Abstract
Granulomatosis with polyangiitis (GPA) is characterized by necrotizing vasculitis of small and medium sized vessels and is rarely present in the pediatric population. Cardiac manifestations in pediatric patients with GPA are extremely uncommon, with only two known reported cases associated with coronary artery aneurysms (Rehani and Nelson in Pediatrics 147:e20200932, 2021, 10.1542/peds.2020-0932;Aghaei Moghadam et al. in Case Rep Cardiol 2020:3417910, 2020, 10.1155/2020/3417910). We report a case of a 14-year-old male who presented with a 1 month history of fatigue and shortness of breath. He ultimately was found to have multiple giant coronary aneurysms in both the left and right coronaries including a giant aneurysm in the posterior descending; this has not been previously reported. The case highlights the need for complete multi-modality imaging of the coronary arteries in patients with GPA.
Keywords: Granulomatosis with polyangiitis, Coronary aneurysms, Vasculitis
Introduction
GPA (formerly known as Wegener’s Granulomatosis) is known to primarily affect the upper respiratory tract, lungs, and kidneys. It is rare in the pediatric population with an estimated incidence of 1.8 cases/million person-years [1]. The 1-year mortality is estimated to be close to 90% in untreated patients [2]. Affected systems in pediatric patients at the time of diagnosis are ears, nose and throat (91%), constitutional (89%), respiratory (79%), mucosa and skin (64%), musculoskeletal (59%) and eyes (35%) [2]. Cardiac manifestations are present in only 6% of adults with GPA and are likely even less common in the pediatric population [3–5]. We highlight a rare case of GPA in a 14-year-old male with multi-system involvement and multiple giant coronary artery aneurysms. The extent of his coronary involvement was only appreciated with advanced imaging and cardiac catheterization.
Case Report
A 14-year-old obese male with no significant medical issues presented for an outpatient cardiology evaluation with 1 month of cough, dyspnea, diaphoresis, fatigue, conjunctivitis, and bilateral knee pain. He denied fever, chest pain, palpitations, rash or recent COVID-19 exposure. He also had an unintentional 20-pound weight loss over the previous month. His vital signs were significant for sinus tachycardia at 110 bpm with a normal systemic oxygen saturation, temperature, and blood pressure. Physical examination revealed bilateral conjunctival injection and significant dyspnea. His electrocardiogram was unremarkable except for the sinus tachycardia. His echocardiogram, however, revealed dilation of the proximal left anterior descending (LAD) coronary artery (6 mm; Z-score 4.6) and a small pericardial effusion. Subsequent laboratory studies were remarkable for elevated inflammatory markers (ESR 98 mm/h, CRP 84 mg/L, procalcitonin 0.57 ng/mL), high-sensitivity troponin I (366 ng/L), and NT-pro-BNP (5388 pg/mL). Multisystem inflammatory syndrome in children (MIS-C) was considered, however, he did not have recent COVID-19 exposure and his SARS-CoV-2 rapid PCR nasopharyngeal swab and serum IgG were both negative. The differential was expanded to other forms of vasculitis. In the setting of his continued dyspnea, he was referred for a chest computed tomography (CT) angiogram. The CT scan demonstrated multiple, giant saccular and fusiform aneurysms throughout the left and right coronary artery systems (Fig. 1). The largest aneurysm was located in the posterior descending artery (PDA) and measured 16 mm × 13 mm in diameter (Fig. 2a). The patient was then admitted to the hospital and anticoagulation with enoxaparin and aspirin was started.
Fig. 1.
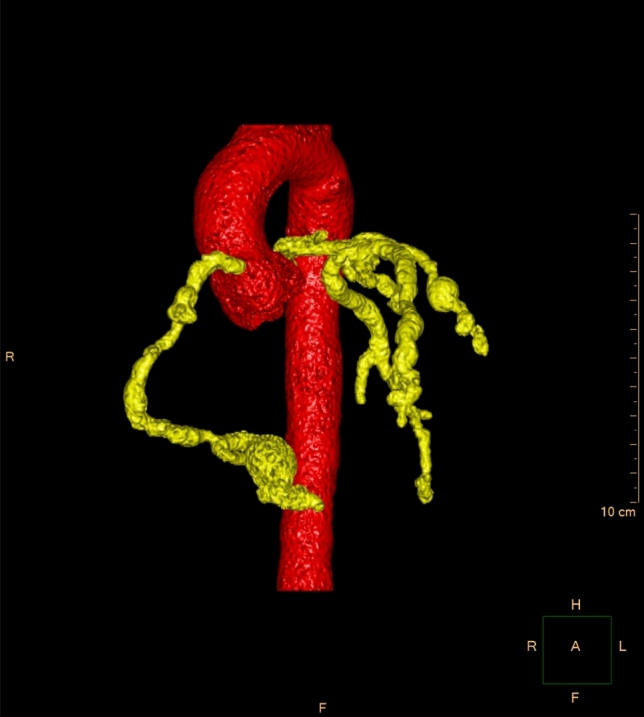
Segmented 3-D reconstruction from the cardiac CT scan, demonstrating numerous saccular and fusiform aneurysms of both the right and left coronary artery systems
Fig. 2.
a Coronal view of the chest CT showing the PDA aneurysm, b coronal view of the chest CT showing increased size of the aneurysm 10 days later
His vasculitis workup revealed positive c-ANCA and targeting proteinase 3 (PR3) consistent with a diagnosis of GPA. He was treated with prednisone and rituximab with improvement in his symptoms throughout his hospital course. However, his high-sensitivity troponin I remained elevated (416 ng/L) and a repeat echocardiogram on hospital day 10 showed progression in the dimensions of the proximal LAD aneurysm to 7.4 mm (Fig. 3). A CT angiogram was repeated and revealed interval dilation of several of the coronary aneurysms, as well as the development of patchy stenosis involving the distal right and left anterior descending coronaries. The largest coronary aneurysm, located in the PDA, had increased in diameter to 17 mm × 24 mm and there was concern for intraluminal thrombus (Fig. 2b). His ECG was not suggestive of ischemia, but given the concern for thrombosis on the CT angiogram he was taken to the catherization lab for further evaluation and consideration of local thrombolytic therapy.
Fig. 3.
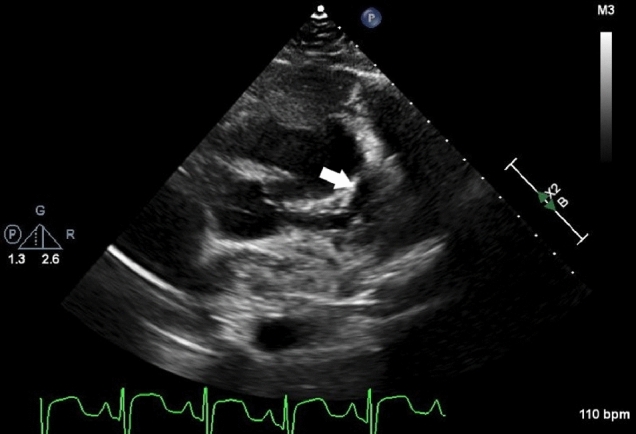
2D transthoracic echocardiography image from the parasternal short axis view. White arrow represents one of the proximal LAD aneurysms
The left main coronary artery appeared normal in size, but there were at least 13 saccular and tubular aneurysms involving the LAD with the largest one measuring 13 × 9 mm (Fig. 4a). The proximal circumflex appeared normal but there were several aneurysms with a bead-like appearance. The right coronary artery (RCA) had a series of 14 aneurysms including the giant aneurysm in the PDA. (Fig. 4b). Sporadic distal stenoses were visualized in the distal LAD and RCA which correlated with the CT scan. Sluggish flow was visualized in the large PDA aneurysm, but there was no evidence of thrombosis.
Fig. 4.
AP image performed using JL 4.0 catheter and a glide catheter. a Coronary angiogram of the left coronary artery showing multiple aneurysms in the left anterior descending artery and left circumflex artery. Mild stenosis of the mid third of the left anterior descending between 2 aneurysms, b coronary angiogram of the right coronary artery showing multiple aneurysms in the right coronary artery and the giant aneurysm in the PDA
Systemic anticoagulation with enoxaparin and aspirin was continued and treatment with cyclophosphamide was initiated following the catheterization. CT angiography of the head, neck, chest, abdomen and pelvis revealed multiple microaneurysms including in the carotid arteries, kidneys, left hepatic lobe and posterior wall of the stomach. Kidney biopsy showed pauci-immune crescentic glomerulonephritis consistent with his diagnosis of GPA. He had an isolated asymptomatic run of non-sustained ventricular tachycardia prior to discharge and was started on a beta blocker and was discharged home with a wearable cardioverter defibrillator (ZOLL Cardiac Diagnostics, Pittsburgh PA). Three months following hospital discharge, he remained asymptomatic and had begun evaluation for the possibility of cardiac transplantation.
Discussion
Pediatric coronary artery aneurysms are commonly associated with Kawasaki disease, Takayasu arteritis and, more recently, MIS-C. Only two known cases of pediatric GPA with coronary artery aneurysms have been reported in the literature, neither of which had coronary artery involvement as extensive as that reported here [6, 7]. In one series of 117 children with GPA, the only cardiovascular complication noted was venous thrombosis in three patients (2.6%) [4]. In another cohort of 183 children with GPA, venous thrombosis was again the only cardiovascular manifestation observed (n = 3, 1.6%) [5]. Since this degree of coronary involvement was not reported in either case series, it is difficult to estimate a true prevalence.
Cardiac manifestations occurred in 3–13% of adult patients with GPA [8, 9]. Common cardiac manifestations include pericarditis, cardiomyopathy, ischemic coronary artery disease, valvular disease and conduction disorders [8, 9]. There is no direct mention of coronary artery aneurysms in the adult literature. Adult patients with GPA are at increased risk of morbidity and mortality from ischemic heart disease [10]. Patients with GPA have an increased observed-to-expected ratio of acute myocardial infarction of 3.6 compared to the general population [10]. In children with Kawasaki disease, patients with giant aneurysms are at increased risk of thrombosis [11]. Postmortem, patients with giant aneurysms had chronic thrombus lining their vessels [11]. As there are no formal guidelines for the treatment of coronary artery aneurysms in GPA, the anticoagulation strategy used in our patient was based on guidelines for Kawasaki disease [12]. The long-term outcome of patients with large coronary artery aneurysms in GPA is not known. Given the extensive size and number of coronary aneurysms, the presence of patchy coronary artery stenosis, and the subsequent development of non-sustained ventricular tachycardia in this patient, he was referred for consideration of cardiac transplantation.
Apart from the unique presentation of severe coronary disease in a rare vasculitis, this case illustrates the importance of CT imaging and invasive angiography in the diagnosis and management of coronary aneurysms. Although echocardiography is often the initial, primary diagnostic imaging modality for the evaluation of coronary artery aneurysms in children, this may be insufficient in the setting of an uncooperative patients, poor sonographic windows, or aneurysms limited to the distal coronary segments. The limitations of echocardiography are acknowledged in the 2017 AHA Kawasaki guidelines, particular in children of larger size and older age [12]. The presence and extent of coronary artery stenosis or thrombosis is also challenging to evaluate with echocardiography alone. Although catheterization is not routinely suggested for patients with acute Kawasaki disease due to the risk of adverse events, there may be utility for invasive imaging when the CT angiogram findings are equivocal [13]. In our case, concern was raised from the CT scan for the possibility of thrombosis in the posterior descending aneurysm, necessitating further evaluation.
Conclusion
While rare, coronary artery aneurysms have been associated with pediatric GPA and may be extensive in both size and number. Advanced cardiac imaging should be considered in this setting to adequately assess the extent of coronary involvement.
Acknowledgements
No grants or other financial support were used in the preparation of this case report.
Author Contributions
All the authors have seen and approved of the final draft.
Declarations
Conflict of interest
There are no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Panupattanapong S, Stwalley DL, White AJ, Olsen MA, French AR, Hartman ME. Epidemiology and outcomes of granulomatosis with polyangiitis in pediatric and working-age adult populations in the United States: analysis of a large national claims database. Arthritis Rheumatol. 2018;70(12):2067–2076. doi: 10.1002/art.40577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohm M, Gonzalez Fernandez MI, Ozen S, et al. Clinical features of childhood granulomatosis with polyangiitis (Wegener's granulomatosis) Pediatr Rheumatol Online J. 2014;12:18. doi: 10.1186/1546-0096-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocco G, Gasparyan AY. Myocardial ischemia in Wegener's granulomatosis: coronary atherosclerosis versus vasculitis. Open Cardiovasc Med J. 2010;4:57–62. doi: 10.2174/1874192401004020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabral DA, Uribe AG, Benseler S, O'Neil KM, Hashkes PJ, Higgins G, Zeft AS, Lovell DJ, Kingsbury DJ, Stevens A, McCurdy D, Chira P, Abramson L, Arkachaisri T, Campillo S, Eberhard A, Hersh AO, Huber AM, Kim S, Klein-Gitelman M, Levy DM, Li SC, Mason T, Dewitt EM, Muscal E, Nassi L, Reiff A, Schikler K, Singer NG, Wahezi D, Woodward A, ARChiVe (A Registry for Childhood Vasculitis: e-entry) Investigators Network (2009) Classification, presentation, and initial treatment of Wegener's granulomatosis in childhood. Arthritis Rheum 60(11):3413–3424. 10.1002/art.24876 [DOI] [PubMed]
- 5.Cabral DA, Canter DL, Muscal E, Nanda K, Wahezi DM, Spalding SJ, Twilt M, Benseler SM, Campillo S, Charuvanij S, Dancey P, Eberhard BA, Elder ME, Hersh A, Higgins GC, Huber AM, Khubchandani R, Kim S, Klein-Gitelman M, Kostik MM, Lawson EF, Lee T, Lubieniecka JM, McCurdy D, Moorthy LN, Morishita KA, Nielsen SM, O'Neil KM, Reiff A, Ristic G, Robinson AB, Sarmiento A, Shenoi S, Toth MB, Van Mater HA, Wagner-Weiner L, Weiss JE, White AJ, Yeung RS, ARChiVe Investigators Network within the PedVas Initiative (2016) Comparing presenting clinical features in 48 children with microscopic polyangiitis to 183 children who have granulomatosis with polyangiitis (Wegener's): an ARChiVe Cohort Study. Arthritis Rheumatol 68(10):2514–2526. 10.1002/art.39729 [DOI] [PubMed]
- 6.Rehani C, Nelson JS. Coronary artery aneurysms as a feature of granulomatosis with polyangiitis. Pediatrics. 2021;147(1):e20200932. doi: 10.1542/peds.2020-0932. [DOI] [PubMed] [Google Scholar]
- 7.Aghaei Moghadam E, Aslani N, Mojtabavi H, Larti F, Ghamari A, Ziaee V. Giant thrombosis at left anterior descending artery aneurysm in a 10-year old boy with granulomatosis with polyangiitis. Case Rep Cardiol. 2020;2020:3417910. doi: 10.1155/2020/3417910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGeoch L, Carette S, Cuthbertson D, et al. Cardiac involvement in granulomatosis with polyangiitis. J Rheumatol. 2015;42(7):1209–1212. doi: 10.3899/jrheum.141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P, French Vasculitis Study Group (FVSG) (2011) The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort, Medicine 90(1):19–27. 10.1097/MD.0b013e318205a4c6 [DOI] [PubMed]
- 10.Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum. 2009;60:1187–1192. doi: 10.1002/art.24386. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, Russo PA, Mierau GW, de Chadarévian JP, Perlman EJ, Trevenen C, Rotta AT, Kalelkar MB, Rowley AH. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE. 2012;7:e38998. doi: 10.1371/journal.pone.0038998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 13.Gurofsky RC, Sabharwal T, Manlhiot C, Redington AN, Benson LN, Chahal N, McCrindle BW. Arterial complications associated with cardiac catheterization in pediatric patients with a previous history of Kawasaki disease. Catheter Cardiovasc Interv. 2009;73(6):809–813. doi: 10.1002/ccd.21892. [DOI] [PubMed] [Google Scholar]




