Keywords: disuse, immobilization, pericyte, recovery, skeletal muscle
Abstract
Extended bed rest or limb immobilization can significantly reduce skeletal muscle mass and function. Recovery may be incomplete, particularly in older adults. Our laboratory recently reported that vascular mural cell (pericyte) quantity is compromised after immobilization and appropriate replacement immediately before remobilization can effectively recover myofiber size in mice. Identification of a single cell surface marker for isolation of the most therapeutic pericyte would streamline efforts to optimize muscle recovery. The purpose of this study was to compare the capacity for neural/glial antigen 2 (Cspg4/NG2+) and melanoma cell adhesion molecule (Mcam/CD146+) positive pericytes to uniquely recover skeletal muscle post-disuse. A single hindlimb from adult C57BL/6J mice was immobilized in full dorsiflexion via a surgical staple inserted through the center of the foot and body of the gastrocnemius. Fourteen days after immobilization, the staple was removed and pericytes, either NG2+CD45−CD31−[Lin−], CD146+NG2−Lin−, or CD146+Lin− pericytes, were injected into the atrophied tibialis anterior (TA) muscle. TA muscles were excised 14 days after transplantation and remobilization. Pericyte transplantation did not significantly improve muscle mass or myofiber cross-sectional area (CSA) after 14 days of remobilization. However, injection of CD146+ pericytes significantly increased Type IIa quantity, capillarization, and collagen remodeling compared with NG2+ pericytes (P < 0.05). Our results suggest that selection of pericytes based on CD146 rather than NG2 results in the isolation of therapeutic mural cells with high capacity to positively remodel skeletal muscle after a period of immobilization.
NEW & NOTEWORTHY In this study, pericytes were isolated from mouse skeletal muscle based on cell surface marker expression of neural/glial antigen 2 (NG2) or melanoma cell adhesion molecule (Mcam/CD146) and then compared for the capacity to recover skeletal muscle after a period of immobilization in recipient mice. We report that CD146+Lin− pericytes exhibit higher capacity than NG2+Lin− pericytes to recover Type IIa fiber quantity, capillary content, and collagen turnover after disuse.
INTRODUCTION
Skeletal muscle mass comprises ∼40% of the body weight in a healthy individual and is essential for force production/movement, glucose disposal, and thermoregulation. Thus, maintenance of skeletal muscle over the lifespan is paramount to whole body health. Injury or illness can result in extended periods of bed rest or limb immobilization, which can induce marked changes in body composition due to loss of skeletal muscle and accumulation of fat mass (1, 2). Physical rehabilitation is commonly prescribed to recover skeletal muscle mass and function. However, physical therapy may not be effective in populations with limited mobility, including older adults (3–6). Alternative strategies are highly desired to improve recovery following disuse atrophy.
Pericytes are mural cells that intimately surround and support microvessels and are identified based on localization and expression of cell surface markers such as neural/glial antigen 2 (Cspg4/NG2+), melanoma cell adhesion molecule (Mcam/CD146+), and platelet-derived growth factor receptor β (PDGFRβ) (7). The canonical role of pericytes is to regulate vessel diameter, permeability, and angiogenesis, yet recent studies suggest the capacity for pericytes to regenerate muscle in response to injury and ischemia (8–12), maintain baseline skeletal muscle mass (13), and promote mechanical load-induced growth (14). The potential for pericytes to regulate muscle mass suggests that these cells may also influence loss and gain of muscle mass in the context of disuse atrophy and reload. Munroe et al. (15) recently reported a decrease in pericyte quantity in mouse skeletal muscle after 14 days of hindlimb immobilization. Replacement of pericytes via intramuscular transplantation successfully recovered the myofiber cross-sectional area (CSA) during remobilization without differentiation or fusion with existing fibers, whereas deficits were still observed in control mice 14 days later. The results from this study suggest that pericytes may represent an effective vascular mural cell-based strategy for recovery of muscle mass after disuse. However, transplantation of a mixture of NG2+ and CD146+ pericytes in the Munroe et al. study (15) did not provide any insight regarding which pericyte type provided the greatest benefit. Thus, identification of a single cell surface marker for isolation of the most therapeutic pericyte would streamline efforts to develop an effective strategy to recover muscle mass after disuse.
Minimal information exists regarding pericyte function in skeletal muscle based on cell surface marker expression (CD146 vs. NG2). We recently examined coexpression in muscle-derived pericytes under baseline conditions and reported that CD146+ pericytes represent the largest relative fraction of lineage (Lin) negative (CD45−CD31−) mononuclear cells (∼50%–60%) when compared with NG2+ pericytes (<10%) (14, 15). Interestingly, >90% of NG2+ pericytes coexpress CD146, whereas only 10%–20% of CD146+ pericytes coexpress NG2. In addition, a relatively high percentage of NG2+Lin− cells express the fibro-adipogenic cell marker PDGFRα (∼50%), whereas coexpression was limited to <15% in CD146+Lin− cells. These data suggest that NG2 is expressed in a small fraction of CD146+ pericytes in muscle, and that these cells may also possess fibro-adipogenic potential. Based on this information, we chose to investigate the therapeutic potential of CD146+ pericytes with depletion of NG2+ (denoted CD146+NG2−Lin−) and NG2+ pericytes without depletion of CD146 (due to limited yield) (denoted NG2+Lin−). Depletion of NG2+ cells from the larger CD146+ pericyte fraction may be unnecessary and cost prohibitive. Therefore, we also examined the therapeutic potential of CD146+ pericytes without depletion of NG2+ cells (CD146+NG2+/−Lin− or simply CD146+Lin−).
Here, we report that selection of CD146+ mononuclear cells from the lineage negative pool, with or without depletion of NG2+ mononuclear cells, allows for optimal retrieval of muscle-resident pericytes with capacity to positively remodel skeletal muscle after disuse.
MATERIALS AND METHODS
Animals
Adult (4-mo-old), male C57BL/6J mice were ordered from the Charles River Laboratories (Wilmington, MA). Mice were housed at the Beckman Institute for Advance Science and Technology, Biological Resources at the University of Illinois at Urbana-Champaign, Illinois. Mice were kept under a 12-h light/dark cycle (lights on 0700–1900) in a pathogen free, temperature-controlled facility and given ad libitum access to food, standard laboratory chow, and water. Mouse strain and age were selected to reproduce results from Munroe et al. (15). Protocols for animal use were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Illinois at Urbana-Champaign, and National Institutes of Health guidelines for the care and use of laboratory animals were strictly followed.
Experimental Design
Mice were subjected to 14 days of hindlimb immobilization (IM) and 14 days of remobilization (RM) as previously described (15). Mice were weighed, then anesthetized using isoflurane (∼2.5%). Buprenorphine was acquired from the University of Illinois at Urbana-Champaign (UIUC) Division of Animal Resources (DAR) and was administered subcutaneously at 0.1 mg/kg. The right mouse hindlimb was shaved, then immobilized using Covidien Royal AutoSuture 35 W skin stapler (eSutures, Mokena, IL). One tine of the staple was inserted through the plantar of the foot. The other tine was inserted through the distal gastrocnemius, restraining the foot in full dorsiflexion. This both shortened and immobilized the tibialis anterior (TA) muscle. Every 12 h for 48 h following the procedure, mice received a buprenorphine injection subcutaneously to mitigate any pain. During this time, mouse weights were recorded, and their surgical sites were assessed for bleeding or injury. Pericytes from age-matched (4 mo) donor mice were isolated using fluorescence-activated cell sorting (FACS) as described below and illustrated in Fig. 1B. Cells were cultured for 10 days to allow for recovery after FACS. Following 14 days of immobilization, mice were anesthetized with isoflurane, and the surgical staple was removed. Mice then received an intramuscular injection of either 1× PBS (30 μL) or pericytes (5 × 104 pericytes/30 μL) in the TA muscle of the immobilized leg via a 29-g needle (n = 6 per group; 4 groups) (Fig. 1A). The mobile limb was used as a baseline control and was not injected. Mice were euthanized at 14 days postremobilization via carbon dioxide asphyxiation and confirmed by cervical dislocation. The TA muscles from both legs were excised and frozen in 2-methyl-butane cooled in liquid nitrogen and stored at −80°C until analysis.
Figure 1.
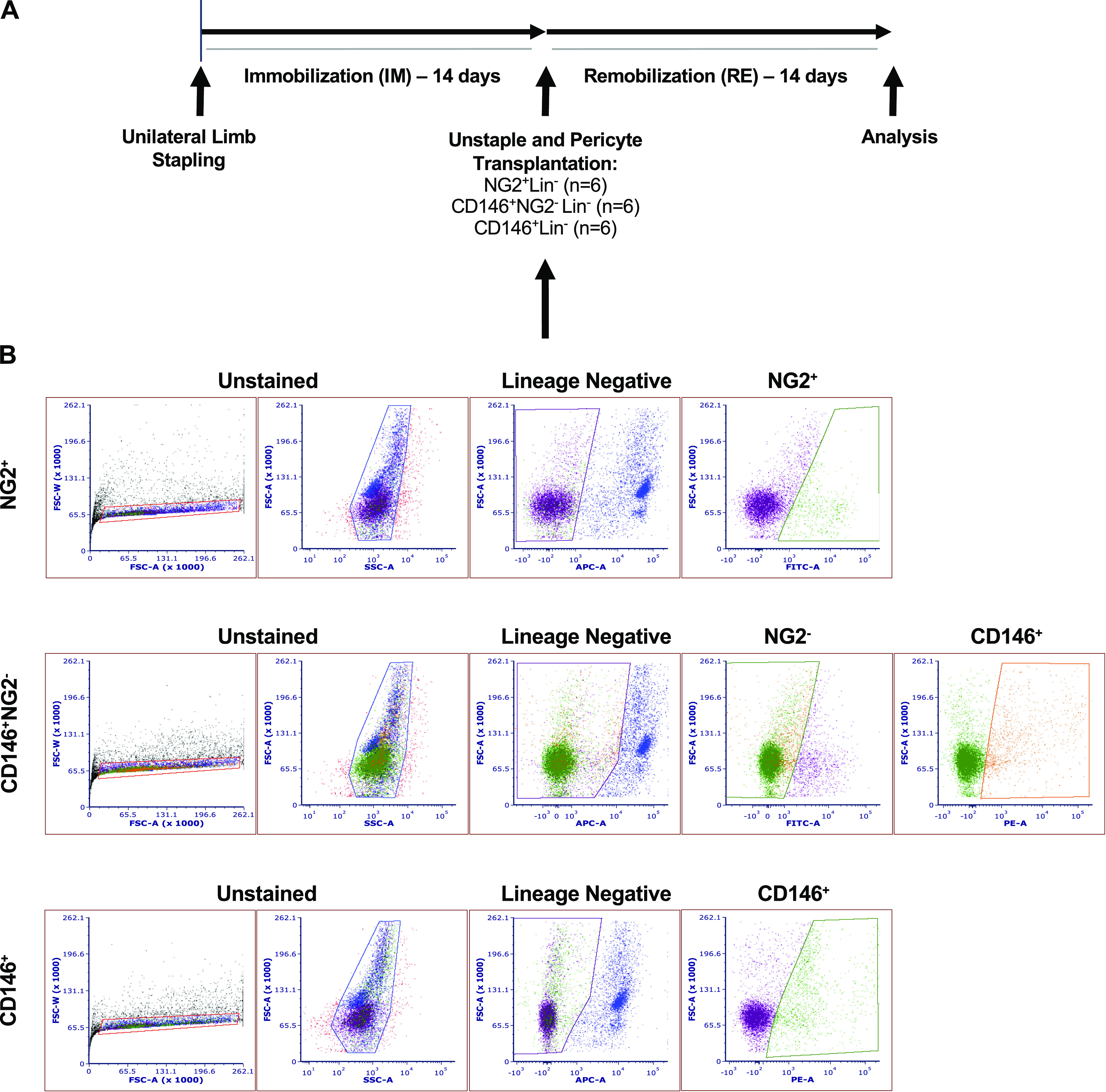
Study design and representative gating strategy for isolation of pericyte types using fluorescence-activated cell sorting (FACS). A: pericytes were isolated from age-matched, mobile, C57BL/6J male donor mice 9–10 days before transplantation. Fourteen days after unilateral hindlimb immobilization, the staple was removed and either PBS or pericytes (NG2+Lin−, CD146+NG2-Lin− or CD146+Lin−) were directly injected into the immobilized tibialis anterior (TA) muscle. The contralateral control served as the uninjected mobile control. Muscles were dissected and evaluated 14 days after remobilization, and a paired difference of zero indicates full recovery compared with the mobile control. B: the mononuclear cell population was identified based on forward scatter (FSC) and side scatter (SSC) size. After exclusion of debris and cell doublets, NG2+, CD146+NG2−, and CD146+ pericytes were gated from the lineage negative single cells (CD31−CD45−). Cells were recovered in culture for up to 10 days without splitting before transplantation. IM, immobilization; Lin, lineage; NG2, neural/glial antigen 2; PE, phycoerythrin; RE, remobilization.
Pericyte Isolation from Skeletal Muscle via Fluorescence-Activated Cell Sorting
Age-matched C57BL6/J donor mice (n = 3 per pericyte type; 3 groups) were euthanized 10 days before transplantation to obtain primary pericytes. Tibialis anterior, gastrocnemius-soleus complex, and quadriceps complex muscles were excised from both legs and washed in cold 1× PBS with penicillin-streptomycin antibiotic (P/S; Thermo Fisher Scientific, Grand Island, NY). The tissue was mechanically minced and enzymatically digested for ∼60 min at 37°C. The enzyme solution contained 250 U/mL collagenase Type 2 (Worthington-Biochemical Corp., Lakewood, NJ), 2.4 U/mL neutral protease (Dispase) (Worthington-Biochemical Corp.), 60 U/mL DNase I (Sigma-Aldrich, St. Louis, MO), and 2.5 mM CaCl2 in 1× PBS. Enzyme solution was inhibited with media containing 1× DMEM (Corning; No. 10013CV), 20% FBS, 1 mM EDTA (UltraPure 0.5 M EDTA, pH 8.0; Thermo Fisher Scientific; No. 15575-038) and P/S. The tissue solution was filtered (70 μm and 40 μm), resulting in a mononuclear cell suspension. Cells were centrifuged at 300 relative centrifugal force (RCF) for 5 min, supernatant was decanted, and resuspended in 2% FBS solution. Cells were incubated for 10 min with anti-mouse CD16/CD32 (e-bioscience; clone 93; Thermo Fisher Scientific; No. 14–0161-81) at 4°C to block Fc-mediated nonspecific binding. Cells were washed by adding 500 μL of 2% FBS, then filtered (40 μm). Aliquots were obtained for control tubes, and all other cells were incubated with a cocktail of antibodies designed for FACS (Table 1).
Table 1.
Antibodies used for isolation of pericytes via FACS
| PE-CD146 | FITC-NG2 | APC-CD31 | APC-CD45 | |
|---|---|---|---|---|
| CD146+Lin− | ||||
| Unstained | − | − | − | − |
| APC | − | − | + | + |
| PE | + | − | − | − |
| Sample | + | − | + | + |
| NG2+Lin− | ||||
| Unstained | − | − | − | − |
| APC | − | − | + | + |
| FITC | − | + | − | − |
| Sample | − | + | + | + |
| CD146+NG2−Lin− | ||||
| Unstained | − | − | − | − |
| APC | − | − | + | + |
| FITC | − | + | − | − |
| PE | + | − | − | − |
| PE FMO | − | + | + | + |
| FITC FMO | + | − | + | + |
| APC FMO | + | + | − | − |
| Sample | + | + | + | + |
APC, allophycocyanin; FACS, fluorescence-activated cell sorting; FMO, fluorescence minus one; Lin, lineage; NG2, neural/glial antigen 2; PE, phycoerythrin.
Mononuclear cells were incubated for 60 min at 4°C with combinations of the following antibodies diluted in 2% FBS in 1× PBS: conjugated anti-mouse CD146 (melanoma cell adhesion molecule)-phycoerythrin (PE) (Clone ME-9F1, 1:50; BioLegend, San Diego, CA; No. 134704), NG2 (chondroitin sulfate proteoglycan-4)-Alexa Fluor 488 (1:50; Millipore-Sigma, Burlington, MA; AB5320A4), CD31 (PECAM-1)-allophycocyanin (APC; Clone MEC 13.3, 1:100; BD Biosciences; No. 561814), and CD45-APC (Clone 30-F11, 1:100; BD Biosciences; No. 561018) (Table 1). Following antibody incubation, cells were washed with 500 μL of 2% FBS, centrifuged for 5 min at 300 RCF and 4°C, and their supernatant was discarded. Cell pellet was resuspended in 2% FBS in 1× PBS, filtered through a 40-μm filter, and placed on ice.
CD146+Lin−, NG2+Lin−, and CD146+NG2−Lin− skeletal muscle pericytes were sorted using a BD FACS Aria II sorter by the Flow Cytometry Center at the UIUC Roy J. Carver Biotechnology Center (Urbana, IL) (Fig. 1B). Lineage negative (Lin−) is defined here as CD31−CD45− cells. Unstained and single-stained control samples were analyzed to account for potential spectral overlap between fluorophores and compensate accordingly. Fluorescence minus one (FMO) controls were used to establish proper gating parameters. Isolated cells were collected in pericyte growth media (10% FBS, 1% P/S in DMEM). Cells were centrifuged at 300 RCF for 5 min, supernatant removed, and cells resuspended in pericyte growth media to remove any sheath fluid. Cells were cultured in 100-mm dishes and cell media was changed every 3–4 days before transplantation.
Immunofluorescence
Frozen tissues were transferred from −80°C to the cryostat (Leica CM3050S, Wetzlar, Germany). Tissue was then cut transversely at the mid-belly. Half of the tissue was embedded in optimum cutting temperature (OCT; Tissue-Tek; Thermo Fisher Scientific), and the other was returned to −80°C. Sections were collected at 10 μm thickness onto Superfrost microscope slides (Thermo Fisher Scientific) and stored at −80°C until staining.
Slides were removed from −80°C and thawed to room temperature. Sections were fixed in −20°C acetone for 5 min and washed with three times with 1× PBS. Sections were blocked for 1 h at room temperature with AffiniPure Fab Fragment goat anti-mouse IgG (H + L) (55 μg/mL; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; No. 115-007-003) in 5% bovine serum albumin (BSA) with 0.05% Tween-20 (Fisher Bioreagents; No. BP337-500) in 1× PBS.
To assess total myofiber CSA (CSA) and muscle capillarization, sections were incubated with rat anti-mouse CD31 (clone 390) (1:100; Thermo Fisher Scientific; No. 14–0311-85) and rabbit anti-mouse dystrophin (1:100; Abcam, Cambridge, MA; ab15277) in Wash Buffer (1% BSA with 0.05% Tween-20 in 1× PBS) simultaneously for 1 h at room temperature. Alexa Fluor 488 goat anti-rabbit IgG (1:100; Thermo Fisher Scientific; No. A-11034) and Alexa Fluor 633 goat anti-rat IgG (1:100) (Thermo Fisher, No. A-21094) secondary antibodies were applied simultaneously for 1 h at room temperature to visualize dystrophin and CD31. Finally, sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 3 min at room temperature (1:20,000; Sigma-Aldrich, St. Louis, MO) to identify nuclei. Slides were mounted with VECTASHIELD mounting media (Vector Laboratories, Burlingame, CA; No. H-1000) and stored at 4°C.
To assess collagen remodeling, slides were prepared as described previously, but without Fab fragment during the 1-h block. Sections were washed in 1× PBS. Then, 5 μM CHP solution [collagen hybridizing peptide, 5-carboxyfluorescein (5-FAM) conjugate (F-CHP); 3Helix, Salt Lake City, UT; No. FLU300] was prepared in 1× PBS and warmed for 5 min at 80°C. Solution was immediately placed on ice for 1 min to cool, then added to sections. Slides were incubated overnight at 4°C. Slides were washed with 1× PBS, DAPI was added, and slides were sealed with VECTASHIELD.
For assessment of myosin heavy chain (MHC) myofiber type, sections were initially prepared as previously described, then blocked with AffiniPure Fab Fragment goat anti-mouse IgG (H + L) (55 μg/mL; Jackson ImmunoResearch Laboratories) in 0.5% bovine serum albumin (BSA) with 0.5% Triton X-100 in 1× PBS. Sections were washed in 1× PBS and incubated with either mouse IgG1 anti-Type IIa MHC (Sc-71) (1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mouse IgM anti-Type IIb MHC (BF-F3) (1:50; Hybridoma), or mouse IgM anti-Type IIx MHC (6H1) (1:50; Hybridoma) in 0.5% bovine serum albumin (BSA) with 0.5% Triton X-100. Sections were costained with rabbit anti-mouse dystrophin (1:100, Abcam; ab15277). Alexa Fluor 633 goat anti-rabbit (1:100; Thermo Fisher Scientific; No. A-21070) and either Alexa Fluor 488 conjugated anti-mouse IgG subclass 1 (1:100; Jackson Immunoresearch; No. 115–545-205) or Alexa 488 anti-mouse IgM μ-chain specific (1:100; Jackson Immunoresearch; No. 115–545-075) secondary antibodies were applied in 0.5% bovine serum albumin (BSA) with 0.5% Triton X-100 to visualize dystrophin and Type IIa or Type 2X and 2B fibers, respectively. Secondary antibodies were applied for 1 h at room temperature. Sections were mounted using VECTASHIELD HardSet with DAPI (1.5 μg/mL; Vector Laboratories; No. H-1500) and stored at 4°C.
Imaging and Analysis
Tissue sections prepared for myofiber CSA, fiber type-specific CSA, capillary density, capillary-to-fiber ratio, and CHP analysis were visualized using an inverted fluorescent microscope. Images were acquired at ×10 magnification for global and fiber type-specific CSA analysis and ×20 magnification for capillary and CHP analysis. Images were captured with a Zeiss Axiocam digital camera and Axiovision software (Zeiss, Thornwood, NY). Images were acquired in separate color channels to allow for independent analysis of images.
To assess total myofiber CSA, entire sections were analyzed using ×10 dystrophin-Alexa Fluor 488 images. Semiautomatic muscle analysis was completed using segmentation of histology (SMASH). Images were uploaded to SMASH, and fibers were automatically outlined on the green channel (dystrophin-Alexa Fluor 488). Any incomplete fiber outlines were manually edited to ensure their inclusion. Improperly selected fibers (areas of interstitium) were manually excluded. Fibers above 0.98 eccentricity or below 0.65 convexity were excluded from the analysis for illegitimate fiber morphology. Fibers less than 250 μm2 were also excluded.
Muscle capillarization was determined using five, random ×20 images per sample as described previously (16). Measures of capillary-to-fiber ratio (number of capillaries per total number of myofibers) and capillary density (number of capillaries per muscle fiber area) were determined for each image by quantifying the total number of transversely cut capillaries, the number of myofibers, and the total area (mm2) of the imaged muscle section.
Collagen remodeling was quantified with a threshold intensity program from ImageJ (National Institutes of Health). RGB channels were separated and the green channel was thresholded to remove background to determine the percent of degraded collagen observed within each imaged muscle section. Four random ×10 images were analyzed per sample.
Fiber type-specific CSA was assessed using three ×10 images per section. The average number of fibers analyzed per section per fiber type is as follows: IIa, ∼170; IIx, ∼360; IIb, ∼300. Images were uploaded to SMASH and fiber CSA was assessed as described for total myofiber CSA. SMASH outlined all fibers on the red channel (dystrophin-Alexa Fluor 633), then all fibers not expressing Alexa Fluor 488 were excluded. This was repeated for each fiber type.
For all assessments, samples were coded and investigators were blinded to sample information until after data collection was complete.
Western Blotting
The proximal half of TA muscles were placed directly in 400 µL of ice-cold lysis buffer containing HEPES (20 mM), Triton X-100 (1%), glycerol (10%), and protease inhibitor (Complete Mini; Roche) and phosphatase inhibitor (PhosSTOP; Roche) tablets. The tissue was homogenized using a handheld homogenizer and the homogenates were rotated at 4°C for 1 h and centrifuged at 14,000 g for 5 min at 4°C. Supernatants were collected and the concentration of protein was determined with the Bradford assay (Bio-Rad) using bovine serum albumin (BSA) for the standard curve. Equal amounts of protein (30 μg) were subjected to SDS-PAGE using 8% or 10% acrylamide gels and transferred to nitrocellulose membranes. Equal protein loading was verified by Ponceau S staining. Membranes were blocked in Tris-buffered saline (pH 7.8) with 0.1% Tween 20 (TBS-T) containing 5% BSA for 1 h at room temperature, then incubated overnight with primary antibodies of phospho-AktSer473 (1:1,000, Cell Signaling No. 9271), Akt (1:1,000, Cell Signaling No. 9272), phospho-Foxo3aThr32 (1:1,000, Cell Signaling No. 9464), Foxo3a (1:1,000, Cell Signaling No. 2497), Atrogin-1 (1:1,000, ECM Biosciences No. AP2041), or Ubiquitin (1:1000, Cell Signaling No. 3933) suspended in 2% BSA in TBS-T. Horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA; Cell Signaling, Danvers, MA) were applied for 1 h at 1:2,000 in TBS-T containing 2.5% nonfat dry milk (NFDM). Bands were detected using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific) and a Bio-Rad ChemiDoc Imaging System (Bio-Rad, Hercules, CA). Quantification was completed using Quantity One software (Bio-Rad). All target bands were normalized to all bands detected by Ponceau S. For phospho/total measures, the phospho and total were run on separate membranes and each normalized to Ponceau S before calculating the ratio.
Statistical Analysis
All data are presented as means ± standard error. Two-way ANOVA was completed to determine immobilization-remobilization (IM + RE) × pericyte treatment (PBS, NG2+Lin−, CD146+NG2−Lin−, CD146+Lin−) interaction and main effects. One-way ANOVA was used to examine the paired differences between IM + RE and mobile legs (IM + RE-Mobile). Games–Howell and Tukey’s post hoc analyses were completed when appropriate. Statistical analyses were completed using SPSS v. 18 (IBM, Chicago, IL). Differences were considered statistically significant at P ≤ 0.05.
RESULTS
Impact of Pericyte Transplantation on Recovery of Muscle Mass and Myofiber CSA
No differences in body weight were detected between treatment groups at the end of the study (data not shown). Skeletal muscle wet weight decreased as a result of immobilization and remobilization (IM + RE) (main effect, P = 0.001) and pericyte treatment (main effect, P < 0.001) (Fig. 2A, top). Paired differences between the mobile control and IM + RE limbs revealed no changes in wet weight according to pericyte treatment (Fig. 2A, bottom).
Figure 2.
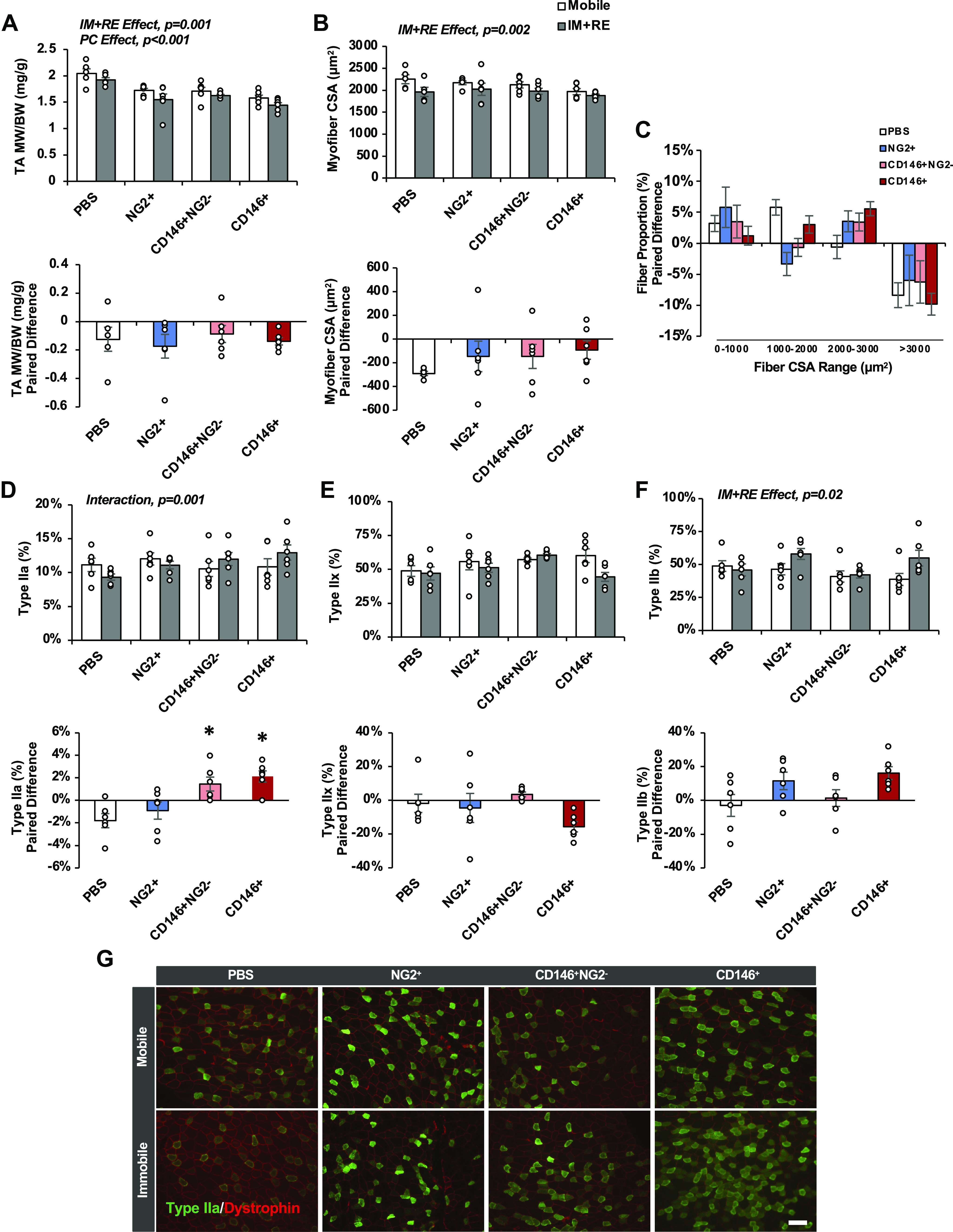
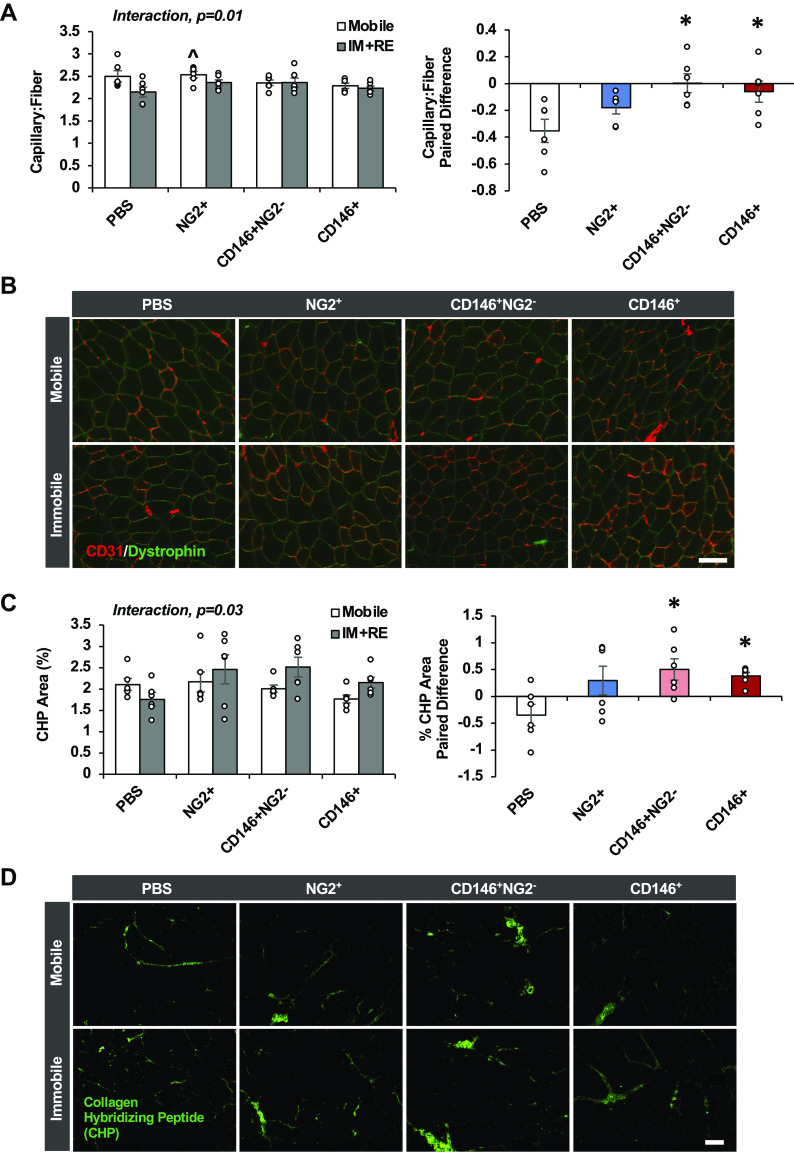
Impact of pericyte transplantation on recovery of muscle mass, fiber size, and fiber type after 14 days of immobilization and 14 days of remobilization. Tibialis anterior (TA, A) muscle weight normalized to body weight, myofiber cross-sectional area (CSA, B), fiber size distribution (C), Type IIa relative quantity (D), Type IIx relative quantity (E), and Type IIb relative quantity (F). G: representative images of Type IIa fibers for each treatment group. Scale bar 100 μm. n = 5 or 6 male mice. All values expressed as means ± SE. Post hoc analyses were performed after interaction or significant one-way ANOVA was detected. *P < 0.05 vs. PBS. I + R, immobilization and remobilization; NG2, neural/glial antigen 2; PC, pericyte treatment.
Muscle fiber CSA decreased as a result of IM + RE (main effect, P = 0.002) (Fig. 2B, top). Paired differences between the mobile control and IM + RE limbs revealed no significant changes in muscle fiber CSA according to pericyte treatment (P > 0.05; PBS: −241.19 ± 50.62 μm2, NG2+: −146.37 ± 129.07 μm2; CD146+NG2−: −146.49 ± 101.52 μm2, CD146+: −92.25 ± 78.47 μm2) (Fig. 2B, bottom). Upon examination of the myofiber size distribution, the quantity of myofibers ranging from 2,000–3,000 μm2 appeared higher in all groups receiving pericyte transplantation compared with saline, with CD146+Lin− pericyte treatment approaching significance compared with PBS control (one-way ANOVA, P = 0.07) (Fig. 2C). Muscle fiber type-specific CSA was decreased as a result of IM + RE for Type IIx and IIb fibers (Type IIx: main effect, P = 0.001; Type IIb: main effect, P = 0.001), and paired differences between limbs revealed no significant changes according to pericyte treatment (data not shown). Unexpectedly, CD146+ pericyte treatment, with or without exclusion of NG2, significantly altered the relative quantity of oxidative Type IIa fibers during recovery (interaction, P = 0.001) (Fig. 2D, top). Paired differences between limbs revealed significant increases compared with NG2+ pericyte treatment and PBS (one-way ANOVA, P = 0.001) (Fig. 2D, bottom). No significant interactions were observed in the relative quantities of Type IIx or IIb fibers (Fig. 2, E and F). However, a trend for an increase was noted for Type IIb fibers (interaction, P = 0.06; I + R main effect, P = 0.02), and a trend for a pericyte main effect was noted for Type IIx fibers (P = 0.08).
CD146+ Pericyte Transplantation Recovers Capillary Quantity in Skeletal Muscle after Disuse
The capillary-to-fiber ratio (C:F) was altered as a result of IM + RE and pericyte treatment (interaction, P = 0.01) (Fig. 3, A and B). Paired differences between limbs revealed significant improvements in C:F for CD146+NG2− and CD146+ injected mice compared with NG2+ pericyte treatment and PBS (one-way ANOVA, P = 0.01). Capillary density was not altered as a result of IM + RE and pericyte treatment (interaction, P = 0.16).
Figure 3.
CD146+NG2− and CD146+ pericyte transplantation effectively recovers capillary content and collagen remodeling after 14 days of immobilization and 14 days of remobilization. Capillary-to-fiber ratio (C:F, A), representative images of C:F for all treatment groups (B), degraded collagen based on collagen hybridizing peptide (CHP) area (C), and representative CHP images for all treatment groups (D).Scale bar 100 μm. n = 6 male mice. All values expressed as means ± SE. Post hoc analyses were performed after interaction or significant one-way ANOVA was detected. ^P < 0.05 vs. PBS IM + RE, *P < 0.05 vs. PBS. I + R, immobilization and remobilization; NG2, neural/glial antigen 2; PC, pericyte treatment.
CD146+ Pericyte Transplantation Enhances Remodeling of the Extracellular Matrix after Disuse
CHP content, which reflects collagen degradation and turnover, was altered as a result of IM + RE and pericyte treatment (interaction, P = 0.03) (Fig. 3, C and D). Paired differences between limbs revealed significant improvements for CD146+NG2− and CD146+ injected mice compared with NG2+ pericyte treatment and PBS (one-way ANOVA, P = 0.05).
Pericyte Transplantation Significantly Increases Akt Phosphorylation after Disuse
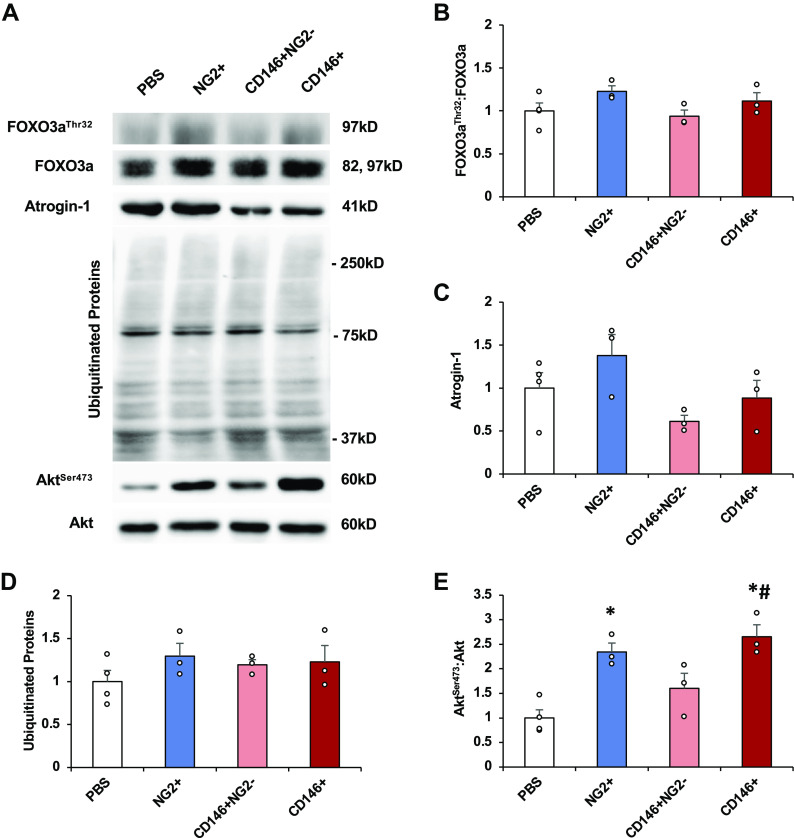
Mechanical unloading can result in decreased Akt phosphorylation, as well as decreased phosphorylation of the downstream substrate forkhead box O3a (FOXO3a) on threonine 32, ultimately allowing translocation of FOXO3a to the nucleus to induce atrogene gene expression (17, 18). In addition, ubiquitination levels can increase during disuse and can remain elevated in aged rats during reload, suggesting prolonged muscle degradation during recovery (19). No differences were observed between groups for FOXO3aThr32:FOXO3a (one-way ANOVA, P = 0.15) (Fig. 4, A and B), atrogin-1 (one-way ANOVA, P = 0.69) (Fig. 4, A and C), or ubiquitinated proteins (one-way ANOVA, P = 0.30) (Fig. 4, A and D) at this late time point. However, differences were observed between groups for AktSer473:Akt (one-way ANOVA, P = 0.002). NG2+ and CD146+ pericyte transplantation significantly increased AktSer473:Akt compared with PBS treatment 14 days after remobilization (Fig. 4, A and E).
Figure 4.
CD146+ pericyte transplantation significantly increases Akt phosphorylation. TA muscles obtained 14 days after transplantation and remobilization were homogenized in lysis buffer and examined for measures of protein synthesis and degradation. Representative immunoblots (A). FOXO3aThr32:FOXO3a (B), atrogin-1 total protein (C); ubiquitinated proteins (D), and AktSer473:Akt (E). All results were normalized to Ponceau S. n = 3 or 4 male mice. All values expressed as means ± SE. Post hoc analyses were performed after significant one-way ANOVA was detected. *P < 0.05 vs. PBS, #P < 0.05 vs. CD146+NG2−. FOXO3a, forkhead box O3a; NG2, neural/glial antigen 2; TA, tibialis anterior.
DISCUSSION
The purpose of this study was to compare the capacity for CD146+Lin− and NG2+Lin− pericytes to enhance recovery of skeletal muscle mass after a period of hindlimb immobilization. CD146+ pericytes, with or without exclusion of NG2 expression, did not significantly increase muscle mass or fiber size, but significantly improved several early measures of muscle recovery (Type IIa fiber quantity, capillarization, and collagen degradation) compared with NG2+ pericyte treatment and PBS controls. Overall, these data suggest that CD146+Lin− pericytes demonstrate higher potential to recover muscle mass after disuse compared with NG2+Lin− pericytes.
CD146+ and NG2+ pericytes consistently improve muscle regeneration in mouse models of injury, ischemia, and disease (8–12, 20, 21). NG2+ pericytes also appear to be essential for maintenance of the baseline muscle mass (13). Despite these intriguing results, studies have not directly compared the capacity for pericytes to regulate skeletal muscle mass and function based on the primary cell surface marker used for isolation—either NG2 or CD146. We recently reported that CD146+ pericytes comprise the majority (>50%) of perivascular stem/stromal cells relative to NG2+ pericytes (<10%) in mouse skeletal muscle after elimination of the lineage negative fraction (CD45−CD31−) (14, 15). In addition, CD146+ and NG2+ pericytes differ in their ability to express PDGFRα, which suggests that divergent functional properties may exist. Properly addressing the cell surface marker that yields sufficient pericyte quantity with high capacity to regulate muscle mass is essential to design cell-based strategies to effectively rehabilitate muscle after injury, disease, and disuse.
NG2 is an integral membrane proteoglycan that is expressed by several different types of immature proliferating cells, particularly multipotential glial precursor cells (O2A progenitors) (22, 23) and mesenchymal stem/stromal cells (MSCs) (24). Interestingly, NG2 identifies rapidly dividing bone marrow-derived (BM)-MSCs with high colony-forming efficiency (24). On the other hand, high expression of the cell surface glycoprotein CD146 on BM-MSCs does not appear to impact proliferation, but enhances secretory capacity, including the release of immunomodulatory factors that promote M1-to-M2 macrophage polarization and factors that reduce fibrosis (24, 25). These findings are consistent with our recent study results demonstrating that muscle resident CD146+ pericytes possess higher secretory capacity in response to exercise and promote muscle growth to a greater extent following mechanical strain than NG2+ pericytes (14). Thus, NG2+ pericytes may represent immature proliferating cells that may replenish glial cells in the perivascular niche, whereas CD146+ pericytes predominantly secrete factors necessary to recover muscle mass immediately after disuse.
In our first study published in 2019 (15), lack of information regarding pericyte functionality based on cell surface marker expression prompted us to inject a mixture (50/50) of CD146+ and NG2+ pericytes to evaluate recovery following disuse (15). The pericyte mixture successfully improved all measures evaluated, yet the relative contribution of each cell type to enhanced myofiber size, capillarization, and collagen remodeling remained unknown. In the current study, three pericyte fractions (NG2+CD45−CD31−[Lin−], CD146+NG2−Lin−, or CD146+Lin−) were separately injected and evaluated to address this gap in knowledge. Consistent with our prior observation using this model, deficits in TA mass and fiber size remained present between the immobilized and mobile legs with PBS treatment after remobilization, whereas the deficit was less apparent with the injection of CD146+ pericytes and unaltered with NG2+ pericytes. Comparison of the paired differences between treatments, unfortunately, did not reach statistical significance by one-way ANOVA. It will be important to complete a follow-up study to examine the impact of CD146+ pericytes on recovery using a larger sample size. Despite our enthusiasm regarding the potential for pericytes to decrease the deficit in myofiber size between treated and untreated legs, a pericyte treatment main effect was observed, suggesting the ability for pericytes to reduce fiber size in both limbs. A similar effect was observed for relative muscle mass. Mice were randomly placed in groups at the beginning of the study without regard for body mass size. Therefore, in the absence of a total sham control group, it is difficult to ascertain whether baseline differences existed or whether pericytes negatively impacted the contralateral control legs. The average fiber size of 1,750 μm2 in the mobile legs of the CD146+ pericyte-treated group aligns with our previously reported data for the TA muscle without treatment (14). In addition, the combination of improvements in Type IIa fiber quantity, capillary content, and increased collagen turnover observed only in the treated leg suggest that it is unlikely that pericyte treatment negatively impacted the injected or contralateral TA muscles. Follow-up studies using sham controls will be required to address any concerns regarding a contralateral effect.
Loss of Type IIa fibers, decreased capillary content, and accumulation of collagen are commonly observed with immobilization and microgravity (15, 26–29). In the current study, injection of CD146+ pericytes, with or without exclusion of NG2, reversed all these changes compared with PBS and NG2+ pericytes. Although one study suggested that pericytes can directly differentiate into endothelial cells in response to ischemia (30), others have reported maintenance of cellular identity in diverse pathological settings (31). The nonspecific nature of CD146 expression makes it difficult to conduct lineage tracing experiments to determine CD146+ pericyte fate. Regardless, CD146+ pericytes are highly secretory in response to contraction compared with NG2+ pericytes, releasing factors such as proangiogenic factors and matrix metalloproteinases (14, 32). Thus, it is likely that these perivascular cells serve the purpose of continuously secreting factors that remodel the microenvironment in a manner that supports myofiber structure and function.
Skeletal muscle disuse not only inhibits anabolic signaling but can stimulate catabolic processes that result in myofibrillar degradation, resulting in dramatic decreases in muscle fiber size. The initiation of muscle contraction upon reload may similarly induce catabolism, which may provide the basis for delayed recovery after disuse (33). In the current study, our time point for evaluation of cellular signaling was very late (14 days postremobilization), and not surprisingly, we did not observe any significant changes in FOXO3a phosphorylation, atrogin-1, or ubiquitinated proteins with pericyte transplantation. Additional experiments at earlier time points are necessary to determine if CD146+ pericytes can effectively target catabolic pathways in the first few days following remobilization. NG2+ and CD146+ pericyte treatment significantly enhanced AktSer473. The importance of Akt phosphorylation to recovery is not clear given prior studies that demonstrate significant elevations in Akt activation and protein synthesis early during the reload period despite lack of recovery (19, 34, 35). Further mechanistic studies are essential for understanding the basis for pericyte-mediated myofiber recovery post-disuse, and these studies may can now be pursued with greater confidence using CD146 as a marker for identification and isolation.
Conclusions
In conclusion, this study is the first to compare pericyte functionality (NG2+ vs. CD146+) on recovery of skeletal muscle following disuse. Whereas NG2+ pericytes did not exhibit any beneficial effects, CD146+ pericytes, with or without elimination of NG2 expression, significantly recovered Type IIa content, capillarization, and collagen remodeling. These results are consistent with our prior results suggesting that CD146+ pericytes possess high capacity to secrete immunoregulatory, proangiogenic and antifibrotic factors in response to contraction (14). Overall, this information is essential to streamline efforts to design an effective strategy to improve muscle mass recovery in humans.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grant NIAMS R01 AR072735A (to M.D.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.F.W., S.L., S.D., and M.D.B. conceived and designed research; Y.F.W., S.L., S.D., G.G., M.K., A.T., and L.D. performed experiments; Y.F.W., S.L., S.D., and M.D.B. analyzed data; Y.F.W., S.L., S.D., and M.D.B. interpreted results of experiments; Y.F.W., S.L., and M.D.B. prepared figures; Y.F.W., S.L., and M.D.B. drafted manuscript; Y.F.W., S.L., S.D., and M.D.B. edited and revised manuscript; Y.F.W., S.L., S.D., G.G., M.K., A.T., L.D., and M.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Madeleine Meehan and Madeleine Subach for assistance with data analysis.
REFERENCES
- 1.Dirks ML, Wall BT, Van De Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, Van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65: 2862–2875, 2016. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 2.Dirks ML, Wall BT, van Loon LJ. Interventional strategies to combat muscle disuse atrophy in humans: focus on neuromuscular electrical stimulation and dietary protein. J Appl Physiol (1985) 125: 850–861, 2018. doi: 10.1152/japplphysiol.00985.2016. [DOI] [PubMed] [Google Scholar]
- 3.Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ørtenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol (1985) 109: 1628–1634, 2010. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- 4.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 5.Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591: 3789–3804, 2013. doi: 10.1113/jphysiol.2013.257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pišot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Reggiani C, Toniolo L, di Prampero PE, Passaro A, Narici M, Mohammed S, Rittweger J, Gasparini M, Gabrijelcˇicˇ BM, Šimunič B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2wk of bed rest and recovery. J Appl Physiol (1985) 120: 922–929, 2016. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 7.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Birbrair A, Zhang T, Wang Z-M, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22: 2298–2314, 2013. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birbrair A, Zhang T, Wang Z-M, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericytes subtypes differ in their differentiation potential. Stem Cell Res 10: 67–84, 2013. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, Saggio I, Robey PG, Riminucci M, Bianco P. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep 6: 897–913, 2016. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persichini T, Funari A, Colasanti M, Sacchetti B. Clonogenic, myogenic progenitors expressing MCAM/CD146 are incorporated as adventitial reticular cells in the microvascular compartment of human post-natal skeletal muscle. PLoS One 12: e0188844, 2017. doi: 10.1371/journal.pone.0188844. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chen T, Ye B, Tan J, Yang H, He F, Khalil RA. CD146+ mesenchymal stem cells treatment improves vascularization, muscle contraction and VEGF expression, and reduces apoptosis in rat ischemic hind limb. Biochem Pharmacol 190: 114530, 2021. doi: 10.1016/j.bcp.2021.114530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development 142: 1242–1253, 2015. doi: 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- 14.Dvoretskiy S, Garg K, Munroe M, Pincu Y, Mahmassani ZS, Coombs C, Blackwell B, Garcia G, Waterstradt G, Lee I, Drnevich J, Rhodes JS, Boppart MD. The impact of skeletal muscle contraction on CD146+Lin− pericytes. Am J Physiol Cell Physiol 317: C1011–C1024, 2019. doi: 10.1152/ajpcell.00156.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munroe M, Dvoretskiy S, Lopez A, Leong J, Dyle MC, Kong H, Adams CM, Boppart MD. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J 33: 7694–7709, 2019. doi: 10.1096/fj.201802580R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huntsman HD, Zachwieja N, Zou K, Ripchik P, Valero MC, De Lisio M, Boppart MD. Mesenchymal stem cells contribute to vascular growth in skeletal muscle in response to eccentric exercise. Am J Physiol Heart Circ Physiol 304: H72–H81, 2013. doi: 10.1152/ajpheart.00541.2012. [DOI] [PubMed] [Google Scholar]
- 17.Talbert EE, Smuder AJ, Min K, Kwon OS, Szeto HH, Powers SK. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J Appl Physiol (1985) 115: 529–538, 2013. doi: 10.1152/japplphysiol.00471.2013. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski JM, Hord JM, Lee Y, Guzzoni V, Rodriguez D, Lawler MS, Garcia-Villatoro EL, Holly D, Ryan P, Falcon K, Garcia M, Gomes MJ, Fluckey JD, Lawler JM. Effect of Eukarion-134 on Akt-mTOR signalling in the rat soleus during 7 days of mechanical unloading. Exp Physiol 103: 545–558, 2018. doi: 10.1113/EP086649. [DOI] [PubMed] [Google Scholar]
- 19.Baehr LM, West DWD, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P-N, Traas J, Schugar R, Deasy BM, Badylak S, Buhring H-J, Giacobino J-P, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Dahlin KJ, Stallcup WB. The expression of NG2 proteoglycan in the developing rat limb. Development 111: 933–944, 1991. doi: 10.1242/dev.111.4.933. [DOI] [PubMed] [Google Scholar]
- 23.Levine JM, Nishiyama A. The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells. Perspect Dev Neurobiol 3: 245–259, 1996. [PubMed] [Google Scholar]
- 24.Russell KC, Tucker HA, Bunnell BA, Andreeff M, Schober W, Gaynor AS, Strickler KL, Lin S, Lacey MR, O'Connor KC. Cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in heterogeneous cultures of marrow-derived mesenchymal stem cells. Tissue Eng Part A 19: 2253–2266, 2013. doi: 10.1089/ten.TEA.2012.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowles AC, Kouroupis D, Willman MA, Perucca Orfei C, Agarwal A, Correa D. Signature quality attributes of CD146+ mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. Stem Cells 38: 1034–1049, 2020. doi: 10.1002/stem.3196. [DOI] [PubMed] [Google Scholar]
- 26.Latroche C, Gitiaux C, Chrétien F, Desguerre I, Mounier R, Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology (Bethesda) 30: 417–427, 2015. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- 27.Tyml K, Mathieu-Costello O. Structural and functional changes in the microvasculature of disused skeletal muscle. Front Biosci 6: D45–D52, 2001. doi: 10.2741/tyml. [DOI] [PubMed] [Google Scholar]
- 28.Kaneguchi A, Ozawa J, Kawamata S, Kurose T, Yamaoka K. Intermittent whole-body vibration attenuates a reduction in the number of the capillaries in unloaded rat skeletal muscle. BMC Musculoskelet Disord 15: 315, 2014. doi: 10.1186/1471-2474-15-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thijssen DHJ, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875, 2010. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes KL, Messina LM, Schwartz LM, Yan J, Burnside AS, Witkowski S. Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice. Am J Physiol Cell Physiol 314: C534–C544, 2018. doi: 10.1152/ajpcell.00158.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans S. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20: 345–359, 2017. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng Y-C, Porfirio-Sousa AL, Ribeiro GM, Arend MC, da Silva Meirelles L, Chen ES, Rosa DS, Han SW. Analyses of the pericyte transcriptome in ischemic skeletal muscles. Stem Cell Res Ther 12: 183, 2021. doi: 10.1186/s13287-021-02247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang C, Goodman CA, Hornberger TA, Ji LL. PGC-1a overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J 29: 4092–4106, 2015. doi: 10.1096/fj.14-266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol 64: 17–32, 2015. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberson PA, Shimkus KL, Welles JE, Xu D, Whitsell AL, Kimball EM, Jefferson LS, Kimball SR. A time course for markers of protein synthesis and degradation with hindlimb unloading and the accompanying anabolic resistance to refeeding. J Appl Physiol (1985) 129: 36–46, 2020. doi: 10.1152/japplphysiol.00155.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.