibodies used in Western blotting, see Table 1. Antibodies were validated according to the Rules of Antibody Validation (53). The antibodies were considered valid if they produced a band (or bands) of the expected molecular weight(s) for the target protein without nonspecific binding and cross reactivity, were properly documented by the manufacturer (application protocols were provided), had been applied successfully in multiple published projects, had a good binding strength, and were accompanied with good ratings and comments from users on different “open science” platforms. If available from the manufacturer, blocking peptides were used in control experiments. All antibody validation information is consolidated in Tables 1 and 2.
Table 1.
List of antibodies used for Western Blot
| Antibody Name | Dilution | Vendor, Country, Catalog Number | Validation and References |
|---|---|---|---|
| H1R | 1:500 | ProteinTech, USA, 13413-1-AP | Validated in knockout animals (45); validated by vendor in multiple applications; (46); |
| H2R | 1:1,000 | Alomone, Israel, AHR-002 | (47) Validated in immunocytochemistry, Western blot; |
| H3R | 1:1,000 | Abcam, USA, EPR5631 (ab124732) | (48) Validated in immunocytochemistry, Western blot; |
| H4R | 1:1,000 | Sigma Aldrich (Merck), USA, AB5663P | Vendor performed their independent validation in ELISA assay and Western blot; (49, 50); |
| HNMT | 1:1,000 | Thermo Fisher Sci, USA, PIPA557289 | Validated by vendor in multiple applications |
| HDC | 1:1,000 | Thermo Fisher Sci, USA, PIPA597354 | Validated by vendor in multiple applications |
| DAO | 1:1,000 | ProteinTech, USA, 13273-1-AP | Validated by vendor in multiple applications; (55) |
| αENaC | 1:1,000 | StressMarq, Canada, SPC-403 | Western blot and immunofluorescence validation (51, 52) |
ENaC, epithelial Na+ channels; HR, histamine receptor.
Table 2.
Antibodies used in immunocytofluorescence
| Antibody Name | Vendor, Country, Catalog Number | Validation and References |
|---|---|---|
| H1R | Alomone, Israel, AHR-001 | Validated in immunocytochemistry, immunofluorescence (54, 47); blocking peptide applied |
| H2R | Alomone, Israel, AHR-002 | see Table 1 |
| H3R | Abcam, USA, EPR5631 (ab124732) | Used in immunofluorescence (56); also see Table 1 |
| H4R | Sigma Aldrich (Merck), USA, AB5663P | see Table 1 |
| αENaC | StressMarq, Canada, SPC-403 | Used in immunocytochemistry, immunofluorescence (51) |
| Secondary Antibody | Thermo Fisher Sci, USA, A-11008 | Validated by vendor in multiple applications |
DAO, diamine oxidase; ENaC, epithelial Na+ channels; HDC, histidine decarboxylase; HNMT, histamine N-methyltransferase; HR, histamine receptor.
Immunocytochemistry
mpkCCDcl4 cells were stained to visualize expression of HRs with primary and secondary antibodies using a standard procedure (57, 58). Briefly, cells grown on permeable Transwell supports (6.5 or 12 mm diameter) were excised from these inserts, placed on Parafilm, washed with PBS, fixed in 3.7% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X100 for 10 min. Then cells were incubated with primary anti-HRs antibodies for 18–20 h at +4°C, and fluorescence-labelled secondary antibodies for 1 h at room temperature in the dark. Samples were mounted on coverslips using Vectashield mounting medium (Vector Laboratories) and visualized with a Leica TCS SP5 confocal microscope (Leica Microsystems GmBH, Germany) using an ×40 oil objective (NA 1.4). The cell membrane was stained with CellBright Membrane Stain (Biotium, 1/500, 30 min) and the nucleus was stained with Hoechst (H3570, 1/1,000, 10 min). The starting dilution of primary antibodies was 1:1,000, and 1:400–1:500 for the secondary antibodies. The images were quantified in ImageJ software (Fig. 1) using Plot Profile function, and obtained intensity plots were graphed in Origin. The appearance of cells before and after the introduction of histamine was quantitatively compared using the Mean Gray Value function in ImageJ. For the catalogue numbers of the antibodies used in immunofluorescence experiments, see Table 2.
Figure 1.
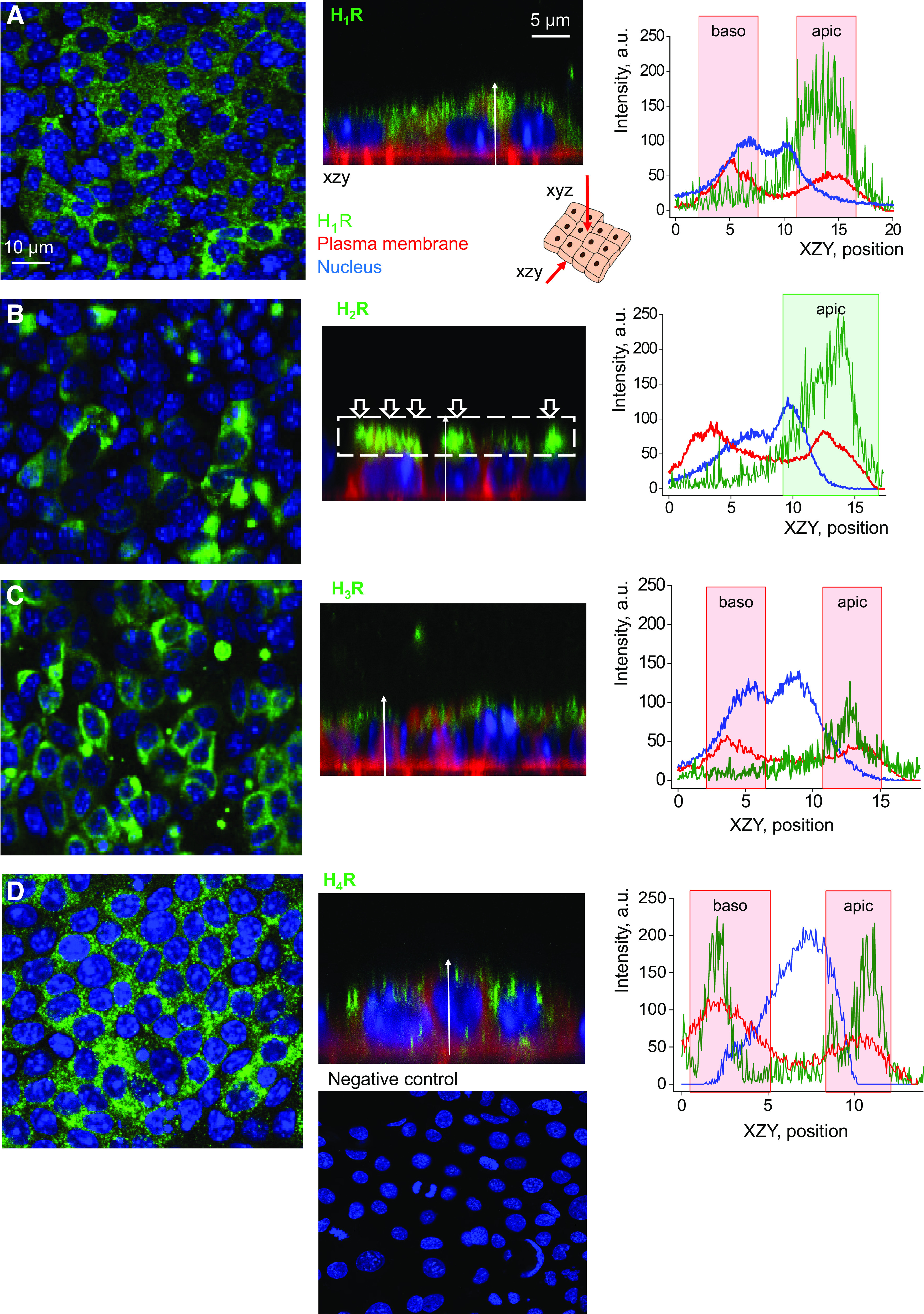
Histamine receptors are detectable in the basolateral and apical membrane of mpkCCDcl4 cells. Shown is the top-down (xyz) and side (xzy) view of immunocytochemistry performed to visualize histamine receptors’ expression [H1R (A), H2R (B), H3R (C), and H4R (D)] in mpkCCDcl4 cells. Scale bar is shown in A, and is the same for all images. Graphs demonstrate representative quantification of fluorescence intensity distribution of the receptors’ level (green), plasma membrane localization (red), and the nucleus (blue) in the xzy view along the white arrow. Baso, basolateral membrane; apic, apical membrane. Shown are representative images, n = 3–5 total independent staining experiments. Bottom panel shows negative control (secondary antibodies in the absence of primary antibodies and Hoechst stain). mpkCCDcl4, mouse cortical collecting duct cell line.
Live Imaging and Detection of Intracellular Ca2+ Level Change
The Leica TCS SP5 (Leica Microsystems GmBH, Germany) confocal microscope system was used to test the degree of Ca2+ increase after introduction of histamine, similarly to what was detailed in previous publications (59, 60). Briefly, polarized mpkCCDcl4 cells were grown on Mat-Tek glass-bottom dishes (P35G-1.5–14-C, Mattek Corp), then incubated with Fluo-8, AM (cell permeable fluorescent dye, Ex488/Em520 nm, Aat Bioquest) and pluronic acid for ∼40 min at room temperature on a rotating shaker. For imaging, dishes were mounted on the Leica TCS SP5 microscope, and growth media was changed to DMEM/F12 containing no supplements but antibiotics. Cells were imaged every 4 s at 1,024 × 1,024 resolution. Background fluorescence level was obtained for 30 s to 1 min, and experimental drugs were added to elicit a Ca2+ response. A ×40 objective with oil immersion and a NA of 1.4 was used to capture the associated images. Image analysis was done using ImageJ open software (59, 60).
cAMP Level Quantification
Cyclic AMP (cAMP) levels in mpkCCDcl4 cells were measured using a commercial kit, according to the manufacturer’s instructions (Cayman 581001). The cells were incubated with varying concentrations of histamine (0, 25, 50, 100, and 200 μM) for 4 h, and the values between different concentrations were compared using the one-way ANOVA statistical test with Holm–Sidak post hoc test.
Quantitative PCR
Mature mpkCCDcl4 cells were grown to full confluency on 60 mm cell culture dishes, harvested and homogenized in a lysis buffer using a 26 G syringe needle. RNA was isolated with the RNeasy kit (Qiagen, 74104). cDNA was subsequently synthesized using a SuperScript III kit (Thermo Fisher Scientific, 18080051) and qPCR completed using PrimePCR primers from Bio-Rad in the Bio-Rad CFX96 Thermocycler (Bio-Rad). The negative controls for the qPCR were RNA samples lacking the addition of reverse transcriptase during cDNA synthesis, while the positive controls were the PrimePCR templates provided by Bio-Rad. β-actin (Actb) was used as a housekeeping gene. RNA purity was verified using 260/230 nm ratios in a spectrophotometer (Nanodrop, Thermo Fisher Scientific); PCR products were separated and visualized in an agarose gel. Primers used in qPCR experiments included β-actin gene and Scnn1a, encoding for the α-subunit of ENaC (see catalogue numbers in Table 3).
Table 3.
Primers used in qPCR. Scnn1a encodes for the α-subunit of ENaC
| Primer for | Catalog Number | Context Sequence |
|---|---|---|
| β-actin (Actb) | Bio-Rad qMmuCEP0039589 | TGCTCGAAGTCTAGAGCAACATAGCACAGCTTCTCTTTGATGTCACGCACGATTTCCCTCTCAGCTGTGGTGGTGAAGCTGTAGCCACGCTCGGTCAGGATCTTCATGAGGTAGTCTGTCAGGTCCCGGCCAGCCAGGT |
| Scnn1a | Bio-Rad qMmuCEP0055781 | TCTGGGCGGTGCTCTGGCTCTGCACCTTCGGCATGATGTACTGGCAGTTTGCTT TGCTGTTCGAGGAATACTTCAGCTACCCCGTGAGTCTCAACATCAACCTCAATTC GGACAAGCTGGTC |
| Positive control | Bio-Rad, Cat no. 10031285 |
ENaC, epithelial Na+ channels.
Statistical Analysis
One-way ANOVA with a Holm–Sidak test post hoc and one-way repeated-measures ANOVA with a Holm–Sidak test or paired Student t test were used when applicable. Data are expressed as box plots, with whiskers being SDs, the box representing SE, and the line showing the median. Values of P < 0.05 were considered statistically significant. Origin 2021 b software was used for all statistical analysis.
RESULTS
Histamine Receptors Are Expressed in the mpkCCDcl4 Cells
First, we tested if HRs are expressed in mpkCCDcl4 cells, an established model of mouse CCDs that polarize when put on permeable supports and exhibit all typical properties of renal epithelium (37, 38). To determine cellular localization of HRs, immunocytochemical (ICC) staining for HRs 1–4 was conducted in combination with confocal microscopy. All HRs were found to be expressed in the apical membrane of mpkCCD cells, with H4R additionally expressed in the basolateral membrane, as shown in Fig. 1. Then, Western blots were conducted to assess and verify the expression of H1R, H2R, H3R, H4R, HNMT, HDC, and DAO, as shown in Fig. 2A. Next, to determine the functional effect of histamine on HR expression, we conducted immunocytochemical experiments on polarized mpkCCDcl4 cells exposed to a 4-h incubation with 200 μM of histamine or vehicle. The mean gray value of the ICC fluorescence was subsequently quantified using ImageJ. Histamine caused a significant increase in the expression of both receptors H1R and H4R [fluorescence intensity increased in response to histamine from 30.6 ± 4.4 (n = 5) and 29.2 ± 1.4 (n = 8) to 47.1 ± 5.3 (n = 4) and 70.5 ± 4.6 a.u. (n = 8) for H1R and H4R, respectively (Fig. 2B)]. H2R and H3R expression was similar between groups incubated with vehicle or histamine [H2R: 27.9 ± 0.5 (n = 9) vs. 29.3 ± 0.6 (n = 12), H3R: 29.6 ± 0.2 (n = 12) and 29.8 ± 0.3 a.u. (n = 12) pre- and post-histamine application, respectively].
Figure 2.
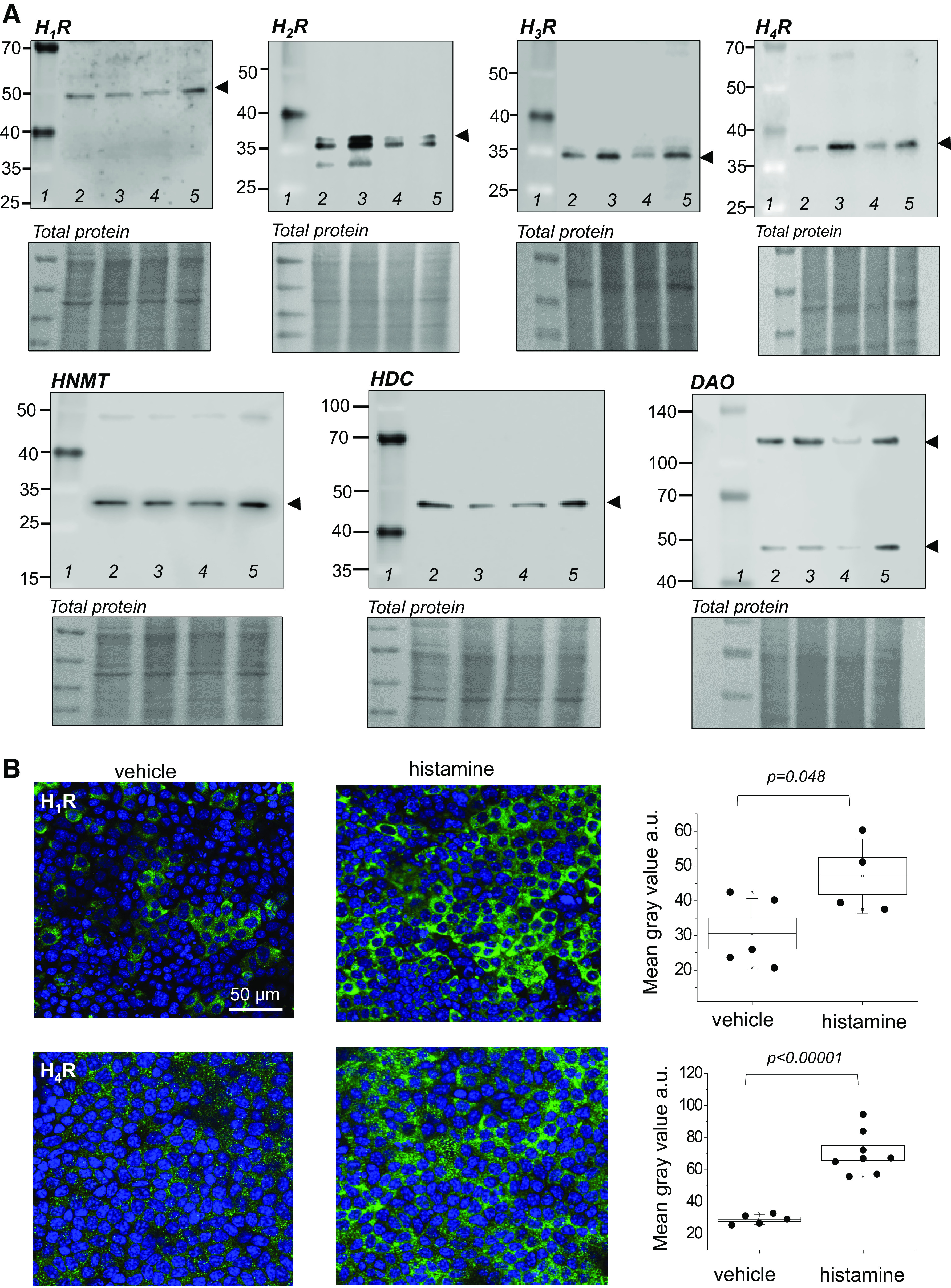
The components of the histaminergic system and all histamine receptors are present in the collecting duct cells. A: Western blot analysis showing H1R, H2R, H3R, H4R, histamine-N-methyltransferase (HNMT), histidine decarboxylase (HDC), and diamine oxidase (DAO) expression in mpkCCDcl4 cells. Each lane on the Western blot image pertains to an independently grown cell culture dish to illustrate that proteins are consistently expressed in independent cell samples; no treatments were performed. Lane 1 in each Western is a weight marker; lanes 2–5 are individual independent lysates. B: representative immunofluorescence staining of H1R and H4R expression in the apical membrane of the mpkCCDcl4 cells following treatment with vehicle or 200 μM histamine for 4 h; shown are topside view images (XYZ) at ×63. Graphs show quantification of H1R and H4R expression (based on fluorescence intensity, normalized to background fluorescence) between treatment and experimental groups. n = 3 experiments from independent cell samples, n = 4–8 fields of view were quantified. Box illustrated means ± SE, whiskers are SD. Two-sample Student’s t test was used to test for statistical significance in B. Scale bar is shown in B for H1R and is the same for all images. mpkCCDcl4, mouse cortical collecting duct cell line.
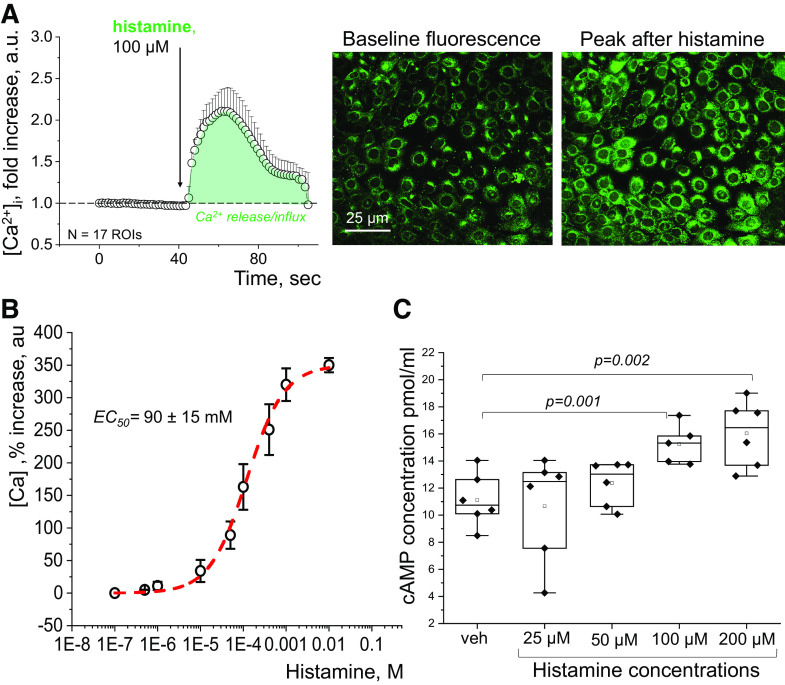
Histamine Evokes Ca2+ Transients and cAMP Production in mpkCCDcl4 Cells
Ca2+ handling is one of the major components of the intracellular pathways downstream of HR activation (27). The functional effect of histamine on intracellular Ca2+ levels ([Ca2+]i), of mpkCCDcl4 cells was assessed by loading cells with a Fluo-8 Ca2+ dye; changes in [Ca2+]i in response to histamine were imaged in confocal microscopy. Fluorescence of Fluo-8, indicative of the Ca2+ level, was evaluated at baseline and after an acute addition of different concentrations of histamine. As shown in Fig. 3A, histamine evoked an acute increase in fluorescence of Fluo-8, thus suggesting an increase in [Ca2+]i. The response of [Ca2+]i to histamine was dose-dependent, as shown in Fig. 3B. The dose-response curve allowed us to calculate an EC50 value of 90 ± 15 μM for histamine in this setting. To further evaluate the functional ramification of histamine application in the CCD, we tested the effect of histamine on the production of another known component of HR cascades, cAMP (27). mpkCCDcl4 cells were treated with different concentrations (25, 50, 100, and 200 μM) of histamine or vehicle (control) for 4 h, and the intracellular cAMP level was quantified postincubation. As shown in Fig. 3C, cAMP concentration increased in a dose-dependent manner.
Figure 3.
Effect of histamine on Ca2+ release and cAMP production in mpkCCDcl4 cells. A: representative quantification of an intracellular Ca2+ transient in response to an application of 100 μM of histamine, along with representative images demonstrating an increase in Fluo-8 fluorescence posthistamine, indicative of a Ca2+ level elevation. n = 17 regions of interest (ROIs, individual cells) were quantified to obtain the representative graph. B: dose-dependency curve illustrates the increase in intracellular Ca2+ level in response to an acute addition of histamine, in a range from 10 nM to 10 mM, in mpkCCDcl4 cells. Data were obtained via confocal imaging, the graph is showing an increase (in percent) in influx of Ca2+ (measured at the peak of the response vs baseline) on the y-axis, and concentration of histamine on the x-axis. n = at least 3 experimental replicates per concentration; an average of 30–50 cells were analyzed for each concentration tested. Data are shown as means and SD. The EC50 was found to be 90 ± 15 μM (obtained using the nonlinear curve fitting function in OriginPro). C: effect of histamine on cAMP production. cAMP concentration analyzed by cAMP ELISA kit in mpkCCDcl4 cells in control, and after treatment with 25, 50, 100, and 200 μM of histamine groups. n = 5 to 6 independent individually collected cell samples per group; one-way ANOVA with Tukey’s post hoc was used for statistical analysis. mpkCCDcl4, mouse cortical collecting duct cell line.
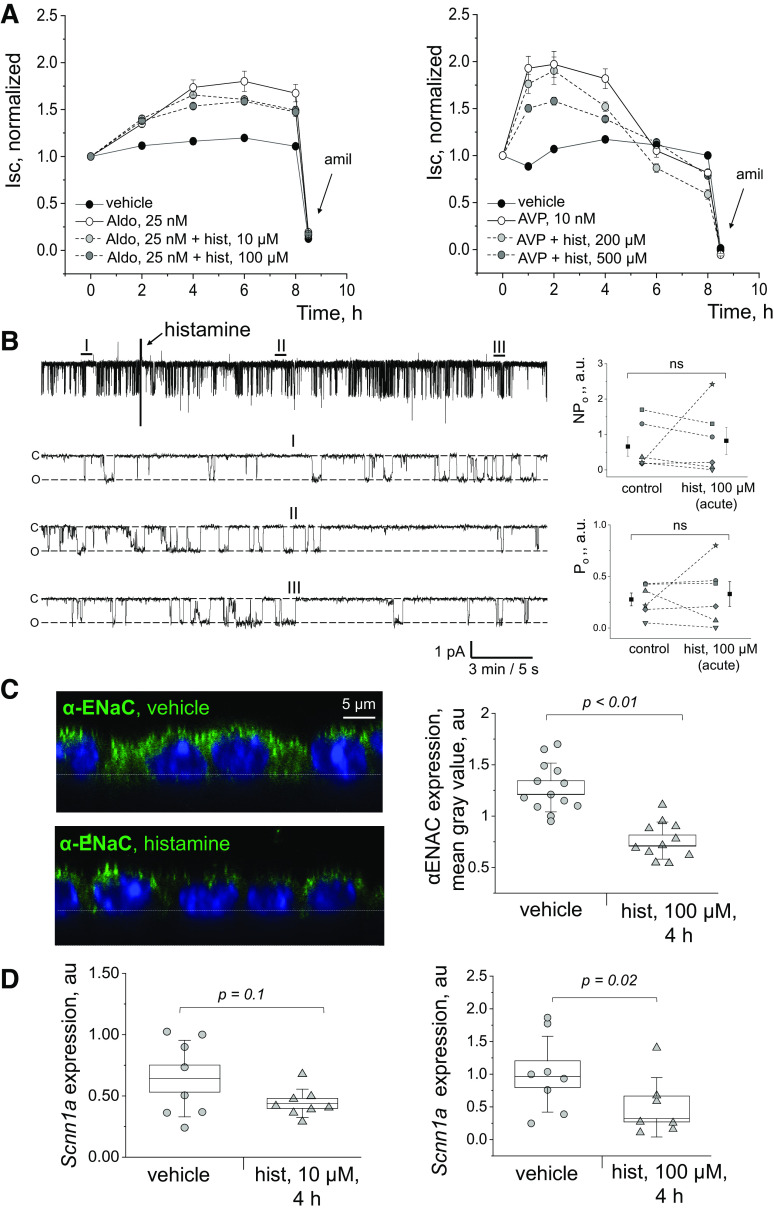
Incubation with Histamine Suppresses ENaC-Mediated Currents and Decreases αENaC Expression in mpkCCDcl4 Cells
To determine the effect of histamine on Na+ reabsorption in the CCD, ENaC-mediated currents were measured in short-circuit current experiments conducted in mpkCCDcl4 cells grown on permeable supports. As shown in Fig. 4A, it was confirmed that AVP and aldosterone, known activators of ENaC, stimulate Na+ currents in mpkCCDcl4 cells. It was further found that the addition of histamine to AVP-stimulated mpkCCDcl4 cells (10 nM) suppresses ENaC-mediated current at a 200-μM concentration and further at a 500-μM concentration. The effect of a combination of aldosterone (25 nM) and histamine on ENaC-mediated currents was also examined. The addition of histamine to aldosterone-stimulated mpkCCDcl4 cells suppressed ENaC-mediated currents at the 10 μM histamine concentration and further at the 100 μM histamine concentration. Single-channel ENaC activity was probed with the patch-clamp technique in a cell-attached configuration to test the acute effects of histamine at the single-channel level. Figure 4B illustrates a typical example of single-channel currents through ENaC, recorded in the cell-attached mode at different time points during the experiment, and corresponding summary graphs of ENaC activity (NPo) in control and after histamine addition (100 μM). Our data revealed that acute application of histamine does not affect ENaC activity (Fig. 4B, P value > 0.3; n = 6; paired Student’s t test).
Figure 4.
Effect of histamine on epithelial Na+ channel (ENaC) currents and expression. A: effects of histamine on ENaC-mediated Isc currents (short-circuit currents) in mpkCCDcl4 cells. Shown is the effect of aldosterone (aldo) alone and in combination with histamine (hist) on ENaC-mediated currents, as well as the effect of vasopressin (AVP) alone and in combination with histamine (hist) on ENaC-mediated currents. n = 6 independent wells/group/time point. B: acute application of histamine does not affect ENaC activity in the apical membrane of mpkCCDcl4 principal cells. Left: representative current trace of single channel ENaC currents recorded in the mpkCCDcl4 principal cells in response to addition of 100 μM histamine. Areas before (I), after 5 min (II), and 20 min (III) of the histamine treatment are shown below with an expanded time scale. Closed state of the channel is denoted by c, open state by o. Patch was held at a −60 mV test potential during the course of the experiment. Right: summary graphs of total ENaC activity (NPo; top) and the open probability of individual channels (Po; bottom) in cell-attached patches obtained in mpkCCDcl4 cells before (control) and after histamine application (histamine, 100 μM). The data before/after application of histamine were compared using Student’s paired t test. ns, nonsignificant; a.u., arbitrary units; pA, picoAmps. n = 6 independent experimental recording from n = 3 different days. C: effect of histamine on αENaC expression. Representative immunofluorescent staining for αENaC expression in the apical membrane of the mpkCCDcl4 cells following treatment with 100 μM histamine or vehicle for 4 h; the images are taken from a side view (XZY) at ×63 with a digital zoom. Also shown is the quantification of αENaC expression (based on fluorescence intensity, normalized to background fluorescence) between treatment and experimental groups. n = 13 and n = 11 fields of view randomly selected from at least three independent staining experiments were quantified, respectively (at least 4 cells in each field of view). Scale bar shown is the same for all images. D: effect of histamine on the expression Scnn1a, encoding for the alpha subunit of the ENaC. Shown are results of qPCR for Scnn1a tested in mpkCCDcl4 cells treated with histamine or vehicle (10 and 100 μM) for 4 h (values normalized to nuclear-encoded Actb). Each datapoint shown is an average of three technical replicates, n = 3 independent experiments were performed (n = 8 samples/group). Data were compared using a one-way ANOVA with Holm–Sidak post hoc test. mpkCCDcl4, mouse cortical collecting duct cell line.
The effect of histamine on expression of the gene encoding for the α-subunit of ENaC (Scnn1a) and αENaC protein was tested using qPCR and ICC experiments. We first tested the effect of histamine on the expression of αENaC protein in the apical membrane of the mpkCCDcl4 cells using ICC (Fig. 4C). mpkCCD cells were incubated for 4 h with 200 μM of histamine or vehicle. n = 13 and n = 11 different fields of view were quantified for each experimental group, with at least 4 cells/field of view. We report that treatment with histamine significantly reduced the expression of αENaC in the apical membrane of the cells. This was in line with our qPCR data: we found that 100 μM of histamine caused a significant decrease in expression of Scnn1a (Fig. 4D).
DISCUSSION
In this study, using an integrative approach including molecular, pharmacological, and electrophysiological methods, we confirmed the expression and functionality of histamine receptors in mpkCCDcl4 cells. First, using Western blot we have shown that the expression of histaminergic system components (HNMT, HDC, and DAO) and all four HR proteins in the collecting duct cells (Fig. 2A). In response to a 4-h incubation with histamine, we detected an increase in H1R and H4R expression in polarized CCD cells (Fig. 2B). Previous studies found that histamine elicited an upregulation of H1R, H2R, and H3R in astrocytes accompanied with an increased neuroprotective effect during inflammatory processes (61). In the kidney, there is very limited data available regarding the expression and interdependence of the components of the histaminergic system. Both transcriptomic [KIT project (62–64), Kidney Systems Biology Project (65–67), and proteomic Human Protein Atlas] databases suggest low-to-moderate expression of H1R and H2R, and lower expression of other histamine receptors in the CD. Expression of the other components of the histaminergic system has also been observed, with low expression of HDC and DAO and moderate expression of HNMT. Pini et al. (68) previously reported increased expression of H3R in CCD cells of diabetic versus non-diabetic rat kidneys. Relatively low levels of H4R mRNA were detected in the kidneys of healthy Wistar rats with a significant upregulation in diabetic kidneys, mainly in the loop of Henle (69). The presence of all four HRs was detected in human primary immortalized renal tubular epithelial cells (TECs) from the renal cortex and the HK-2 cell line by Veglia et al. (70), consistent with our results in the cultured mpkCCDcl4 cells. While previous studies reported that only H3R is localized apically in rat CCD cells, our immunofluorescence experiments revealed pronounced differential expression of HRs in the apical and basolateral membranes of the polarized mpkCCDcl4 monolayers. Interestingly, H2R expression was more pronounced in the apical membrane of the polarized cells. Our data on spatial localization (apical, basal) are somewhat similar to the results previously reported from human renal tubules: H3R immunolabeling was detected at the apical membrane of tubular epithelium, and H4R at the basal membrane of tubular epithelial cells (70).
Using varying concentrations of histamine in conjunction with confocal fluorescence Ca2+ imaging and a cAMP quantification assay, we confirmed the functional importance of HRs in mpkCCDcl4 cells, showing that their activation elicited a downstream intracellular signaling response. In live Ca2+ measurements of mpkCCDcl4 cells, we found that histamine evoked an increase of the intracellular Ca2+ level in a dose-dependent manner (Fig. 3). These results complement published data that downstream signaling cascades for all four HRs may lead to an increase in intracellular Ca2+ levels (71, 72). The important role of histamine in intracellular Ca2+ pathways was described in various cell types. For instance, histamine-induced Ca2+ increase in cultured human smooth muscle cells was found to be mediated by distinct histamine receptors (H1R, H2R, H3R, separated by specific agonists) (72). Similarly, an increased intracellular Ca2+ release was induced by histamine (at a concentration of 10 μM) or activation of all HR subtypes in cultured rat goblet cells using HR agonists histamine dimaleate (for H1R), amthamine (for H2R), a-methylhistamine (for H3R), or 4-methylhistamine (for H4R) (73). In human embryonic kidney 293 (HEK 293) cells 100 μM histamine was only shown to affect calcium handling via H1R and H2R (41). Here we demonstrated that histamine induces dose-dependent changes in Ca2+ release with an EC50 of 90 ± 15 μM. Further research using HR-specific agonists and antagonists is needed to determine the involvement of a particular receptor subtype in the CD cells. However, given that the affinity of the H1R and H2R for histamine is lower (micromolar range) compared with the H3R and H4R (5–10 nM), and the lack of effects of nanomolar histamine on [Ca2+]i in our hands, it can be speculated that histamine-evoked Ca2+ changes are possibly mediated by H1R and H2R in mpkCCDcl4 cells. Future studies should also help discern whether the changes in [Ca2+]i are due to an influx of Ca2+ from the extracellular space and/or the intracellular store depletion.
Furthermore, our results demonstrated a histamine-induced increase in intracellular level of cAMP (Fig. 3C). These data are consistent with previous studies that showed the involvement of all HR subtypes in the cAMP signaling pathway. Histamine, acting on H1R, was shown to inhibit cAMP accumulation in U373 MG astrocytoma cells in a Ca2+-dependent manner (74). Activation of H2R, on the other hand, induced cAMP accumulation in various cell lines including HL-60 promyelocytes, neutrophils, U937 promonocytes, HGT-1 gastric cancer cells, and monocyte-derived dendritic cells (75). Forskolin-mediated cAMP production has been shown to be inhibited by H3R activation in the SK-N-MC cell line (76). H4R may potentially also affect cAMP due to the similarity of its downstream pathways to H3R (27, 76); however, no effect of H4R stimulation on cAMP production was found in human monocyte-derived dendritic cells (77). Since our data indicate that histamine induced an increase in cAMP, this effect in our CD model system may be mediated in part via H2R, which is known to propagate cAMP signaling. Given the possibility that the level of cAMP that we observe in our experiments is the result the coordinated work of all four receptors and compensation by activation of HR subtypes, more detailed studies with HR inhibitors of greater specificity are required.
CCD cells mediate the reabsorption of Na+ via ENaC channels, which allow for precise regulation of electrolyte balance and thus blood pressure (6, 7). Currently, there are no published data about the direct action of histamine on ENaC. Our short-circuit data demonstrated that ENaC amiloride-sensitive currents stimulated by vasopressin or aldosterone are decreased after the addition of histamine. The observed effect may be due to changes in the apical expression of ENaCs or their open probability. Immunofluorescence experiments confirmed the reduced expression of αENaC in the apical membrane of polarized mpkCCDcl4 monolayers, and qPCR analysis further supported these data. Single-channel cell-attached patch-clamp studies corroborated the lack of the immediate changes of ENaC activity in response to an acute histamine application. However, short circuit current experiments demonstrated an attenuation of ENaC-mediated currents in the mpkCCD cells within a time frame of several hours post histamine application. These observations prompt us to suggest that histamine likely affects Na+ reabsorption through the modulation of ENaC expression rather than activity. However, more studies are needed to assess the long-term effects of histamine on ENaC using the high-resolution single-channel patch-clamp analysis, as we cannot exclude that histamine might influence ENaC open probability in the long-term perspective. To summarize, we have established here a new histamine-mediated regulatory mechanism that inhibits ENaC in CCD, and thus can affect Na+ reabsorption in this segment of the nephron.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants R01 HL148114 (to D.V.I.), NHLBI R01HL148114-02S1 (to D.V.I. and D.R.S.), the Augusta University, Department of Physiology startup funds (to D.V.I.), and the AHA Postdoctoral Fellowship (to D.R.S.).
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.V.I. conceived and designed research; A.V.S., M.V.F., R.F.S., Y.Z., S.P., M.D., M.S., D.V.L., D.R.S., and D.V.I. performed experiments; A.V.S., M.V.F., R.F.S., Y.Z., S.P., M.D., M.S., D.V.L., and D.R.S. analyzed data; A.V.S., M.V.F., R.F.S., Y.Z., S.P., M.D., D.V.L., D.R.S., and D.V.I. interpreted results of experiments; A.V.S., M.V.F., R.F.S., Y.Z., S.P., D.V.L., D.R.S., and D.V.I. prepared figures; A.V.S., M.V.F., D.R.S., and D.V.I. drafted manuscript; A.V.S., M.V.F., R.F.S., S.P., M.D., M.S., D.V.L., D.R.S., and D.V.I. edited and revised manuscript; A.V.S., M.V.F., R.F.S., Y.Z., S.P., M.D., M.S., D.V.L., D.R.S., and D.V.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Andrey V. Ilatovskiy helpful discussions of the initial idea for the project.
REFERENCES
- 1.Zhuo JL, Li XC. Proximal nephron. Compr Physiol 3: 1079–1123, 2013. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem 58: 680–689, 2012. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676–687, 2015. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki O, Ishizawa K, Hirohama D, Fujita T, Shibata S. Electrolyte transport in the renal collecting duct and its regulation by the renin-angiotensin-aldosterone system. Clin Sci (Lond) 133: 75–82, 2019. doi: 10.1042/CS20180194. [DOI] [PubMed] [Google Scholar]
- 6.Ware AW, Rasulov SR, Cheung TT, Lott JS, McDonald FJ. Membrane trafficking pathways regulating the epithelial Na+ channel. Am J Physiol Renal Physiol 318: F1–F13, 2020. doi: 10.1152/ajprenal.00277.2019. [DOI] [PubMed] [Google Scholar]
- 7.Feraille E, Dizin E. Coordinated control of ENaC and Na+,K+-ATPase in renal collecting duct. J Am Soc Nephrol 27: 2554–2563, 2016. doi: 10.1681/ASN.2016020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoenig MP, Zeidel ML. Homeostasis, the milieu interieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9: 1272–1281, 2014. doi: 10.2215/CJN.08860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleyman TR, Eaton DC. Regulating ENaC's gate. Am J Physiol Cell Physiol 318: C150–C162, 2020. doi: 10.1152/ajpcell.00418.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noreng S, Bharadwaj A, Posert R, Yoshioka C, Baconguis I. Structure of the human epithelial sodium channel by cryo-electron microscopy. eLife 7: e39340, 2018. doi: 10.7554/eLife.39340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579: 95–132, 2016. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enslow BT, Stockand JD, Berman JM. Liddle's syndrome mechanisms, diagnosis and management. Integr Blood Press Control 12: 13–22, 2019. doi: 10.2147/IBPC.S188869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlov TS, Palygin O, Isaeva E, Levchenko V, Khedr S, Blass G, Ilatovskaya DV, Cowley AW Jr, Staruschenko A. NOX4-dependent regulation of ENaC in hypertension and diabetic kidney disease. FASEB J 34: 13396–13408, 2020. doi: 10.1096/fj.202000966RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutchler SM, Kirabo A, Kleyman TR. Epithelial sodium channel and salt-sensitive hypertension. Hypertension 77: 759–767, 2021. doi: 10.1161/HYPERTENSIONAHA.120.14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez de la Rosa D, Navarro-Gonzalez JF, Giráldez T. ENaC modulators and renal disease. Curr Mol Pharmacol 6: 35–43, 2013. doi: 10.2174/1874467211306010005. [DOI] [PubMed] [Google Scholar]
- 16.Pavlov TS, Levchenko V, Ilatovskaya DV, Palygin O, Staruschenko A. Impaired epithelial Na+ channel activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats. Pediatr Res 77: 64–69, 2015. doi: 10.1038/pr.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marunaka R, Marunaka Y. Interactive actions of aldosterone and insulin on epithelial Na+ channel trafficking. Int J Mol Sci 21: 3407, 2020. doi: 10.3390/ijms21103407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilatovskaya DV, Levchenko V, Brands MW, Pavlov TS, Staruschenko A. Cross-talk between insulin and IGF-1 receptors in the cortical collecting duct principal cells: implication for ENaC-mediated Na+ reabsorption. Am J Physiol Renal Physiol 308: F713–F719, 2015. doi: 10.1152/ajprenal.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haerteis S, Krappitz M, Bertog M, Krappitz A, Baraznenok V, Henderson I, Lindström E, Murphy JE, Bunnett NW, Korbmacher C. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch 464: 353–365, 2012. doi: 10.1007/s00424-012-1138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotin D, Staub O. Function and regulation of the epithelial Na+ channel ENaC. Compr Physiol 11: 2017–2045, 2021. doi: 10.1002/cphy.c200012. [DOI] [PubMed] [Google Scholar]
- 21.Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A. High salt activates CD11c+ antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74: 555–563, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- 23.Thai TL, Yu L, Galarza-Paez L, Wu MM, Lam HY, Bao HF, Duke BJ, Al-Khalili O, Ma HP, Liu B, Eaton DC. The polarized effect of intracellular calcium on the renal epithelial sodium channel occurs as a result of subcellular calcium signaling domains maintained by mitochondria. J Biol Chem 290: 28805–28811, 2015. doi: 10.1074/jbc.M115.668293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao US, Baker JM, Pluznick JL, Balachandran P. Role of intracellular Ca2+ in the expression of the amiloride-sensitive epithelial sodium channel. Cell Calcium 35: 21–28, 2004. doi: 10.1016/s0143-4160(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 25.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative Consensus XWG . Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27: 371–379, 2016. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matoba K, Takeda Y, Nagai Y, Kawanami D, Utsunomiya K, Nishimura R. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int J Mol Sci 20: 3393, 2019. doi: 10.3390/ijms20143393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudarikova AV, Fomin MV, Yankelevich IA, Ilatovskaya DV. The implications of histamine metabolism and signaling in renal function. Physiol Rep 9: e14845, 2021. doi: 10.14814/phy2.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15: 290–300, 2019. doi: 10.1038/s41581-019-0121-z. [DOI] [PubMed] [Google Scholar]
- 29.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm 2018: 9524075, 2018. doi: 10.1155/2018/9524075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, Haas HL. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67: 601–655, 2015. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiligada E, Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol 177: 469–489, 2020. doi: 10.1111/bph.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev 71: 1–51, 1991. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Gill DS, Fonseca VA, Barradas MA, Balliod R, Moorhead JF, Dandona P. Plasma histamine in patients with chronic renal failure and nephrotic syndrome. J Clin Pathol 44: 243–245, 1991. doi: 10.1136/jcp.44.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grange C, Gurrieri M, Verta R, Fantozzi R, Pini A, Rosa AC. Histamine in the kidneys: what is its role in renal pathophysiology? Br J Pharmacol 177: 503–515, 2020. doi: 10.1111/bph.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- 38.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol 18: 1652–1661, 2007. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 39.Bhargava KP, Kulshrestha VK, Santhakumari G, Srivastava YP. Mechanism of histamine-induced antidiuretic response. Br J Pharmacol 47: 700–706, 1973. doi: 10.1111/j.1476-5381.1973.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaeva EI, Tikhonova TB, Magazanik LG, Tikhonov DB. Histamine selectively potentiates acid-sensing ion channel 1a. Neurosci Lett 632: 136–140, 2016. doi: 10.1016/j.neulet.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 41.van Unen J, Rashidfarrokhi A, Hoogendoorn E, Postma M, Gadella TW Jr., Goedhart J. Quantitative Single-Cell Analysis of Signaling Pathways Activated Immediately Downstream of Histamine Receptor Subtypes. Mol Pharmacol 90: 162–176, 2016, [Erratum in Mol Pharmacol 90: 413, 2016]. doi: 10.1124/mol.116.104505. [DOI] [PubMed] [Google Scholar]
- 42.Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J 25: 2688–2699, 2011. doi: 10.1096/fj.10-167262. [DOI] [PubMed] [Google Scholar]
- 43.Levchenko V, Zheleznova NN, Pavlov TS, Vandewalle A, Wilson PD, Staruschenko A. EGF and its related growth factors mediate sodium transport in mpkCCDc14 cells via ErbB2 (neu/HER-2) receptor. J Cell Physiol 223: 252–259, 2010. doi: 10.1002/jcp.22033. [DOI] [PubMed] [Google Scholar]
- 44.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol 21: 833–843, 2010. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valiya Veettil M, Krishna G, Roy A, Ghosh A, Dutta D, Kumar B, Chakraborty S, Anju TR, Sharma-Walia N, Chandran B. Kaposi’s sarcoma-associated herpesvirus infection induces the expression of neuroendocrine genes in endothelial cells. J Virol 94: e01692-19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su WJ, Zhang T, Jiang CL, Wang W. Clemastine alleviates depressive-like behavior through reversing the imbalance of microglia-related pro-inflammatory state in mouse hippocampus. Front Cell Neurosci 12: 412, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apolloni S, Fabbrizio P, Amadio S, Napoli G, Verdile V, Morello G, Iemmolo R, Aronica E, Cavallaro S, Volonté C. Histamine regulates the inflammatory profile of SOD1-G93A microglia and the histaminergic system is dysregulated in amyotrophic lateral sclerosis. Front Immunol 8: 1689, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Hu XY. Inhibition of histamine receptor H3R suppresses prostate cancer growth, invasion and increases apoptosis via the AR pathway. Oncol Lett 16: 4921–4928, 2018. doi: 10.3892/ol.2018.9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun 279: 615–620, 2000. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- 50.Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem 275: 36781–36786, 2000. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 51.Rein JL, Heja S, Flores D, Carrisoza-Gaytán R, Lin NYC, Homan KA, Lewis JA, Satlin LM. Effect of luminal flow on doming of mpkCCD cells in a 3D perfusable kidney cortical collecting duct model. Am J Physiol Cell Physiol 319: C136–C147, 2020. doi: 10.1152/ajpcell.00405.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavlov TS, Levchenko V, Ilatovskaya DV, Moreno C, Staruschenko A. Renal sodium transport in renin-deficient Dahl salt-sensitive rats. J Renin Angiotensin Aldosterone Syst 17: 147032031665385, 2016. doi: 10.1177/1470320316653858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weller MG. Ten basic rules of antibody validation. Anal Chem Insights 13: 1177390118757462, 2018. doi: 10.1177/1177390118757462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raveendran VV, Smith DD, Tan X, Sweeney ME, Reed GA, Flynn CA, Tawfik OW, Milne G, Dileepan KN. Chronic ingestion of H1-antihistamines increase progression of atherosclerosis in apolipoprotein E−/− mice. PLoS One 9: e102165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seki T, Sato M, Konno A, Hirai H, Kurauchi Y, Hisatsune A, Katsuki H. d-Cysteine promotes dendritic development in primary cultured cerebellar Purkinje cells via hydrogen sulfide production. Mol Cell Neurosci 93: 36–47, 2018. [DOI] [PubMed] [Google Scholar]
- 56.Wang N, Ma J, Liu J, Wang J, Liu C, Wang H, Liu Y, Yan H, Jiang S. Histamine H3 receptor antagonist enhances neurogenesis and improves chronic cerebral hypoperfusion-induced cognitive impairments. Front Pharmacol 10: 1583, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chubinskiy-Nadezhdin VI, Sudarikova AV, Shilina MA, Vasileva VY, Grinchuk TM, Lyublinskaya OG, Nikolsky NN, Negulyaev YA. Cell cycle-dependent expression of Bk channels in human mesenchymal endometrial stem cells. Sci Rep 9: 4595, 2019. doi: 10.1038/s41598-019-41096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chubinskiy-Nadezhdin VI, Vasileva VY, Vassilieva IO, Sudarikova AV, Morachevskaya EA, Negulyaev YA. Agonist-induced Piezo1 activation suppresses migration of transformed fibroblasts. Biochem Biophys Res Commun 514: 173–179, 2019. doi: 10.1016/j.bbrc.2019.04.139. [DOI] [PubMed] [Google Scholar]
- 59.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Single-channel analysis and calcium imaging in the podocytes of the freshly isolated glomeruli. J Vis Exp 100: e52850, 2015. doi: 10.3791/52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spires D, Ilatovskaya DV, Levchenko V, North PE, Geurts AM, Palygin O, Staruschenko A. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol 315: F1091–F1097, 2018. doi: 10.1152/ajprenal.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Zhang X, Qian Q, Wang Y, Dong H, Li N, Qian Y, Jin W. Histamine upregulates the expression of histamine receptors and increases the neuroprotective effect of astrocytes. J Neuroinflammation 15: 41, 2018. doi: 10.1186/s12974-018-1068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, Humphreys BD. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun 12: 2190, 2021. doi: 10.1038/s41467-021-22368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheval L, Pierrat F, Rajerison R, Piquemal D, Doucet A. Of mice and men: divergence of gene expression patterns in kidney. PLoS One 7: e46876, 2012. doi: 10.1371/journal.pone.0046876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, Chou CL, Knepper MA. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol 32: 897–912, 2021. doi: 10.1681/ASN.2020101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Lee JW, Chou C-L, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pini A, Chazot PL, Veglia E, Moggio A, Rosa AC. H3 receptor renal expression in normal and diabetic rats. Inflamm Res 64: 271–273, 2015. doi: 10.1007/s00011-015-0808-y. [DOI] [PubMed] [Google Scholar]
- 69.Rosa AC, Grange C, Pini A, Katebe MA, Benetti E, Collino M, Miglio G, Bani D, Camussi G, Chazot PL, Fantozzi R. Overexpression of histamine H4 receptors in the kidney of diabetic rat. Inflamm Res 62: 357–365, 2013. doi: 10.1007/s00011-012-0587-7. [DOI] [PubMed] [Google Scholar]
- 70.Veglia E, Grange C, Pini A, Moggio A, Lanzi C, Camussi G, Chazot PL, Rosa AC. Histamine receptor expression in human renal tubules: a comparative pharmacological evaluation. Inflamm Res 64: 261–270, 2015. doi: 10.1007/s00011-015-0807-z. [DOI] [PubMed] [Google Scholar]
- 71.Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 9: 1873, 2018. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neuhaus J, Weimann A, Stolzenburg JU, Dawood W, Schwalenberg T, Dorschner W. Histamine receptors in human detrusor smooth muscle cells: physiological properties and immunohistochemical representation of subtypes. World J Urol 24: 202–209, 2006. doi: 10.1007/s00345-006-0079-x. [DOI] [PubMed] [Google Scholar]
- 73.Li D, Carozza RB, Shatos MA, Hodges RR, Dartt DA. Effect of histamine on Ca2+-dependent signaling pathways in rat conjunctival goblet cells. Invest Ophthalmol Vis Sci 53: 6928–6938, 2012. [Erratum in Invest Ophthalmol Vis Sci 53: 7380, 2012]. doi: 10.1167/iovs.12-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong MP, Cooper DM, Young KW, Young JM. Characteristics of the Ca2+-dependent inhibition of cyclic AMP accumulation by histamine and thapsigargin in human U373 MG astrocytoma cells. Br J Pharmacol 130: 1021–1030, 2000. doi: 10.1038/sj.bjp.0703411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci 34: 33–58, 2013. doi: 10.1016/j.tips.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieland K, Bongers G, Yamamoto Y, Hashimoto T, Yamatodani A, Menge WM, Timmerman H, Lovenberg TW, Leurs R. Constitutive activity of histamine h(3) receptors stably expressed in SK-N-MC cells: display of agonism and inverse agonism by H(3) antagonists. J Pharmacol Exp Ther 299: 908–914, 2001. [PubMed] [Google Scholar]
- 77.Gutzmer R, Diestel C, Mommert S, Köther B, Stark H, Wittmann M, Werfel T. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol 174: 5224–5232, 2005. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]