Abstract
Oocyte maturation disorder and decreased quality are the main causes of infertility in women, and granulosa cells (GCs) provide the only microenvironment for oocyte maturation through autocrine and paracrine signaling by steroid hormones and growth factors. However, chronic inflammation and oxidative stress caused by ovarian hypoxia are the largest contributors to ovarian aging and GC dysfunction. Therefore, the amelioration of chronic inflammation and oxidative stress is expected to be a pivotal method to improve GC function and oocyte quality. In this study, we detected the protective effect of chitosan oligosaccharides (COS), on hydrogen peroxide- (H2O2-) stimulated oxidative damage in a human ovarian granulosa cell line (KGN). COS significantly increased cell viability, mitochondrial function, and the cellular glutathione (GSH) content and reduced apoptosis, reactive oxygen species (ROS) content, and the levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), 4-hydroxynonenal (4-HNE), hypoxia-inducible factor-1α (HIF-1α), and vascular endothelial-derived growth factor (VEGF) in H2O2-stimulated KGN cells. COS treatment significantly increased levels of the TGF-β1 and IL-10 proteins and decreased levels of the IL-6 protein. Compared with H2O2-stimulated KGN cells, COS significantly increased the levels of E2 and P4 and decreased SA-β-gal protein expression. Furthermore, COS caused significant inactivation of the HIF-1α-VEGF pathway in H2O2-stimulated KGN cells. Moreover, inhibition of this pathway enhanced the inhibitory effects of COS on H2O2-stimulated oxidative injury and apoptosis in GCs. Thus, COS protected GCs from H2O2-stimulated oxidative damage and apoptosis by inactivating the HIF-1α-VEGF signaling pathway. In the future, COS might represent a therapeutic approach for ameliorating disrupted follicle development.
1. Introduction
Due to environmental pollution, life pressure, radiation and chemotherapy administered to patients with cancer, and other factors that damage female ovarian function and shorten human life, infertility, premature ovarian failure (POF), and aging have become major medical problems and social problems threatening human health. The speed of ovarian aging is significantly faster than that of other organs of the body. Some scholars have considered ovarian aging as pacemakers of female body aging. Ovarian aging is the initial factor triggering cardiovascular and cerebrovascular diseases, neurodegenerative diseases, and other chronic diseases [1]. Unfortunately, few methods are available to protect the ovary and delay ovarian aging in healthy aged women. In pathological conditions, treatments include administration of gonadotrophin-releasing hormone (GnRH) analogs, transposition of the ovaries outside of the fields of radiation in cancer patients, and cryopreservation of the ovarian cortical tissue. All these methods are either less effective or are still being investigated [2]. The fundamental reason is the mechanism of ovarian aging. Chemicals that potentially protect the oocyte and its feeder cells from injury would be highly useful.
Ovogenesis has a close relationship with the follicle growth and its maturity. Primary oocytes are originated from primordial germ cells [3, 4]. Primary oocytes interact with the surrounding monolayer of follicular cells and gradually developed into primitive follicles. The primitive follicle terminates the dormancy in certain condition; next, it enters the growth state [5]. During the normal follicle development process, oocytes gradually develop and mature, while granulosa cells (GCs) surrounding oocytes also proliferate and differentiate continuously [4]. Nevertheless, more than 99% of mammalian follicles evolve into atresia state in physiological phenomenon, they degenerate in a period of growth and development, and only a few follicles have the ability to accomplish the development process [6–8]. Follicular atresia is attributed to the programmed cell death of ovarian GCs. GCs communicate with and form a protective barrier between the oocyte and follicle microenvironment by transmitting nutrients, energy, and signals to oocytes and play roles in the androgen to estrogen conversion and progesterone synthesis; thus, a decrease in GC quantity and function leads to decreased production of estradiol (E2) and progesterone (P4) and may trigger follicular atresia [9, 10]. The morphology and quantity of GCs have been used as biomarkers of developmental ability and pregnancy outcomes [11]. Therefore, the disorder of GCs caused by various factors is the key cause of disrupted oocyte maturation.
The mechanisms underlying decreased GC function and subsequent ovarian aging are not wholly clear. Previous studies have shown that chronic inflammation and oxidative stress (OS) caused by ovarian hypoxia are the main causes of ovarian aging and GC dysfunction [12, 13]. Hypoxia, via a hypoxia-inducible factor-1α- (HIF-1α-) dependent manner, promotes GC expression of more than 70 downstream genes (including nuclear factor kappa-B (NF-κB), nitric oxide synthase (NOS), and VEGF), resulting in the overproduction of IL-6 and ROS [14–18]. ROS promote the formation of DNA adducts such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) and lipid peroxidation such as 4-hydroxynonenal (4-HNE). Both of these adducts and inflammatory factors jointly cause the accumulation of DNA damage, epigenetic changes, abnormal gene expression and altered cell signaling pathways in GCs, leading to cell and organ aging [19–22]. At present, our group and others' studies have shown that ovarian aging is delayed by improving mild inflammatory and OS environment in the ovary [23, 24]. Therefore, the search for natural materials and chemicals to improve the inflammatory and OS environment of GCs is expected to provide a novel method to rejuvenate the ovary via GC function restoration. COS might be the target chemical meeting these properties. COS is a derivative of chitin that is mainly derived from crustaceans, fungi, insects, and algae cell membranes [25]. COS, water-soluble alkaline polysaccharide with positively charged, has good biocompatibility and is widely used in food, medicine, the chemical industry, environmental protection, cosmetics, agriculture, and other applications [26]. It is known as the “sixth life element” after protein, fat, sugar, vitamin, and mineral in the biomedical field. COS can be used as a functional food and is known as “soft gold.” COS is an oligomer composed of β-(1 ➔ 4)-linked d-glucosamine, and it is an oligosaccharide obtained by the hydrolysis of chitosan (CTS) [27]. COS has distinct characteristics, such as a low molecular weight, noncytotoxicity, good water solubility, and easy absorption in the intestine. Meanwhile, COS possesses various diverse biological features, including anti-inflammatory, radical-scavenging, antioxidant, and antidiabetic effects [28]. Currently, products of health care and skincare and other applications have been applied COS as a supplement.
The role of reactive oxygen species (ROS) concentrations in follicular fluid in gynecological diseases and assisted reproduction is relevant to its role in reproductive outcomes [29, 30]. Patients with premature ovarian failure, polycystic ovarian syndrome (PCOS), and physiologically aging ovaries experience a decrease in follicle quality and disrupted oocyte maturation. These tissues are all in chronic low-grade inflammation state caused by OS [31–33]. Tiwari and Chaube found that a gentle increase in ROS levels in the follicle is advantageous for meiotic resumption and first polar body extrusion [34]. In contrast, the excess accumulation of ROS such as H2O2 and superoxide anion radicals leads to OS, and progressive OS generally induces an inflammatory state [35, 36]. High levels of ROS in the follicle microenvironment prevent the recovery of meiosis of diploid terminated oocyte and GC function, causing poor communication between GCs and oocytes by reducing the supply of nutrition, and eventually negatively affect oocyte quality and maturation [37–40].
Hypoxia-inducible factor (HIF) expression is induced by hypoxia and by other pathological environments associated with inflammation, aging, infectious microorganisms, and tumors [41–44]. It is composed of three subunits: 1α, 2α, and 1β. HIF-1α is the main subunit that is widely expressed and regulates a variety of target genes, such as glucose transporters, and VEGF [45]. Recent research has reported that inflammation may lead to cumulate HIF-1α protein in macrophages via a mechanism relative to ROS [46]. In addition, KC7F2 is an HIF-1α protein inhibitor but does not affect the protein degradation rate or mRNA transcription. TGF-β (transforming growth factor-β), along with its superfamily number anti-Müllerian hormone (AMH), bone morphogenetic protein-15 (BMP-15), and growth differentiation factor-9 (GDF-9), plays important roles in influencing the developing follicle microenvironment through autocrine and paracrine manner [47]. In ovary and other organs, hypoxia significantly inhibited GSH activities and superoxide dismutase (SOD) and enhanced the production of malondialdehyde (MDA) [48–50].
However, it's not clear whether COS has a role in GC development and function by controlling chronic low-grade inflammation and OS. We studied the direct effects of different COS concentrations on a human ovarian granulosa cell line (KGN) as a first step to examine the potential protective effect of COS on H2O2-stimulated GC dysfunction. Also, we studied the effects of COS on KGN cells mediated by the HIF-1α signaling pathway.
2. Materials and Methods
2.1. Experimental Set-Up
The human ovarian granulosa cell line (KGN) was obtained from Dr. Yang Zou (Jiangxi Maternal and Child Health Hospital, China). The culture medium used was DMEM/F12 (Wisent, China) with 10% FBS (Gibco, USA) and 1% antibiotics (Solarbio, China). KGN cells were incubated with 5% CO2 at 37°C.
Phase A: we investigated the H2O2-stimulted KGN OS model. KGN cells were seeded in 96-well plates at a density of 8 × 103 cells/well. Then, cells were treated with 100, 200, 400, or 800 μM H2O2 (Wako Pure Chemical Industries, China) for 1, 2, 3, or 4 h. The OD value was detected using the CCK-8 method, and the cell survival (%) was calculated. A cell survival close to 50% was required to determine the optimal concentration and time for subsequent experiments.
Phase B: we investigated the dose- and time-dependent effects of a broad range of concentrations of COS (0, 100, 200, and 300 μg/ml) (Glycobio, China, purity ≥90%) on H2O2-stimulated OS damage in KGN cells.
Phase C: the optimal concentration of COS was studied to explore the mechanism underlying the protective effect.
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
The centrifuged supernatant of the medium was determined using ELISA kits (Westang, China) for the levels of IL-6, IL-10 (cell lysates), E2, and P4, according to the manufacturer's recommendations.
2.3. Immunofluorescence Staining
2.3.1. Measurement of the Mitochondrial Quantity
KGN cells were incubated with 200 nM MitoTracker (AAT Bioquest, USA) diluted with medium (without FBS) for 30 min at 37°C to specifically assess mitochondrial numbers.
2.3.2. Detection of ROS Production
The Reactive Oxygen Species Assay Kit (Solarbio, China) was used to determine ROS level. After COS treatment, KGN cells were loaded with 10 μM DCFH-DA in FBS-free medium for 20 min at 37°C. Then, the medium containing DCFH-DA was removed, and KGN cells were washed to remove unconjugated DCFH-DA.
2.3.3. Measurement of the Mitochondrial Membrane Potential (MMP, ΔΨm)
MMP was detected by MMP assay kit with JC-1 (Beyotime, China). KGN cells were incubated with 1 ml staining working solution (8 ml ultrapure water + 50 μl JC − 1 (200X) + 2 ml JC − 1 staining buffer (5X)) and 1 ml FBS-free medium per well (6-well plates) at 37°C in the dark for 20 min, then washed with JC-1 iced staining buffer (1X), and maintained in FBS-free medium while being examined.
All of the fluorescence intensity was examined under an Olympus 1X71 microscope and analyzed with ImageJ. The results are from 3 replications.
2.4. Cell Counting Kit-8
Cell viability was measured using the Cell Counting Kit-8 assay (CCK-8, APExBIO, USA) in 96-well plates at a density of 8 × 103 cells/well. The mixture liquid (10 μl CCK − 8 assay reagent + 90 μl FBS − free medium) was added to each well and incubated in the dark for 2 h at 37°C. The optical density (OD) was assessed by a microplate reader (Bio-Rad, USA).
2.5. Flow Cytometry
KGN cells were resuspended in annexin V binding buffer with FITC-conjugated annexin V (Bestbio, China). Then, 10 μl of propidium iodide was added. A flow cytometer was used to analyze the KGN cells (Beckman Coulter, USA).
2.6. Western Blotting
KGN cells were incubated with lysis buffer supplemented with a protease inhibitor cocktail (Solarbio, China) on ice. The supernatant was collected after centrifugation at 4°C for 15 min. Protein concentrations were measured using the DC protein assay (Bio-Rad Laboratories Inc.). Equal amounts of total protein (25 μg) were separated on 10% SDS–PAGE gels (Boster, USA) and transferred onto PVDF membranes (Immobilon-P, USA). Membranes were blocked with 5% nonfat milk in TBST and incubated with primary antibodies against TGF-β1 (1 : 500; ab92486; Abcam), HIF-1α (1 : 500; 66730; Proteintech), VEGFα (1 : 1,000; 66828-1; Proteintech), 4-HNE (1 : 1,000; ab46545; Abcam), 8-OHdG (1 : 1,000; 251640; Abbiotec LLC), or β-actin (1 : 1,000; AC006; ABclonal) overnight at 4°C. The corresponding secondary antibody (1 : 3,000; RS0001; ImmunoWay) was incubated with the membrane at room temperature for 1 h. Immunoreactive bands were detected using a western blotting luminescence reagent (ECL) kit (Tiangen, China) and photographed with ChemiDoc XRS (Bio-Rad, USA). The protein band was analyzed with ImageJ software.
2.7. RNA Extraction and RT–qPCR
KGN cell RNA was extracted with TRIzol® reagent (Tiangen, China). cDNAs were generated by reverse transcription with a PrimeScript RT Reagent Kit (Takara, Japan). RT-qPCR using an ABI 7500 real-time PCR instrument (Biosystems, USA) with SYBR® Green PCR Master Mix (Takara, Japan) was performed. The primers for the reference genes were HIF-1α (forward, 5′-AACATAAAGTCTGCAACATGGAAG-3′ and reverse, 5′-TTTGATGGGTGAGGAATGGG-3′), VEGF (forward, 5′-AGTCCAACATCACCATGCAG-3′ and reverse, 5′-TTCCCTTTCCTCGAACTGATTT-3), and GAPDH (forward, 5′-ACATCGCTCAGACACCATG-3′ and reverse, 5′-TGTAGTTGAGGTCAATGAAGGG-3′). The specificity was validated by performing a melting curve analysis, and experiments were repeated at least three times.
2.8. SA-β-Gal Assay
The SA-β-gal assay was carried out according to the manufacturer's instructions (Beyotime, China). Freshly collected KGN cells were fixed for 15 min with β-galactosidase fixative at room temperature, washed, and stained with X-gal solution overnight at 37°C. Cells were photographed under an Olympus 1X71 microscope. The percentage of blue SA-β-gal positive cells was determined using ImageJ software.
2.9. GSH
The cellular total GSH were measured using a GSH Assay Kit (Beyotime, China). KGN cells were collected after centrifugation. Then, the supernatant was removed. M solution for protein removal was added, and the volume was three times the cell pellet volume. The samples were rapidly freeze-thawed three times. Afterward, suspension was centrifuged. The supernatant was collected to measure the total GSH content.
2.10. Statistical Analysis
All data (three independent cultures) are presented as the means ± SEM (standard errors of the means). Statistical analyses were performed using Student's t-test and one-way ANOVA with SPSS software. P < 0.05 were considered statistically significant.
3. Results
3.1. H2O2 Inhibited KGN Cell Survival in a Concentration- and Time-Dependent Manner
CCK-8 results showed that H2O2 inhibited the cell survival of KGN cells in vitro. Various H2O2 concentrations (100, 200, 300, and 400 μM) showed concentration- and time-dependent inhibition of cell survival (Figure 1). When cells were treated with 100 μM H2O2 for 4 h, the cell survival rate was 53.25 ± 3.76%, which was closest to the IC50. Therefore, 100 μM and 4 h were selected for subsequent experiments as the optimal concentration and time, respectively.
Figure 1.
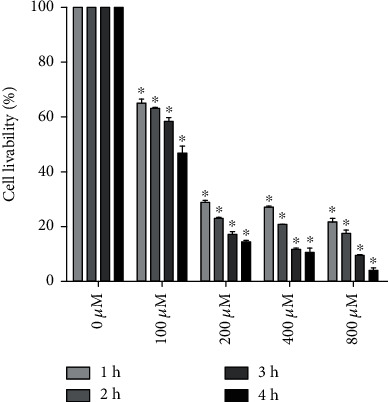
H2O2 inhibited KGN cell survival in a concentration- and time-dependent manner. Data are presented as the means ± SEM (n = 3). ∗P < 0.05 vs. the 0 μM group.
3.2. COS Increased Viability and Inhibited Apoptosis in KGN Cells under Oxidative Stress
H2O2 obviously reduced cell viability (Figure 2(c), P < 0.001), but the viability of COS treatment groups was higher than that of the H2O2 group, except the 200 COS group, but the differences were not significant (P > 0.05). In Figure 2(a), annexin V and PI assays were conducted to evaluate apoptosis. The percentage of apoptotic cells was calculated from H2 (late stage of apoptosis) and H4 (early stage of apoptosis). In Figure 2(b), H2O2 increased the proportion of apoptotic cells (P < 0.001). After treatment with different concentrations of COS, the percentage of apoptotic cells was significantly decreased (P < 0.05 in the H2O2+100 COS group). These results support that COS protects KGN cells from apoptosis stimulated by H2O2.
Figure 2.
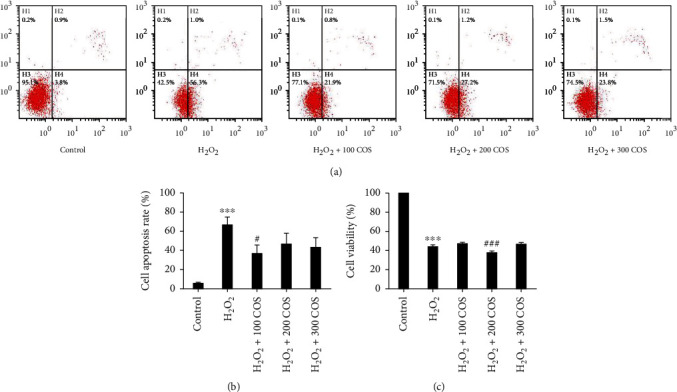
Effect of COS treatment on cell viability and apoptosis in H2O2-stimulated KGN cells. (a) Cell apoptosis rate. (b) Quantification of cell apoptosis rate. (c) Cell viability. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group.
3.3. COS Increased Levels of the TGF-β1 and IL-10 Proteins and Steroidogenesis and Decreased Levels of the IL-6 Protein in H2O2-Stimulated KGN Cells
As depicted in Figure 3(a), H2O2 increased TGF-β1 levels (P < 0.01), similar to previous studies [51, 52]. The increase in IL-6 levels and decrease in IL-10 expression induced by H2O2 (Figures 3(c) and 3 (d), P < 0.001, respectively) were substantially reversed by COS. Additionally, COS significantly increased TGF-β1 protein expression (P < 0.01 in the H2O2+100 COS/300 COS group).
Figure 3.
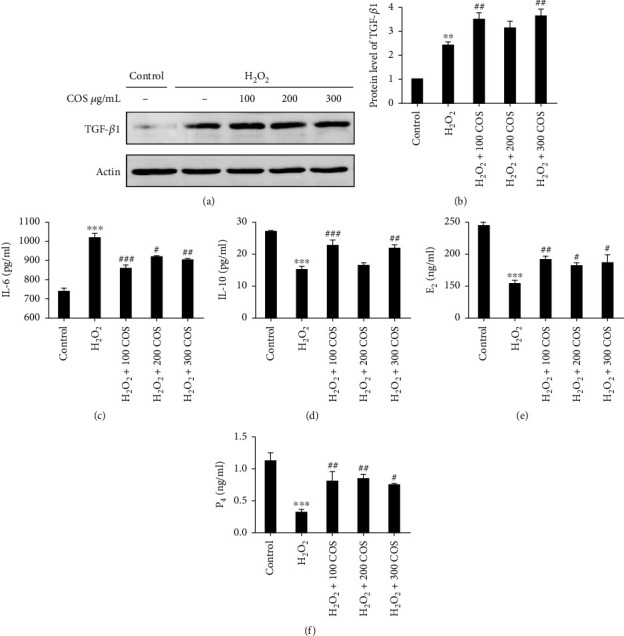
The expression of TGF-β1, IL-6, IL-10, E2, and P4 after COS treatment in H2O2-stimulated KGN cells. (a) The protein expression of TGF-β1. (b) The statistics histogram of western blotting was expressed as band density normalized versus ACTIN. (c–f) The levels of IL-6, IL-10, E2, and P4. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01 or ###P < 0.001 vs. the H2O2 group.
H2O2 decreased steroidogenesis in KGN cells (Figures 3(e) and 3(f), P < 0.001, respectively). The levels of secreted E2 and P4 were increased by COS treatment at different doses ranging from 100 to 300 μg/ml.
Based on these results, COS treatment exerts a positive effect on steroid production and induces anti-inflammatory and proliferative activities that help KGN cells recover from OS.
3.4. COS Inhibited Oxidative Stress in H2O2-Stimulated KGN Cells
The levels of 4-HNE, 8-OHdG, ROS, and GSH were evaluated as markers of OS. In Figures 4(a) and 4(d), the levels of 4-HNE and 8-OHdG and ROS release were markedly increased in KGN cells exposed to H2O2 (P < 0.01 for 4-HNE, P < 0.05 for 8-OHdG, and P < 0.001 for ROS). The increased production of 4-HNE, 8-OHdG, and ROS was significantly attenuated in the H2O2+100 COS group (P < 0.05 for 4-HNE/8-OHdG, P < 0.05 for ROS). The results presented in Figure 4(c) proved that the H2O2-stimulated decrease in GSH activity (P < 0.001) was mitigated by the COS treatment (P < 0.05 in the H2O2+100 COS/300 COS group). Overall, these data show that COS restored the function of H2O2-stimulated KGN cells through attenuating OS level.
Figure 4.
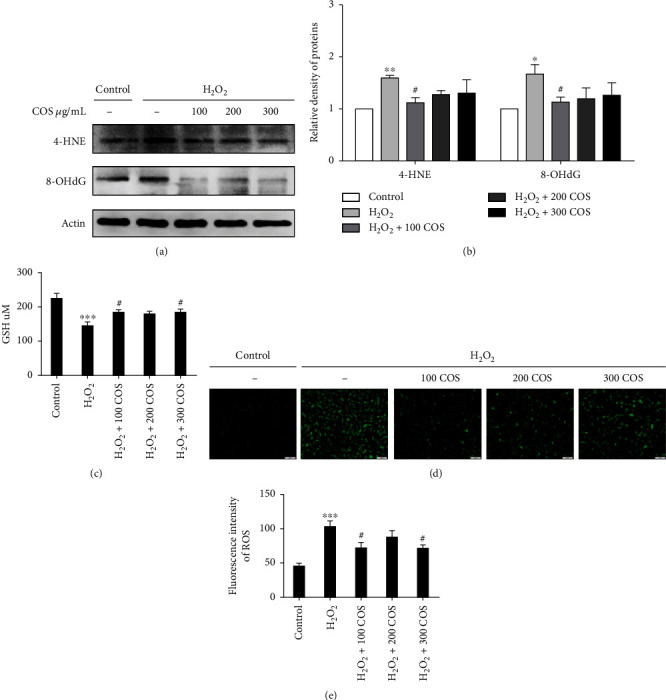
COS inhibited oxidative stress in H2O2-stimulated KGN cells. (a) The protein expressions of 4-HNE and 8-OHdG after COS treatment. (b) The statistics histogram of western blotting is expressed as band density normalized versus actin. (c) Influence of COS on cellular GSH. (d) The cellular production of ROS determined by a fluorescent probe. (e) The statistics histogram of immunofluorescence expressed fluorescent intensity. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group. Scale bar: 100 μm.
3.5. COS Improved Mitochondrial Function in H2O2-Stimulated KGN Cells
Mitochondria are one of the most important organelles and indicators of cell function. The mitochondrial numbers were determined by coloading the cells with a mitochondrion-specific probe, MitoTracker. H2O2 reduced MitoTracker staining. (Figure 5(a), P < 0.001). After COS treatment, the fluorescence intensity was noticeably increased (P < 0.01 in the H2O2+100 COS group; P < 0.05 in the H2O2+200/300 COS group). Thus, COS increased mitochondrial numbers. We also assessed MMP using JC-1 and measuring the red/green ratio. A decrease of MMP in KGN cell exposure to H2O2 was observed (Figure 5(c), P < 0.001). Treatment with COS rescued the cells from the loss of MMP (P < 0.001 in the H2O2+100 COS/300 COS group). These data indicate that COS can improve mitochondrial quantity and MMP in H2O2-stimulated KGN cells.
Figure 5.
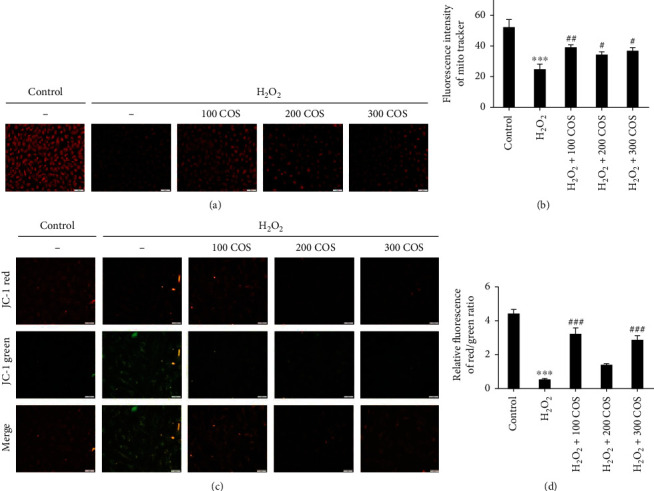
COS improved mitochondrial function in H2O2-stimulated KGN cells. (a) MitoTracker was stained on KGN by immunofluorescence. (b) The statistics histogram of immunofluorescence expressed fluorescent intensity. (c) JC-1 red, JC-1 green, and merge images. (d) Statistics data were expressed in terms of the ratio of JC-1 red to JC-1 green. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group. Scale bar: (a): 100 μm; (c): 50 μm.
3.6. COS Inactivated the HIF-1α/VEGF Pathway, and HIF-1α Inhibition Reversed the Protective Effects of COS on H2O2-Stimulated KGN Cells
Western blotting analysis revealed that H2O2 exposure markedly increased levels of the HIF-1α and VEGFα proteins in KGN cells (Figure 6(a), P < 0.05, respectively), and COS inhibited the increases in HIF-1α and VEGFα protein levels induced by H2O2 in KGN cells. When HIF-1α was inhibited by KC7F2, it also reversed the increases in VEGFα, IL-6 (Figure 6(f)), and TGF-β1 protein levels (Figure 7(a)) and decreases in the levels of IL-10 (Figure 6(g)), E2 (Figure 6(h)), P4 (Figure 6(i)), MitoTracker staining (Figure 8(a)), GSH content (Figure 7(e)), and cell viability (Figure 7(f)) induced by H2O2, as well as the increase in the level of senescence-associated β-galactosidase (SA-β-gal) (Figure 6(d)). Furthermore, the increased levels of 4-HNE and 8-OHdG (Figure 7(a)), ROS release (Figure 8(a)), and cell apoptosis rate (Figure 7(c)) induced by H2O2 were all prevented by KC7F2.
Figure 6.
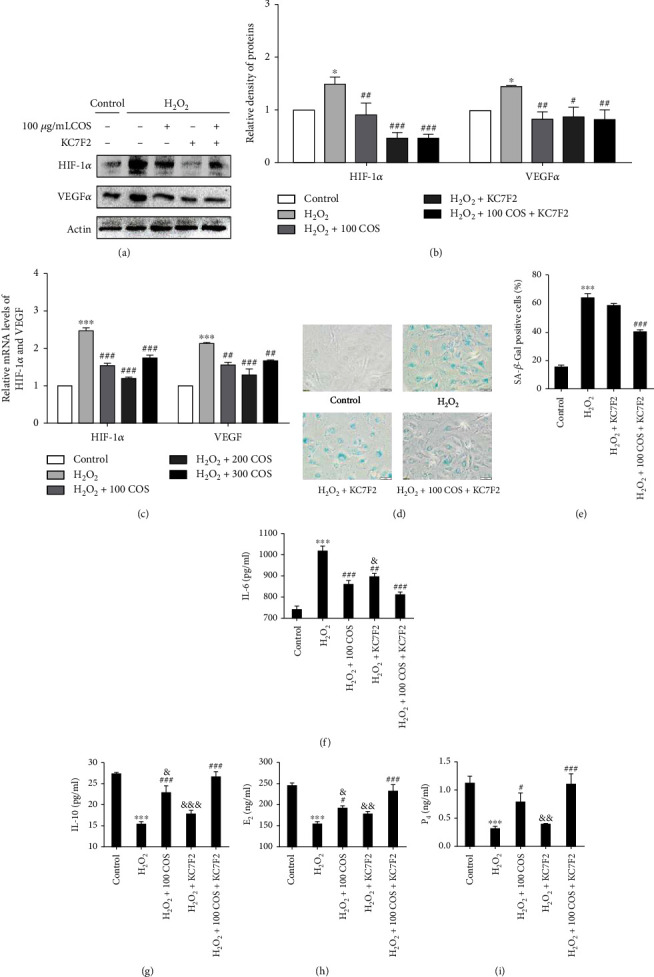
The effect of the COS treatment on the HIF-1α/VEGF pathway in H2O2-stimulated KGN cells. (a) The protein expressions of HIF-1α and VEGF. (b) The statistics histogram of western blotting is expressed as band density normalized versus actin. (c) The mRNA expressions of HIF-1α and VEGF. (d) Determination of senescence in KGN cells by staining for SA-β-gal (200X). (e) Statistical analysis of quantification of the SA-β-gal-positive cells. (f–i) The levels of IL-6, IL-10, E2, and P4. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group; &P < 0.05, &&P < 0.01, or &&&P < 0.001 vs. the H2O2+100 COS+KC7F2 group.
Figure 7.
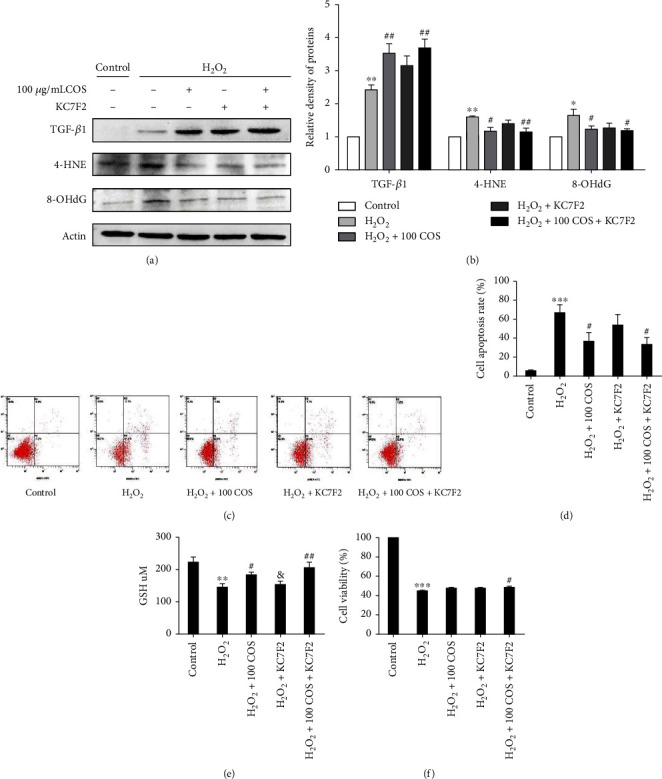
The effect of the COS treatment on the HIF-1α/VEGF pathway in H2O2-stimulated KGN cells. (a) The levels of TGF-β1, 4-HNE, and 8-OHdG. (b) The statistics histogram of western blotting are expressed as band density normalized versus actin. (c) Cell apoptosis rates. (d) Quantitative results of KGN cell apoptosis rate. (e, f) Cellular GSH content and cell viability. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group; &P < 0.05, &&P < 0.01, or &&&P < 0.001 vs. the H2O2+100 COS+KC7F2 group.
Figure 8.
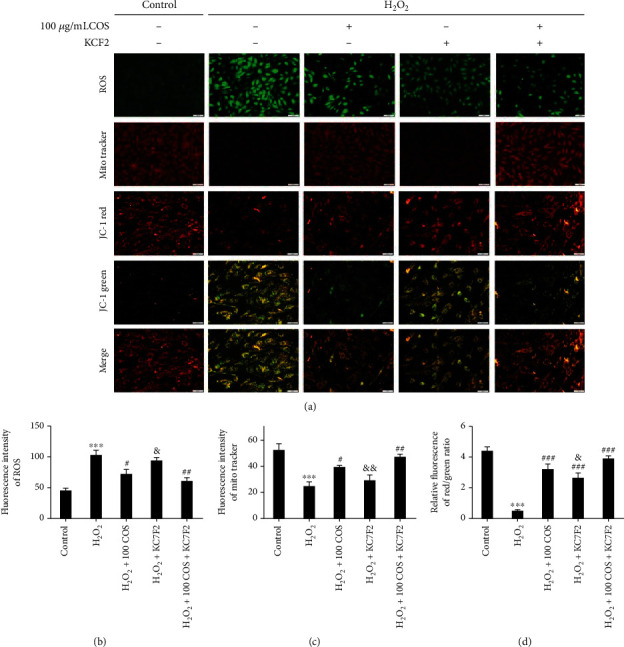
The effect of the COS treatment on the HIF-1α/VEGF pathway in H2O2-stimulated KGN cells. (a) Production of ROS determined by a fluorescent probe. (b) MitoTracker was stained on KGN cells by immunofluorescence. (c) JC-1 red, JC-1 green, and merge image. (d, e) The statistics histogram of immunofluorescence expressed fluorescent intensity. (f) Statistics data were expressed in terms of the ratio of JC-1 red to JC-1 green. Data are presented as the means ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, or ∗∗∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01, or ###P < 0.001 vs. the H2O2 group; &P < 0.05, &&P < 0.01, or &&&P < 0.001 vs. the H2O2+100 COS+KC7F2 group. Scale bar: (a) ROS/MitoTracker: 100 μm; JC-1 red/JC-1 green/merge: 50 μm.
COS and KC7F2 exerted synergistic effects on all of these outcomes. Based on these results, the HIF-1α-VEGF signaling pathway mediated the protective effects of COS on H2O2-stimulated KGN cells.
4. Discussion
Aging, mental stress, endocrine disorders, and chemo- and/or radiotherapy all exert substantial effects on female fertility [53–55]. Currently, few methods are available to preserve ovarian function in human being. Furthermore, these methods are less effective and still under investigated in experimental research. Chemicals that potentially protect the follicle of oocyte and GCs from injury might be the next female fertility-reserving medicine. A vital antioxidant and a forceful free radical scavenger, COS might be this type of agent.
GCs promote oocyte maturation and protect oocytes from OS damage via supplying essential nutrients and maturation-promoting factors; they play crucial roles in folliculogenesis [56]. GCs are susceptible to ROS, which are byproducts produced in the course of the citric acid cycle. The generation of endogenous ROS is through the following: one is various metabolic processes, and the other is the electron transport chain. Mitochondria are intimately involved in cellular respiration and metabolism, and thus, they are the main generator of endogenous ROS and the main target organelle for OS damage. There are two main methods to remove ROS. One is the internal antioxidant system, which consists of enzyme such as SOD and the nonenzymatic antioxidant such as GSH [49, 50]. GSH is a major cellular antioxidant, also recognized key indicator to determine the degree of OS [50]. The other is exogenous antioxidant from supplement to improve the antioxidant capacity [57]. Obviously, it leads to a better effect that endogenous and exogenous antioxidant methods simultaneously remove ROS to resist oxidant damage. ROS is not only considered to be the most critical induction factor of cell damage caused by oxidative stress; meanwhile, free radicals mainly attack macromolecules such as biofilms, membrane system, DNA, and protein. Free radicals easily react with unsaturated fatty acids on the membrane then produce lipid radicals, destroying the original membrane structure [58]. The levels of ROS are markedly accumulative after stimulation and induction OS in GCs, leading to GC dysfunction [59]. Hence, ROS-induced injury of OS and GC decline in quantity are deemed the primary etiological factors of ovarian dysfunction. In this study, H2O2 was applied to induce OS and GC apoptosis [60–62]. We found that COS-mediated GC protection depends on improved mitochondrial function and a decrease in apoptosis. Therefore, our results suggest that COS inhibits OS-induced damage in GCs by ameliorating mitochondrial damage. Numerous animal studies have shown that OS and chronic inflammation promote each other in a vicious cycle: OS leads to an increase in ROS production through NOD-like receptor 3 (NLRP3) and NF-κB, resulting in a series of inflammatory responses and increased secretion of IL-1β, IL-8, and TNF-α, which in turn promote OS and accelerate the organ aging process [63]. So we evaluated the protective effect of COS on H2O2-oxidative damage and cytokine production in KGN cells. COS significantly increased cell viability, the cellular GSH content, and mitochondrial function and reduced ROS production; the levels of 8-OHdG, 4-HNE, IL-6, HIF-1α, and VEGF; and cell apoptosis. COS also significantly increased TGF-β1 and IL-10 expression in the H2O2-stimulated KGN cells, significantly increased E2 and P4 levels, and decreased in SA-β-gal protein expression, implying that COS attenuated oxidative and inflammatory injury in H2O2-stimulated KGN cells (Figure 9). Partial results showed that the effect of the H2O2+200 COS group was lower than that of the other two groups, but the P values between the most optimal concentration group (the H2O2+100 COS group) and the H2O2+200 COS group were not significant except the results in IL-6 and IL-10. It may be related to the higher sensitivity of ELISA than western blotting in quantifying. Meanwhile, it proved that COS was not concentration-dependent in the KGN OS model.
Figure 9.
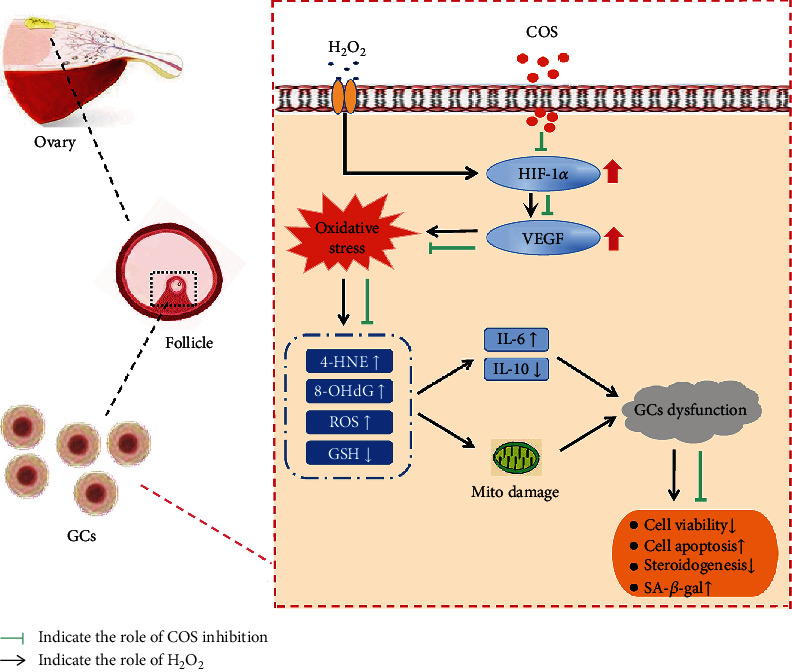
COS attenuated oxidative and inflammatory injury in H2O2-stimulated KGN cells. The protective effects of COS were manifested in inactivating the HIF-1α-VEGF signaling pathway, attenuating OS, decreasing the production of inflammatory cytokines, and improving mitochondrial damage caused by H2O2 in KGN cells.
We were very curious about the triggers of inflammation and OS-induced damage. What is the mechanism of action? We speculated that the mechanism may be related to ovarian hypoxia. Hypoxia, the imbalance between the oxygen supply and demand, is the factor that induces chronic inflammation and OS. Due to the unique tissue construct and large volume of oocyte, the ovary is a hypoxic organ. On the one hand, with the growth and development of follicular oocytes and the proliferation and division of GCs, the oxygen demand gradually increases; on the other hand, continuous ovulation leads to an increase in the amount of fibrous connective tissue and a significant decrease in the number of blood vessels in the ovary, which leads to a decreased oxygen and blood supply in the ovary with aging. In addition, the chronic low-grade inflammatory response caused by repeated ovulation and the accompanying OS further aggravate the imbalance between supply and demand, which reduces the oxygen concentration in the ovary to approximately 1.3%-5.5% [64, 65]. This finding may be an important explanation for the significantly faster ovarian aging rate than that in other organs of the body. Molinari et al.'s transcriptome analysis of human cumulus cells also revealed that hypoxia is a marker and important determinant of follicular senescence [66]. The activity of hydroxylase and the degradation of HIF-1α are prevented in hypoxic circumstances. Stimulation with TNF-α or ROS (such as H2O2) leads to enhance NF-κB transcription, which binds to a distinct element at −197/188 bp of the HIF-1α promoter, thus synthetizing HIF-1α mRNA and protein [67–69]. Hypoxia increases glycolysis metabolism through HIF-1α; the HIF-1α-dependent pathway activates monocytes/macrophages and TH1/TH17 cells through nuclear transcription factor NF-κB and increases the generation of inflammatory factors such as IL-6, IFN7, and TNF-α; ROS can activate NF-κB, which is a significant transcription factor in the complete progress of HIF-1 activation, leading to chronic inflammation of the ovary [49, 70–74]. The relationship between NF-κB and HIF-1 is of major importance for inflammatory diseases. HIF-1 can also activate NF-κB. HIF-1α is a direct target gene of NF-κB [75, 76]. Hypoxia induces excess ROS production, which may cause OS-induced damage to the ovary, including the oxidation of proteins, lipids, and DNA. Oxidative damage promotes the production of adducts: 8-OHdG and 4-HNE [77–79]. Both of the adducts, together with inflammatory factors, cause the accumulation of epigenetic alterations, DNA damage, abnormal gene expression, and dysregulation of cell signaling pathways, leading to cell and organ aging [80–83].
Recently, the Guanghui Liu Laboratory at the Chinese Academy of Sciences (CAS), Shang Fu Laboratory at Peking University, and Qu Jing research group at CAS collaborated and used high-precision single-cell transcriptome sequencing technology to draw the first map of cynomolgus monkey ovarian cell aging and used a human ovarian cell research system at the same time. Aging leads to an imbalance in the cell type-specific redox regulatory network in the ovary, and the decrease in the antioxidant capacity associated with aging is one of the main characteristics of aging in the primate ovary [83]. In this study, we found that COS caused significant inactivation of the HIF-1α-NF-κB-VEGF pathway in H2O2-stimulated KGN cell. Furthermore, inhibition of this pathway strengthened the inhibitory effects of COS on H2O2-stimulated OS damage and apoptosis in KGN cells.
5. Conclusion
In this study, by comparing the oxidative stress damage, we concluded that COS exerts protective effects on H2O2-stimulated oxidative damage and apoptosis in GCs via the inactivation of the HIF-1α-NF-κB–VEGF signaling pathway. Therefore, COS might be an agent for ovarian pathology therapy through its regulatory effect on follicular development.
Acknowledgments
This research was funded by the Natural Scientific Foundation of Jiangxi Province (No. 20192BAB215009), Basic Research Scheme of Shenzhen Science and Technology Innovation Commission, and the National Natural Science Foundation of China (Nos. 81671455, 31460307).
Abbreviations
- GCs:
Granulosa cells
- COS:
Chitosan oligosaccharides
- H2O2:
Hydrogen peroxide
- KGN:
Granulosa cell line
- GSH:
Glutathione
- ROS:
Reactive oxygen species
- 8-OHdG:
8-Hydroxy-2′-deoxyguanosine
- 4-HNE:
4-Hydroxynonenal
- HIF-1α:
Hypoxia-inducible factor-1α
- VEGF:
Vascular endothelial-derived growth factor
- TGF-β1:
Transforming growth factor β-1
- SA-β-Gal:
Senescence-associated β-galactosidase
- POF:
Premature ovarian failure
- GnRH:
Gonadotrophin-releasing hormone
- OS:
Oxidative stress
- CTS:
Hydrolysis of chitosan
- PCOS:
Polycystic ovarian syndrome
- MMP:
Mitochondrial membrane potential
- NLRP3:
NOD-like receptor 3
- NF-κB:
Nuclear factor kappa-B
- NOS:
Nitric oxide synthase
- SOD:
Superoxide dismutase
- MDA:
Malondialdehyde
- GDF-9:
Growth differentiation factor-9
- BMP-15:
Bone morphogenetic protein-15
- AMH:
Anti-Müllerian hormone
- Nrf2:
Nuclear factor erythroid 2-related factor 2.
Contributor Information
Jun Tan, Email: tanjun561127@163.com.
Zhisheng Zhong, Email: panrover@sina.com.
Yuehui Zheng, Email: yuehuizheng@163.com.
Data Availability
The data used to support the finding of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Couzin-Frankel J. Faulty DNA repair linked to ovarian aging in mice and humans. Science . 2013;339(6121):p. 749. doi: 10.1126/science.339.6121.749. [DOI] [PubMed] [Google Scholar]
- 2.Sommezer M., Oktay K. Fertility preservation in female patients. Human Reproduction . 2004;10(3):251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 3.Findlay J. K., Drummond A. E., Dyson M. L., Baillie A. J., Robertson D. M., Ethier J. F. Recruitment and development of the follicle; the roles of the transforming growth factor-β superfamily. Molecular and Cellular Endocrinology . 2002;191(1):35–43. doi: 10.1016/S0303-7207(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez F., Smitz J. Molecular control of oogenesis. Biochimica et Biophysica Acta . 2012;1822(12):1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Li R., Phillips D. M., Mather J. P. Activin promotes ovarian follicle development in vitro. Endocrinology . 1995;136(3):849–856. doi: 10.1210/en.136.3.849. [DOI] [PubMed] [Google Scholar]
- 6.Makrigiannakis A., Coukos G., Blaschuk O., Coutifaris C. Follicular atresia and luteolysis, evidence of a role for N-cadherin. Annals of the New York Academy of Sciences . 2002;2000(900):46–55. doi: 10.1111/j.1749-6632.2000.tb06215.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker T. G. A. A quantitative and cytological study of germ cells in human ovaries. Proceedings of the Royal Society of London. Series B . 1963;158(972):417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 8.Faddy M. J., Gosden R. G., Gougeon A., Richardson S. J., Nelson J. F. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human Reproduction . 1992;7(10):1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 9.Clarke H. Oocytes . Springer; 2017. Control of mammalian oocyte development by interactions with the maternal follicular environment; pp. 17–41. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda F., Inoue N., Manabe N., Ohkura S. J. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. Reproduction Development . 2012;58(1):44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 11.Assou S., Haouzi D., Vos J. D., Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Molecular Human Reproduction . 2010;16(8):531–538. doi: 10.1093/molehr/gaq032. [DOI] [PubMed] [Google Scholar]
- 12.Shen M., Cao Y., Jiang Y., Wei Y., Liu H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation- independent mechanism. Redox Biology . 2018;18:138–157. doi: 10.1016/j.redox.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y., Shen M., Chen Y. Y., Wei Y. H., Tao J. L., Liu H. L. Melatonin represses mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules . 2021;11(7):p. 968. doi: 10.3390/biom11070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H., Niu F., Fan W., Shi J., Zhang J., Li B. Modulating effects of preconditioning exercise in the expression of ET-1 and BNP via HIF-1α in ischemically injured brain. Metabolic Brain Disease . 2019;34(5):1299–1311. doi: 10.1007/s11011-019-00450-z. [DOI] [PubMed] [Google Scholar]
- 15.Ali M. H., Schlidt S. A., Chandel N. S., Hynes K. L., Schumacker P. T., Gewertz B. L. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. American Journal of Physiology . 1999;277(5):L1057–L1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 16.Ding H., Li Y., Li X., Liu X., Zeng H. Treatment with 7% and 10% CO2 enhanced expression of IL-1β, TNF-α, and IL-6 in hypoxic cultures of human whole blood. Journal of International Medical Research . 2020;48(4, article 300060520912105) doi: 10.1177/0300060520912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S. H., Lee Y. J., Han H. J. Effect of arachidonic acid on hypoxia-induced IL-6 production in mouse ES cells: Involvement of MAPKs, NF-κB, and HIF-1α. Journal of Cellular Physiology . 2010;222(3):574–585. doi: 10.1002/jcp.21973. [DOI] [PubMed] [Google Scholar]
- 18.Al-Anazi A., Parhar R., Saleh S., et al. Intracellular calcium and NF-kB regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Science . 2018;212:275–284. doi: 10.1016/j.lfs.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Research . 1984;24(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awasthi Y. C., Sharma R., Cheng J. Z., Yang Y., Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Molecular Aspects of Medicine . 2003;24(4-5):219–230. doi: 10.1016/S0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 21.Yan Z., Dai Y., Fu H., et al. Curcumin exerts a protective effect against premature ovarian failure in mice. Journal of Molecular Endocrinology . 2018;60(3):261–271. doi: 10.1530/JME-17-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim J., Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biology of Reproduction . 2011;84(4):775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M., Ma L. W., Xue L., et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging . 2019;11(3):1030–1044. doi: 10.18632/aging.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y. Q., Ye H. F., Zhu F. Y., Hu C., Zheng Y. H. The role of chito-oligosaccharide in regulating ovarian germ stem cells function and restoring ovarian function in chemotherapy mice. Reproductive Biology and Endocrinology . 2021;19(1):14–30. doi: 10.1186/s12958-021-00699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karthik N., Akanksha K., Pandey A. Production, purification and properties of fungal chitinases--a review. Indian Journal of Experimental Biology . 2014;52(11):1025–1035. [PubMed] [Google Scholar]
- 26.Guan G., Azad M., Lin Y., Kim S. W., Wang H. Biological effects and applications of chitosan and chito-oligosaccharides. Frontiers in Physiology . 2019;10:p. 516. doi: 10.3389/fphys.2019.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naveed M., Phil L., Sohail M., et al. Chitosan oligosaccharide (COS): an overview. International Journal of Biological Macromolecules . 2019;129:827–843. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- 28.Muanprasat C., Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacology & Therapeutics . 2017;170:80–97. doi: 10.1016/j.pharmthera.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A., Gupta S., Sekhon L., Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxidants & Redox Signaling . 2008;10(8):1375–1404. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 30.Askoxylaki M., Siristatidis C., Chrelias C., et al. Reactive oxygen species in the follicular fluid of subfertile women undergoing in vitro fertilization: a short narrative review. Journal of Endocrinological Investigation . 2013;36(11):1117–1120. doi: 10.3275/9097. [DOI] [PubMed] [Google Scholar]
- 31.Feodor N. S., Andersen P. K., Strandberg-Larsen K., Andersen A. M. N. Risk factors for miscarriage from a prevention perspective: a nationwide follow-up study. BJOG - an International Journal of Obstetrics and Gynaecology . 2014;121(11):1375–1385. doi: 10.1111/1471-0528.12694. [DOI] [PubMed] [Google Scholar]
- 32.Naz R. K., Thurston D., Santoro N. Circulating tumor necrosis factor (TNF)-α in normally cycling women and patients with premature ovarian failure and polycystic ovaries. American Journal of Reproductive Immunology . 1995;34(3):170–175. doi: 10.1111/j.1600-0897.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 33.Prattichizzo F., Micolucci L., Cricca M., et al. Exosome-based immunomodulation during aging: a nano-perspective on inflamm- aging. Mechanisms of Ageing & Development . 2017;168:44–53. doi: 10.1016/j.mad.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari M., Chaube S. K. Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. Journal of Obstetrics & Gynaecology Research . 2016;42(5):536–546. doi: 10.1111/jog.12938. [DOI] [PubMed] [Google Scholar]
- 35.Goud A. P., Goud P. T., Diamond M. P., Gonik B., Abu-Soud H. M. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radical Biology & Medicine . 2008;44(7):1295–1304. doi: 10.1016/j.freeradbiomed.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park W. H. Effects of antioxidants and MAPK inhibitors on cell death and reactive oxygen species levels in H2O2-treated human pulmonary fibroblasts. Oncology Letters . 2013;5(5):1633–1638. doi: 10.3892/ol.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiwari M., Prasad S., Tripathi A., et al. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. Reactive Oxygen Species . 2016;1(2):110–116. doi: 10.20455/ros.2016.817. [DOI] [Google Scholar]
- 38.Behrman H. R., Kodaman P. H., Preston S. L., Gao S. Oxidative stress and the ovary. Journal of the Society for Gynecologic Investigation . 2001;8(1 Supplement):S40–S42. doi: 10.1177/1071557601008001S13. [DOI] [PubMed] [Google Scholar]
- 39.Prasad S., Tiwari M., Pandey A. N., Shrivastav T. G., Chaube S. K. Impact of stress on oocyte quality and reproductive outcome. Journal of Biomedical Science . 2016;23(1):1–5. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaube S. K., Shrivastav T. G., Prasad S., et al. Clomiphene citrate induces ROS-mediated apoptosis in mammalian oocytes. Open Journal of Apoptosis . 2014;3(3):52–58. doi: 10.4236/ojapo.2014.33006. [DOI] [Google Scholar]
- 41.Dzhalilova D. S., Kosyreva A. M., Diatroptov M. E., et al. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. Journal Inflammation Research . 2019;Volume 12(12):73–86. doi: 10.2147/JIR.S194581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo E. J. Hypoxia and aging. Experimental & Molecular Medicine . 2019;51(6):1–15. doi: 10.1038/s12276-019-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGettrick A. F., O'Neill L. A. J. The role of HIF in immunity and inflammation. Cell Metabolism . 2020;32(4):524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Lin S., Wan S., Sun L., et al. Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Science . 2012;103(5):904–912. doi: 10.1111/j.1349-7006.2012.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arany T. Z., Huangtt L. E., Eckner R., Bhattacharya S., Jiangt C. An essential role for p300/CBP in the cellular response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America . 1996;93(23):12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palazon A., Goldrath A., Nizet V., Johnson R. HIF transcription factors, inflammation, and immunity. Immunity . 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juengel J. L., McNatty K. P. The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development. Human Reprodution Update . 2005;11(2):144–161. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 48.Liu W., Pu L., Deng B., et al. Intermittent hypobaric hypoxia causes deleterious effects on the reproductive system in female rats. Biomedicine Pharmacotherapy . 2020;130, article 110511 doi: 10.1016/j.biopha.2020.110511. [DOI] [PubMed] [Google Scholar]
- 49.He L., Wang X., Cheng D., Xiong Z., Liu X. Ginsenoside Rg1 improves pathological damages by activating the p21-p53-STK pathway in ovary and Bax-Bcl2 in the uterus in premature ovarian insufficiency mouse models. Molecular Medicine Reports . 2020;23(1):p. 37. doi: 10.3892/mmr.2020.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olaniyan O. T., Bamidele O., Uche S., et al. Ovarian metabolic activity in dehydroepiandrosterone-induced polycystic ovary in Wistar rats treated with aspirin. JBRA Assisted Reproduction . 2020;24(1):41–54. doi: 10.5935/1518-0557.20190059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itagaki T., Shimizu I., Cheng X., et al. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress-induced activation of cultured rat hepatic stellate cells. Gut . 2005;54(12):1782–1789. doi: 10.1136/gut.2004.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dewidar B., Meyer C., Dooley S., Meindl-Beinker A. N. TGF-β in hepatic stellate cell activation and liver fibrogenesis–updated 2019. Cell . 2019;8(11):p. 1419. doi: 10.3390/cells8111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szkodziak F., Krzyżanowski J., Szkodziak P. Psychological aspects of infertility. A systematic review. Journal of International Medical Research . 2020;48(6, article 300060520932403) doi: 10.1177/0300060520932403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adriaens I., Jacquet P., Cortvrindt R., Janssen K., Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology . 2006;228(2-3):333–343. doi: 10.1016/j.tox.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Kranc W., Budna J., Kahan R., et al. Molecular basis of growth, proliferation, and differentiation of mammalian follicular granulosa cells. Journal of Biological Regulators & Homeostatic Agents . 2017;31(1):1–8. [PubMed] [Google Scholar]
- 56.Yang H., Xie Y., Yang D., Ren D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget . 2017;8(15):25310–25322. doi: 10.18632/oncotarget.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buettner G. R. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anti-cancer Agents in Medical Chemistry . 2011;11(4):341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giorgi C., Marchi S., Simoes I. C. M., et al. Mitochondria and reactive oxygen species in aging and age-related diseases. International Review of Cell and Molecular Biology . 2018;340:209–344. doi: 10.1016/bs.ircmb.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du L., Chen E., Wu T., Ruan Y., Wu S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Design Development & Therapy . 2019;Volume 13(13):747–755. doi: 10.2147/DDDT.S179894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saeed-Zidane M., Linden L., Salilew-Wondim D., et al. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS One . 2017;12(11, article e0187569) doi: 10.1371/journal.pone.0187569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J., Luo J., Zhang Y., Tang C., Wang J., Chen C. Silencing of soluble epoxide hydrolase 2 gene reduces H2O2-induced oxidative damage in rat intestinal epithelial IEC-6 cells via activating PI3K/Akt/GSK3β signaling pathway. Cytotechnology . 2020;72(1):23–36. doi: 10.1007/s10616-019-00354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmad S. F., Zoheir K. M. A., Abdel-Hamied H. E., et al. Grape seed proanthocyanidin extract protects against carrageenan-induced lung inflammation in mice through reduction of pro-inflammatory markers and chemokine expressions. Inflammation . 2014;37(2):500–511. doi: 10.1007/s10753-013-9764-2. [DOI] [PubMed] [Google Scholar]
- 63.McGarry T., Biniecka M., Veale D., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radical Biology and Medicine . 2018;125(1):15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 64.Van Blerkom J., Antczak M., Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Human Reproduction . 1997;12(5):1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 65.Robinson R. S., Woad K. J., Hammond A. J., Laird M., Hunter M. G., Mann G. E. Angiogenesis and vascular function in the ovary. Reproduction . 2009;138(6):869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- 66.Molinari E., Bar H., Pyle A. M., Patrizio P. Transcriptome analysis of human cumulus cells reveals hypoxia as the main determinant of follicular senescence. Molecular Human Reproduction . 2016;22(8):866–876. doi: 10.1093/molehr/gaw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonello S., Zähringer C., Belaiba R. S., et al. Reactive oxygen species activate the HIF-1α promoter via a functional NFκB site. Arteriosclerosis, Thrombosis, and Vascular Biology . 2007;27(4):755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 68.Belaiba R. S., Bonello S., Zahringer C., et al. Hypoxia up-regulates hypoxia-inducible factor-1α transcription by involving phosphatidylinositol 3-kinase and nuclear factor κB in pulmonary artery smooth muscle cells. Molecular Biology of the Cell . 2007;18(12):4691–4697. doi: 10.1091/mbc.e07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minet E., Ernest I., Michel G., et al. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and inhibiting sequences located upstream from the transcription initiation site and cis elements located within the 5′UTR. Biochemical and Biophysical Research Communications . 1999;261(2):534–540. doi: 10.1006/bbrc.1999.0995. [DOI] [PubMed] [Google Scholar]
- 70.Sumbayev V. V., Yasinska I., Oniku A. E., Streatfield C. L., Gibbs B. F. Involvement of hypoxia-inducible factor-1 in the inflammatory responses of human LAD2 mast cells and basophils. PLoS One . 2012;7(3, article e34259) doi: 10.1371/journal.pone.0034259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramakrishnan S., Anand V., Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. Journal Neuroimmune Pharmacology . 2014;9(2):142–160. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Gaber T. Hypoxia/HIF modulates immune responses. Biomedicine . 2021;9(3):p. 260. doi: 10.3390/biomedicines9030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D'Ignazio L., Bandarra D., Rocha S. NF-κB and HIF crosstalk in immune responses. The FEBS Journal . 2016;283(3):413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinzii C. M., Lopez L. C. Abnormalities of hydrogen sulfide and glutathione pathways in mitochondrial dysfunction. Journal of Advanced Research . 2021;7(27):79–84. doi: 10.1016/j.jare.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cummins E. P., Berra E., Comerford K. M., et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proceedings of Nations Academy of Sciences U.S.A. . 2006;103(48):18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Görlach A., Bonello S. The cross-talk between NF-κB and HIF-1: further evidence for a significant liaison. Biochemical Journal . 2008;412(3):e17–e19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 77.Gorini F., Scala G., Cooke M. S., Majello B., Amente S. Towards a comprehensive view of 8-oxo-7,8-dihydro-2'-deoxyguanosine: highlighting the intertwined roles of DNA damage and epigenetics in genomic instability. DNA Repair . 2021;97, article 103027 doi: 10.1016/j.dnarep.2020.103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaneko T., Tahara S., Matsuo M. Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidized DNA damage, during aging. Mutation Research . 1996;316(5-6):277–285. doi: 10.1016/S0921-8734(96)90010-7. [DOI] [PubMed] [Google Scholar]
- 79.Dotan Y., Lichtenberg D., Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Progress in Lipid Research . 2004;43(3):200–227. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Ding N., Maiuri A. R., O'Hagan H. M. The emerging role of epigenetic modifiers in repair of DNA damage associated with chronic inflammatory diseases. Mutation Research Reviews in Mutation Research . 2019;780:69–81. doi: 10.1016/j.mrrev.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang N., Sen P. The senescent cell epigenome. Aging . 2018;10(11):3590–3609. doi: 10.18632/aging.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bektas A., Schurman S. H., Sen R., Ferrucci L. Aging, inflammation and the environment. Experimental Gerontology . 2018;105:10–18. doi: 10.1016/j.exger.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S., Zheng Y., Li J., et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell . 2020;180(3):585–600.e19. doi: 10.1016/j.cell.2020.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the finding of this study are included within the article.


