Abstract
Background
Among patients with diabetic retinopathy (DR), no proof was available to confirm the prognostic significance of the neutrophil percentage‐to‐albumin ratio (NPAR). We hypothesized that NPAR plays a role in the incidence of DR in diabetic patients.
Methods
We extracted all diabetes mellitus (DM) data from the National Health and Nutrition Examination Survey (NHANES) database between 1999 and 2018, NPAR was expressed as neutrophil percentage/albumin. Multivariable logistic regression and generalized additive model were utilized for the purpose of examining the correction between NPAR levels and DR. Subgroup analysis of the associations between NPAR and DR was carried out to investigate if the impact of the NPAR varied among different subgroups.
Results
An aggregate of 5850 eligible participants were included in the present research. The relationship between NPAR levels and DR was positive linear. In the multivariate analysis, following the adjustment for confounders (gender, white blood cell, age, monocyte percent, red cell distribution width, eosinophils percent, bicarbonate, body mass index, iron, glucose, basophils percent, total bilirubin, creatinine, and chloride), higher NPAR was an independent risk factor for DR compared to lower NPAR (OR, 95% CI: 1.18, 1.00–1.39; 1.24, 1.04–1.48). For the purpose of sensitivity analysis, we found a trend of consistency (p for trend: 0.0190). The results of the subgroup analysis revealed that NPAR did not exert any statistically significant interactions with any of the other DR risk variables.
Conclusions
Elevated NPAR is associated with an elevated risk of occurrence of DR in diabetic patients.
Keywords: diabetes mellitus, diabetic retinopathy, inflammation, National Health and Nutrition Examination Survey, neutrophil percentage‐to‐albumin ratio
The relationship between neutrophil percentage‐to‐albumin ratio levels and occurrence of diabetic retinopathy was positive linear.
1. INTRODUCTION
Globally, diabetes mellitus (DM) afflicted approximately 415 million individuals in 2015, with the value anticipated to climb to 642 million by the year 2040. 1 With the growing incidence of diabetes and the increase in the population with diabetes having longer life expectancies, the number of people experiencing visual impairment and diabetic retinopathy (DR) as a result of this disease is growing on a global scale. 2 DR has been identified as the major contributor to visual impairment among the working‐age populace in the Western world. 3 Patients with diabetes or DR experience more functional physiological difficulties than those without diabetes, especially profound among those with severe DR. 4
Multiple studies have shown that diabetic control in patients with type 2 DM is associated with serum vitamin D levels, 5 uric acid to high‐density lipoprotein (HDL) cholesterol ratio, 6 and omentin levels. 7 And some of its complications are associated with inflammation, such as platelet‐to‐lymphocyte ratio, 8 neuregulin‐4, 9 and C‐reactive protein to serum albumin Ratio. 10 DR is caused by a variety of pathologic variables that can result in visual impairment, including proliferative vitreoretinopathy, intraocular neovascularization, as well as diabetic macular edema. 11 , 12 Microangiopathy and inflammation jointly perform an integral function in the pathogenic mechanism of DR. 13
The neutrophil percentage‐to‐albumin ratio (NPAR) is a viable biomarker for systemic infection and inflammation that has recently been discovered. According to the findings of several research reports, NPAR might be utilized as a prognostic factor for individuals with acute kidney damage, cardiogenic shock, severe sepsis, and cancer. 14 , 15 , 16 , 17 It is generally recognized that neutrophils perform critical functions in the cellular innate immunity. Prior research has indicated that elevated neutrophil expression levels in the early stages of sepsis were associated with greater severity of the condition. 18 , 19 Moreover, neutrophil‐derived inflammatory markers have been studied in and found to be associated with various inflammatory conditions such as inflammatory bowel disease, 20 irritable bowel disease, 21 diabetes mellitus, 22 atrial fibrillation, 23 thyroiditis, 24 and SARS‐Cov‐2 infection. 25 Albumin is a medium‐sized protein that constitutes the majority of the proteins found in human plasma. Albumin plays an essential role in a wide range of physiological processes. Moreover, it performs a wide range of functions in the body, such as acting as a significant buffer, antidote, immunomodulator, extracellular antioxidant, and transporter in the plasma. 26 , 27 The correlation between NPAR and DR, nevertheless, has received little attention to date. As a result, the purpose of the present research was to examine the function of NPAR in the prediction of DR in diabetic individuals.
2. METHODS
2.1. Data source
The National Health and Nutrition Examination Survey (NHANES) database provides a clustered, stratified, multistage, cross‐sectional probability sample comprising of a population of non‐institutionalized US civilians that is performed by the National Center for Health Statistics (NCHS), which is a branch of the Center for Disease Control and Prevention. The NHANES III survey was performed between 1988 and 1994, and the continuing NHANES survey was carried out between 1999 and 2020, with data published in 2‐year cycles. The NCHS institutional review board granted its approval for the methodology for conducting the NHANES and informed written consent was obtained from all subjects. The Ethics Review Board of the National Center for Health Statistics (NCHS ERB) granted its approval for the NHANES (NCHS IRB/ERB protocols #98‐12, #2005‐06, #2011–17, #2018‐01). Respondents in the NHANES undergo a health assessment at mobile examination centers after an in‐home interview. Participants’ physiological and clinical conditions are evaluated, followed by laboratory examinations. We extracted all DM data from NHANES 1999 to 2018. The exclusion criteria were as follows: participants under 18 years of age, no albumin or neutrophil percentage measured, and having more than 5% missing data.
2.2. Study variables
The extracted data included age, gender, marital status, neutrophil percentage, albumin, mean cell hemoglobin, total cholesterol, eosinophil percent, high‐density lipoprotein, body mass index (BMI), glucose, triglycerides, hematocrit, white blood cell (WBC), diastolic blood pressure (DBP), monocyte percent, glutamyl transpeptidase, systolic blood pressure (SBP), basophils percent, hemoglobin, lymphocyte percent, mean cell volume, red cell distribution width (RDW), platelet, red blood cell, alkaline phosphatase, blood urea nitrogen, globulin, total bilirubin, bicarbonate, uric acid, aspartate aminotransferase (AST), creatinine, sodium, potassium, chloride, phosphorus, total calcium, iron, alanine aminotransferase (ALT), hypertension, and diabetic retinopathy. NPAR was expressed as neutrophil percentage/albumin.
2.3. Statistical analysis
Distribution normality was initially tested through the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± SD or interquartile range (IQR) and medians. Categorical data were presented as percentages or frequencies. For the purpose of investigating whether there were any significant differences among various groups, the Kruskal–Wallis H, one‐way ANOVA, and Chi‐square tests were utilized. The linear correlation between NPAR and the incidence of DR was established with the aid of a generalized additive model. Moreover, a multivariate logistic regression model was conducted to analyze the correlation by identifying possible confounding variables; these findings were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
We integrated the prospective confounding parameters on the basis of epidemiologic and biologic backgrounds and selected only those with a shift in effect estimate of greater than 10% for the purpose of constructing an adjusted model. 28 Two multivariate models were constructed based on NPAR group inclusion according to tertiles. The initial tertile was employed as a point of reference throughout the study. The gender and age of the covariates were subjected to adjustment in model I. In model II, we subsequently adjusted for gender, age, white blood cell, monocyte percent, red cell distribution width, eosinophils percent, bicarbonate, basophils percent, body mass index, iron, glucose, total bilirubin, creatinine, and chloride.
Subgroup analysis of the correlation between NPAR and DR was carried out for the purpose of determining if the impact of the NPAR varied among subgroups. All probabilities were two‐sided and statistical significance was fixed at p < .05. All analyses of statistical data were carried out using the R software (version: 4.00).
3. RESULTS
3.1. Subject characteristics
We identified 5850 diabetic individuals who satisfied our participation requirements and conducted a study on them. The patients were classified into tertiles based on their NPAR scores. Totally, 2829 women, as well as 3021 men, fulfilled the criteria for participation, and 1301 patients underwent a diagnosis of DR (22.2%). Table 1 summarizes the baseline characteristics. Patients who had an elevated NPAR (NPAR ≥ 15.6 ml/g) were more likely to be elderly with a high incidence of DR. Participants with lower NPAR (NPAR < 13.3 ml/g) had higher values of DBP, mean cell hemoglobin, total cholesterol, mean cell volume, hematocrit, hemoglobin, red blood cell, basophil percent, eosinophil percent, monocyte percent, triglycerides, high‐density lipoprotein, lymphocyte percent, ALT, AST, total bilirubin, phosphorus, total calcium, and iron.
TABLE 1.
Characteristics of the study patients according to NPAR
| Characteristics | NPAR, ml/g | |||
|---|---|---|---|---|
| <13.3 (n = 1950) | ≥13.3, <15.6 (n = 1950) | ≥15.6 (n = 1950) | p value | |
| Age, years | 60.10 ± 13.70 | 62.05 ± 13.39 | 62.82 ± 13.92 | 0.022 |
| Gender, n (%) | 0.007 | |||
| Female | 942 (48.31) | 894 (45.85) | 993 (50.92) | |
| Male | 1008 (51.69) | 1056 (54.15) | 957 (49.08) | |
| Marital status, n (%) | 0.033 | |||
| Married | 1104 (56.62) | 1098 (56.31) | 1031 (52.87) | |
| Other | 846 (43.38) | 852 (43.69) | 919 (47.13) | |
| SBP, mmHg | 132.61 ± 20.14 | 133.26 ± 20.52 | 133.80 ± 22.34 | 0.296 |
| DBP, mmHg | 69.65 ± 14.37 | 68.36 ± 14.77 | 67.11 ± 15.31 | <0.001 |
| BMI, kg/m2 | 30.88 ± 6.43 | 31.69 ± 6.82 | 33.69 ± 8.58 | <0.001 |
| NPAR, ml/g | 11.62 ± 1.44 | 14.47 ± 0.65 | 17.71 ± 2.04 | <0.001 |
| Neutrophil percentage, % | 49.82 ± 6.91 | 60.20 ± 4.60 | 68.12 ± 6.06 | <0.001 |
| Albumin, g/dl | 4.29 ± 0.31 | 4.16 ± 0.29 | 3.87 ± 0.36 | <0.001 |
| Total cholesterol, mmol/L | 4.92 ± 1.21 | 4.80 ± 1.13 | 4.64 ± 1.17 | <0.001 |
| High‐density lipoprotein, mmol/L | 1.27 ± 0.38 | 1.24 ± 0.36 | 1.23 ± 0.36 | 0.003 |
| Triglycerides, mmol/L | 2.18 ± 1.91 | 2.17 ± 1.55 | 2.02 ± 1.57 | 0.035 |
| WBC, 109/L | 6.99 ± 2.35 | 7.49 ± 1.97 | 8.26 ± 2.39 | <0.001 |
| Lymphocyte percent, % | 37.63 ± 7.11 | 28.25 ± 4.92 | 21.55 ± 5.62 | <0.001 |
| Monocyte percent, % | 8.48 ± 2.48 | 7.88 ± 2.11 | 7.22 ± 2.10 | <0.001 |
| Eosinophil percent, % | 3.35 ± 2.50 | 3.01 ± 1.96 | 2.51 ± 1.61 | <0.001 |
| Basophil percent, % | 0.78 ± 0.50 | 0.72 ± 0.38 | 0.66 ± 0.42 | <0.001 |
| RBC, 109/L | 4.65 ± 0.52 | 4.62 ± 0.51 | 4.51 ± 0.57 | <0.001 |
| Hemoglobin, g/dl | 13.95 ± 1.47 | 13.85 ± 1.53 | 13.37 ± 1.74 | <0.001 |
| Hematocrit, % | 41.30 ± 4.19 | 41.04 ± 4.29 | 39.79 ± 4.94 | <0.001 |
| Mean cell volume, fL | 89.08 ± 5.64 | 89.07 ± 5.76 | 88.43 ± 6.32 | <0.001 |
| Mean cell hemoglobin, pg | 30.09 ± 2.30 | 30.06 ± 2.33 | 29.70 ± 2.55 | <0.001 |
| RDW, % | 13.34 ± 1.18 | 13.52 ± 1.44 | 13.99 ± 1.68 | <0.001 |
| Platelet, 109/L | 245.07 ± 68.92 | 242.42 ± 71.08 | 245.97 ± 78.37 | 0.351 |
| ALT, U/L | 26.98 ± 16.52 | 25.12 ± 24.00 | 24.04 ± 35.63 | <0.001 |
| AST U/L | 26.40 ± 13.80 | 24.96 ± 15.08 | 24.79 ± 27.63 | <0.001 |
| Alkaline phosphatase, U/L | 74.35 ± 31.30 | 76.75 ± 27.04 | 84.87 ± 41.10 | <0.001 |
| Blood urea nitrogen, mmol/L | 5.57 ± 2.43 | 6.05 ± 2.96 | 6.84 ± 3.95 | <0.001 |
| Globulin, g/L | 30.49 ± 5.03 | 30.41 ± 4.67 | 31.56 ± 5.62 | <0.001 |
| Total bilirubin, µmol/L | 10.83 ± 4.60 | 10.72 ± 4.92 | 10.61 ± 5.37 | <0.001 |
| Bicarbonate, mmol/L | 24.95 ± 2.45 | 24.89 ± 2.40 | 24.93 ± 2.73 | 0.621 |
| GGT, U/L | 37.60 ± 54.78 | 34.40 ± 44.13 | 37.43 ± 50.25 | <0.001 |
| Glucose, mmol/L | 8.03 ± 3.81 | 8.53 ± 4.14 | 9.14 ± 4.59 | <0.001 |
| Uric acid, µmol/L | 332.65 ± 87.59 | 336.81 ± 91.11 | 348.41 ± 105.54 | <0.001 |
| Creatinine, µmol/L | 84.23 ± 52.08 | 89.76 ± 66.57 | 104.20 ± 91.14 | <0.001 |
| Sodium, mmol/L | 138.94 ± 2.75 | 138.92 ± 2.82 | 138.79 ± 3.09 | 0.182 |
| Potassium, mmol/L | 4.06 ± 0.39 | 4.11 ± 0.39 | 4.15 ± 0.44 | <0.001 |
| Chloride, mmol/L | 102.25 ± 3.34 | 102.25 ± 3.57 | 102.18 ± 3.92 | 0.930 |
| Phosphorus, mmol/L | 1.21 ± 0.18 | 1.20 ± 0.19 | 1.19 ± 0.21 | <0.001 |
| Total Calcium, mmol/L | 2.39 ± 0.10 | 2.36 ± 0.10 | 2.32 ± 0.11 | <0.001 |
| Iron, µmol/L | 15.14 ± 5.58 | 14.06 ± 5.55 | 12.39 ± 5.48 | <0.001 |
| Hypertension, n (%) | 0.010 | |||
| No | 1278 (65.91) | 1298 (66.77) | 1384 (70.97) | |
| Yes | 661(34.09) | 646 (33.23) | 566 (29.03) | |
| Diabetic retinopathy, n (%) | <0.001 | |||
| No | 1579 (80.97) | 1512 (77.54) | 1458 (74.77) | |
| Yes | 371 (19.03) | 438 (22.46) | 492 (25.23) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; GGT, glutamyl transpeptidase; NPAR, neutrophil percentage‐to‐albumin ratio; RBC, red blood cell; RDW, red cell distribution width; SBP, systolic blood pressure; WBC, white blood cell.
3.2. Association between NPAR and DR
The relationship between NPAR levels and DR was positive linear (Figure 1). The correlation between NPAR and the prevalence of DR was determined with a logistic multivariate regression model (Table 2). The lower NPAR was used as a reference. In model I, after correcting gender and age, a greater NPAR was related to an elevated risk of DR. In model II, after accounting for confounding variables (gender, age, white blood cell, monocyte percent, red cell distribution width, eosinophils percent, bicarbonate, basophils percent, body mass index, iron, glucose, total bilirubin, creatinine, and chloride), higher NPAR remained an independent risk factor for DR compared to lower NPAR (OR, 95% CI: 1.18, 1.00–1.39; 1.24, 1.04–1.48). After conducting sensitivity analysis, we found a trend of consistency (P for trend: 0.0190).
FIGURE 1.
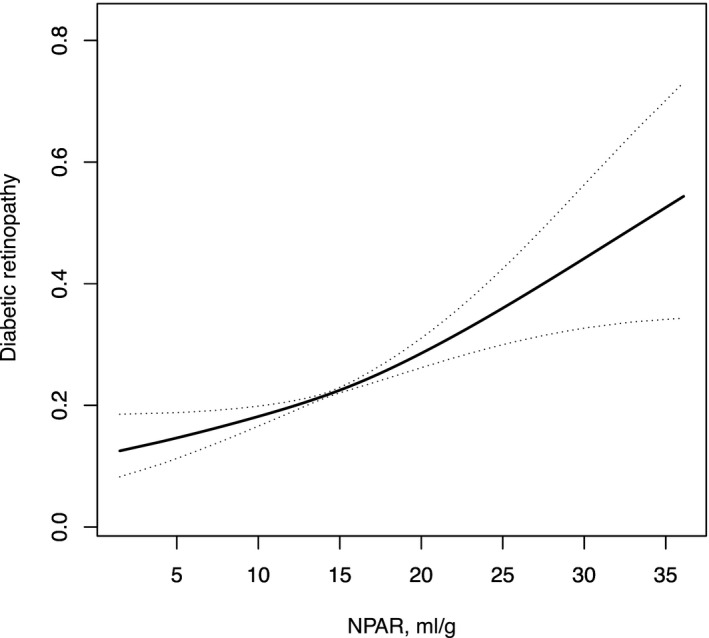
The relationship between NPAR and diabetic retinopathy
TABLE 2.
ORs (95% CIs) for diabetic retinopathy across groups of NPAR level
| RA level, ml/g | Non‐adjusted | Model I | Model II | |||
|---|---|---|---|---|---|---|
| OR (95%CIs) | p value | OR (95%CIs) | p value | OR (95%CIs) | p value | |
| NPAR, ml/g | 1.06 (1.04, 1.08) | <0.0001 | 1.06 (1.04, 1.08) | <0.0001 | 1.04 (1.01, 1.07) | 0.0046 |
| NPAR(Tertiles), ml/g | ||||||
| <13.3 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| ≥13.3, <15.6 | 1.23 (1.06, 1.44) | 0.0082 | 1.22 (1.05, 1.43) | 0.0111 | 1.18 (1.00, 1.39) | 0.0447 |
| ≥15.6 | 1.44 (1.23, 1.67) | <0.0001 | 1.42 (1.22, 1.66) | <0.0001 | 1.24 (1.04, 1.48) | 0.0183 |
| p trend | <0.0001 | <0.0001 | 0.0190 | |||
Models were derived from logistic multivariate regression models. Non‐adjusted model adjusted for: none. Adjust I model adjusted for: age and gender. Adjust II model adjusted for: age, gender, white blood cell, monocyte percent, red cell distribution width, eosinophils percent, bicarbonate, basophils percent, body mass index, iron, glucose, total bilirubin, creatinine, and chloride.
Abbreviations: CI, confidence interval; OR, odds ratio.
3.3. Subgroup analyses
Table 3 shows the results of a subgroup analysis of the correlation between NPAR and the risk of DR, indicating that there was no interplay in these strata (p = .0563–0.9447). Moreover, no statistically significant interactions were discovered between NPAR and any of the other risk variables for DR.
TABLE 3.
Subgroup analysis of the associations between NPAR and diabetic retinopathy
| NPAR, ml/g | p for interaction | |||
|---|---|---|---|---|
| <13.3 | ≥13.3, <15.6 | ≥15.6 | ||
| Age, years | ||||
| <63 | 1.0 (ref) | 1.28 (1.02, 1.60) 0.0304 | 1.53 (1.22, 1.90) 0.0002 | 0.2336 |
| ≥63 | 1.0 (ref) | 1.18 (0.95, 1.47) 0.1327 | 1.35 (1.09, 1.67) 0.0056 | |
| Gender | ||||
| Female | 1.0 (ref) | 1.29 (1.03, 1.62) 0.0286 | 1.49 (1.20, 1.86) 0.0003 | 0.9447 |
| Male | 1.0 (ref) | 1.18 (0.96, 1.46) 0.1216 | 1.39 (1.12, 1.72) 0.0023 | |
| Marital status | ||||
| Married | 1.0 (ref) | 1.18 (0.95, 1.45) 0.1304 | 1.54 (1.25, 1.89) <0.0001 | 0.1742 |
| Other | 1.0(ref) | 1.30 (1.03, 1.64) 0.0243 | 1.32 (1.06, 1.66) 0.0153 | |
| SBP, mmHg | ||||
| <130 | 1.0 (ref) | 1.30 (1.02, 1.65) 0.0338 | 1.29 (1.01, 1.64) 0.0421 | 0.7777 |
| ≥130 | 1.0 (ref) | 1.10 (0.88, 1.38) 0.4058 | 1.46 (1.17, 1.82) 0.0008 | |
| DBP, mmHg | ||||
| <70 | 1.0 (ref) | 1.18 (0.94, 1.50) 0.1593 | 1.34 (1.07, 1.68) 0.0121 | 0.2420 |
| ≥70 | 1.0 (ref) | 1.18 (0.93, 1.49) 0.1745 | 1.39 (1.10, 1.75) 0.0065 | |
| BMI, kg/m2 | ||||
| <30.8 | 1.0 (ref) | 1.13 (0.91, 1.39) 0.2770 | 1.28 (1.03, 1.60) 0.0290 | 0.1261 |
| ≥30.8 | 1.0 (ref) | 1.40 (1.11, 1.77) 0.0051 | 1.55 (1.24, 1.95) 0.0001 | |
| Neutrophil percentage, % | ||||
| <59.9 | 1.0 (ref) | 1.37 (1.13, 1.65) 0.0013 | 2.06 (1.42, 2.99) 0.0001 | 0.4760 |
| ≥59.9 | 1.0 (ref) | 1.52 (0.76, 3.02) 0.2353 | 1.90 (0.96, 3.74) 0.0649 | |
| Albumin, g/dl | ||||
| <4.1 | 1.0 (ref) | 1.22 (0.91, 1.63) 0.1831 | 1.31 (1.00, 1.70) 0.0461 | 0.1271 |
| ≥4.1 | 1.0(ref) | 1.17 (0.97, 1.41) 0.1056 | 1.11 (0.87, 1.41) 0.3959 | |
| Total cholesterol, mmol/L | ||||
| <4.65 | 1.0 (ref) | 1.33 (1.06, 1.68) 0.0149 | 1.72 (1.38, 2.14) <0.0001 | 0.2599 |
| ≥4.65 | 1.0 (ref) | 1.16 (0.94, 1.44) 0.1602 | 1.19 (0.96, 1.48) 0.1120 | |
| High‐density lipoprotein, mmol/L | ||||
| <1.19 | 1.0(ref) | 1.20 (0.96, 1.51) 0.1049 | 1.50 (1.20, 1.86) 0.0003 | 0.6471 |
| ≥1.19 | 1.0 (ref) | 1.26 (1.01, 1.56) 0.0371 | 1.38 (1.11, 1.71) 0.0032 | |
| Triglycerides, mmol/L | ||||
| <1.705 | 1.0 (ref) | 1.25 (0.99, 1.56) 0.0562 | 1.45 (1.16, 1.80) 0.0010 | 0.6652 |
| ≥1.705 | 1.0 (ref) | 1.22 (0.99, 1.51) 0.0673 | 1.44 (1.16, 1.78) 0.0008 | |
| WBC, 109/L | ||||
| <7.3 | 1.0 (ref) | 1.36 (1.10, 1.67) 0.0041 | 1.57 (1.26, 1.96) <0.0001 | 0.0579 |
| ≥7.3 | 1.0 (ref) | 1.11 (0.88, 1.40) 0.3966 | 1.33 (1.06, 1.66) 0.0120 | |
| Lymphocyte percent, % | ||||
| <28.6 | 1.0 (ref) | 1.58 (0.96, 2.59) 0.0724 | 2.05 (1.26, 3.33) 0.0038 | 0.9058 |
| ≥28.6 | 1.0 (ref) | 1.33 (1.10, 1.60) 0.0034 | 1.25 (0.86, 1.81) 0.2397 | |
| Monocyte percent, % | ||||
| <7.6 | 1.0 (ref) | 1.32 (1.04, 1.69) 0.0245 | 1.43 (1.13, 1.80) 0.0027 | 0.9010 |
| ≥7.6 | 1.0 (ref) | 1.19 (0.97, 1.46) 0.0963 | 1.55 (1.26, 1.91) <0.0001 | |
| Eosinophils percent, % | ||||
| <2.5 | 1.0 (ref) | 1.35 (1.07, 1.72) 0.0131 | 1.56 (1.24, 1.96) 0.0001 | 0.5373 |
| ≥2.5 | 1.0 (ref) | 1.17 (0.95, 1.43) 0.1378 | 1.41 (1.14, 1.74) 0.0015 | |
| Basophils percent, % | ||||
| <0.6 | 1.0 (ref) | 1.31 (0.98, 1.76) 0.0691 | 1.64 (1.25, 2.17) 0.0004 | 0.3229 |
| ≥0.6 | 1.0 (ref) | 1.22 (1.01, 1.46) 0.0369 | 1.38 (1.14, 1.66) 0.0007 | |
| RBC, 109/L | ||||
| <4.6 | 1.0 (ref) | 1.27 (1.02, 1.58) 0.0330 | 1.57 (1.27, 1.93) <0.0001 | 0.6137 |
| ≥4.6 | 1.0 (ref) | 1.18 (0.95, 1.47) 0.1347 | 1.23 (0.98, 1.54) 0.0768 | |
| Hemoglobin, g/dl | ||||
| <13.8 | 1.0 (ref) | 1.29 (1.04, 1.61) 0.0221 | 1.40 (1.14, 1.72) 0.0015 | 0.1221 |
| ≥13.8 | 1.0 (ref) | 1.16 (0.93, 1.45) 0.1865 | 1.36 (1.08, 1.71) 0.0086 | |
| Hematocrit, % | ||||
| <40.8 | 1.0 (ref) | 1.28 (1.02, 1.59) 0.0299 | 1.49 (1.21, 1.83) 0.0002 | 0.3860 |
| ≥40.8 | 1.0 (ref) | 1.18 (0.95, 1.47) 0.1282 | 1.28 (1.01, 1.60) 0.0373 | |
| Mean cell volume, fL | ||||
| <89.3 | 1.0 (ref) | 1.24 (0.99, 1.55) 0.0578 | 1.33 (1.07, 1.66) 0.0090 | 0.8333 |
| ≥89.3 | 1.0(ref) | 1.22 (0.99, 1.52) 0.0670 | 1.55 (1.25, 1.92) <0.0001 | |
| Mean cell hemoglobin, pg | ||||
| <30.2 | 1.0 (ref) | 1.30 (1.04, 1.63) 0.0229 | 1.49 (1.20, 1.85) 0.0003 | 0.6937 |
| ≥30.2 | 1.0 (ref) | 1.17 (0.95, 1.45) 0.1421 | 1.38 (1.11, 1.71) 0.0036 | |
| RDW, % | ||||
| <13.3 | 1.0 (ref) | 1.23 (0.99, 1.53) 0.0633 | 1.49 (1.19, 1.88) 0.0005 | 0.2395 |
| ≥13.3 | 1.0 (ref) | 1.23 (0.99, 1.54) 0.0640 | 1.39 (1.13, 1.72) 0.0020 | |
| Platelet, 109/L | ||||
| <236 | 1.0 (ref) | 1.28 (1.02, 1.60) 0.0300 | 1.60 (1.29, 1.98) <0.0001 | 0.0872 |
| ≥236 | 1.0 (ref) | 1.19 (0.96, 1.48) 0.1141 | 1.29 (1.04, 1.60) 0.0182 | |
| ALT, U/L | ||||
| <21 | 1.0 (ref) | 1.17 (0.93, 1.47) 0.1760 | 1.35 (1.09, 1.68) 0.0062 | 0.2653 |
| ≥21 | 1.0 (ref) | 1.27 (1.03, 1.58) 0.0260 | 1.46 (1.17, 1.81) 0.0007 | |
| AST U/L | ||||
| <22 | 1.0 (ref) | 1.16 (0.91, 1.47) 0.2238 | 1.44 (1.15, 1.81) 0.0013 | 0.4812 |
| ≥22 | 1.0 (ref) | 1.30 (1.05, 1.59) 0.0139 | 1.38 (1.12, 1.71) 0.0029 | |
| Alkaline phosphatase, U/L | ||||
| <73 | 1.0 (ref) | 1.30 (1.03, 1.64) 0.0300 | 1.48 (1.16, 1.89) 0.0015 | 0.4791 |
| ≥73 | 1.0 (ref) | 1.20 (0.95, 1.50) 0.1199 | 1.38 (1.11, 1.71) 0.0035 | |
| Blood urea nitrogen, mmol/L | ||||
| <5.36 | 1.0 (ref) | 1.24 (0.97, 1.58) 0.0801 | 1.37 (1.07, 1.76) 0.0120 | 0.3154 |
| ≥5.36 | 1.0 (ref) | 1.17 (0.96, 1.43) 0.1291 | 1.36 (1.12, 1.65) 0.0023 | |
| Globulin, g/L | ||||
| <30 | 1.0 (ref) | 1.20 (0.94, 1.53) 0.1519 | 1.62 (1.27, 2.07) 0.0001 | 0.3020 |
| ≥30 | 1.0 (ref) | 1.26 (1.03, 1.54) 0.0241 | 1.30 (1.07, 1.59) 0.0075 | |
| Total bilirubin, µmol/L | ||||
| <10.26 | 1.0 (ref) | 1.14 (0.90, 1.44) 0.2631 | 1.33 (1.06, 1.67) 0.0127 | 0.7992 |
| ≥10.26 | 1.0 (ref) | 1.29 (1.05, 1.59) 0.0148 | 1.50 (1.22, 1.84) 0.0001 | |
| Bicarbonate, mmol/L | ||||
| <25 | 1.0 (ref) | 1.16 (0.91, 1.47) 0.2275 | 1.29 (1.02, 1.63) 0.0322 | 0.6075 |
| ≥25 | 1.0 (ref) | 1.30 (1.06, 1.59) 0.0132 | 1.55 (1.26, 1.89) <0.0001 | |
| GGT, U/L | ||||
| <24 | 1.0(ref) | 1.14 (0.91, 1.43) 0.2481 | 1.51 (1.21, 1.88) 0.0002 | 0.7339 |
| ≥24 | 1.0(ref) | 1.33 (1.08, 1.65) 0.0080 | 1.36 (1.10, 1.69) 0.0041 | |
| Glucose, mmol/L | ||||
| <7.33 | 1.0 (ref) | 1.17 (0.93, 1.47) 0.1749 | 1.28 (1.02, 1.61) 0.0317 | 0.6292 |
| ≥7.33 | 1.0 (ref) | 1.24 (1.00, 1.54) 0.0481 | 1.47 (1.19, 1.81) 0.0003 | |
| Uric acid, µmol/L | ||||
| <327.1 | 1.0 (ref) | 1.14 (0.91, 1.42) 0.2624 | 1.30 (1.05, 1.63) 0.0187 | 0.2540 |
| ≥327.1 | 1.0 (ref) | 1.33 (1.07, 1.65) 0.0103 | 1.56 (1.26, 1.92) <0.0001 | |
| Creatinine, µmol/L | ||||
| <79.56 | 1.0 (ref) | 1.24 (0.99, 1.55) 0.0605 | 1.19 (0.94, 1.50) 0.1532 | 0.0905 |
| ≥79.56 | 1.0 (ref) | 1.22 (0.98, 1.52) 0.0700 | 1.57 (1.28, 1.93) <0.0001 | |
| Sodium, mmol/L | ||||
| <139 | 1.0 (ref) | 1.31 (1.03, 1.65) 0.0260 | 1.40 (1.11, 1.76) 0.0044 | 0.4889 |
| ≥139 | 1.0 (ref) | 1.17 (0.95, 1.44) 0.1340 | 1.45 (1.19, 1.78) 0.0003 | |
| Potassium, mmol/L | ||||
| <4.1 | 1.0 (ref) | 1.30 (1.03, 1.63) 0.0248 | 1.37 (1.09, 1.72) 0.0074 | 0.3424 |
| ≥4.1 | 1.0 (ref) | 1.15 (0.93, 1.43) 0.1893 | 1.43 (1.16, 1.76) 0.0007 | |
| Chloride, mmol/L | ||||
| <102.2 | 1.0 (ref) | 1.29 (1.04, 1.59) 0.0196 | 1.34 (1.09, 1.66) 0.0060 | 0.0563 |
| ≥102.2 | 1.0 (ref) | 1.16 (0.93, 1.46) 0.1889 | 1.54 (1.23, 1.92) 0.0001 | |
| Phosphorus, mmol/L | ||||
| <1.19 | 1.0 (ref) | 1.08 (0.86, 1.36) 0.4882 | 1.31 (1.05, 1.63) 0.0158 | 0.3220 |
| ≥1.19 | 1.0 (ref) | 1.38 (1.11, 1.70) 0.0031 | 1.56 (1.27, 1.93) <0.0001 | |
| Total Calcium, mmol/L | ||||
| <2.35 | 1.0 (ref) | 1.37 (1.05, 1.78) 0.0193 | 1.70 (1.33, 2.16) <0.0001 | 0.2675 |
| ≥2.35 | 1.0 (ref) | 1.16 (0.96, 1.41) 0.1300 | 1.20 (0.97, 1.48) 0.0979 | |
| Iron, µmol/L | ||||
| <13.1 | 1.0 (ref) | 1.10 (0.88, 1.38) 0.4161 | 1.28 (1.04, 1.59) 0.0221 | 0.7793 |
| ≥13.1 | 1.0 (ref) | 1.33 (1.07, 1.65) 0.0087 | 1.49 (1.19, 1.87) 0.0005 | |
| Hypertension | ||||
| No | 1.0 (ref) | 1.20 (1.00, 1.45) 0.0541 | 1.44 (1.20, 1.73) <0.0001 | 0.0881 |
| Yes | 1.0 (ref) | 1.29 (0.97, 1.71) 0.0762 | 1.34 (1.00, 1.79) 0.0496 | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; GGT, glutamyl transpeptidase; RBC, red blood cell; RDW, red cell distribution width; SBP, systolic blood pressure; WBC, white blood cell.
4. DISCUSSION
A positive linear correlation was observed between NPAR and the risk of incidence of DR. Elevated NPAR levels were found to be correlated with an elevated incidence of DR in the fully adjusted model among diabetic patients. Furthermore, no statistically significant interactions between NPAR and any of the other potential risk factors were observed, indicating that no additional factors had been discovered that could modify the correlation between NPAR and the risk of incidence of DR. As far as we know, this is the first study to highlight the significant correlation between NPAR and DR in diabetic patients.
NPAR was discovered to be a new indicator for systemic infection and inflammation in humans. 14 , 29 The elevated NPAR levels are caused by an increase in neutrophil percentage and/or a reduction in albumin concentrations. Our findings were in line with those of other research reports that examined the prognostic significance of NPARs in various clinical scenarios, such as cardiogenic shock, 15 acute kidney injury, 16 myocardial infarction, 30 and rectal cancer. 31 Inflammation appears to be a significant factor contributing to the occurrence and progression of DR, according to several research reports. 32 , 33 Diabetic patients with DR have elevated levels of several inflammatory chemokines and cytokines in their blood as well as their ocular samples (aqueous and vitreous humor).
Patients with DR and animal models have been shown to exhibit a variety of inflammation‐related characteristics, including tissue edema, enhanced vascular permeability, elevated blood flow, up‐modulation of cytokines, activation of complement and microglial, infiltration of neutrophils and macrophages, and leukostasis. 34 , 35 , 36 Notably, the elevation in these inflammatory factors that are produced by microglia, endothelial cells, macroglia, and later even neurons indicates dramatic increases in the activities of these inflammatory markers in the early stage of DR and the progression of inflammation across all the cell types of the retina. 37 , 38 Some of the cytokines identified, such as interleukin (IL)‐1, IL‐3, and monocyte chemoattractant protein‐1 (MCP‐1) are reported to be involved in angiogenesis, as demonstrated in experimental ischemic mouse models demonstrating that inflammatory responses lead to and predate the progression of neovascularization in proliferative DR. 39 , 40 Moreover, it has been proven that blocking or deleting pro‐inflammatory markers can inhibit the progression of diabetes‐elicited vascular and neuronal pathology in animal models of the DR. 41 , 42
According to the aforementioned results, we hypothesized that NPAR, the blending of albumin and neutrophils, has a high prognostic significance in the progression of DR. NPAR is simplistic, inexpensive, and rapid, which makes it a potential indicator that may be used even in undeveloped medical areas. This indicator facilitates a timely and individualized assessment of the risk of DR in each diabetic patient, which enables more precise decisions on treatment strategies and medical resource allocation. Notably, NPAR increases the prognostic significance of albumin and neutrophil percentage, particularly when those two parameters do not depart remarkably from the normal range, which is something that clinicians frequently ignore when evaluating patients. According to the findings, the NPAR predicts the incidence of DR by the mechanism of combining the distinct processes of albumin levels and neutrophil percentage.
Nevertheless, the present research has several drawbacks. Owing to the cross‐sectional research design, it is impossible to determine if there is a causal relationship. In order to prove causation, prospective studies are required. In addition, the information utilized in the present research was obtained from a single blood test. Since blood cells have a relatively short life span, serial testing might be more feasible as opposed to a single test performed upon admission. Moreover, the depletion of albumin and neutrophils is common, resulting in selection bias.
5. CONCLUSIONS
In diabetic individuals, we revealed that elevated NPAR is correlated with a higher risk of suffering from DR. Nevertheless, these findings need to be validated by prospective multicenter studies.
CONFLICT OF INTEREST
The authors report no conflicts of interest for this work.
AUTHOR CONTRIBUTIONS
XJH and FFD designed the study and collected, analyzed and interpreted the data. XZ collected and analyzed data and drafted the manuscript. JDP designed and supervised the study, obtained funding, and drafted the manuscript. All authors read and approved the final manuscript.
INFORMED CONSENT
The protocols for conduct of NHANES were approved by the NCHS institutional review board and all participants provided informed consent.
He X, Dai F, Zhang X, Pan J. The neutrophil percentage‐to‐albumin ratio is related to the occurrence of diabetic retinopathy. J Clin Lab Anal. 2022;36:e24334. doi: 10.1002/jcla.24334
He and Dai contributed equally to the work.
Funding information
This research was supported by the Scientific Research Foundation of Wenzhou (Grant No. Y20170773)
DATA AVAILABILITY STATEMENT
The data used in the present research were obtained from publicly accessible sources. These data, according to the authors, could be accessible at the following URL: https://www.cdc.gov/nchs/nhanes/.
REFERENCES
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 2. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260‐277. [DOI] [PubMed] [Google Scholar]
- 3. Lin S, Gupta B, James N, Ling RH. Visual impairment certification due to diabetic retinopathy in North and Eastern Devon. Acta Ophthalmol. 2017;95(8):e756‐e762. [DOI] [PubMed] [Google Scholar]
- 4. Nagda D, Mitchell W, Zebardast N. The functional burden of diabetic retinopathy in the United States. Graefe's Archiv Clin Exp Ophthalmol. 2021;259(10):2977‐2986. [DOI] [PubMed] [Google Scholar]
- 5. Erkus E, Aktas G, Kocak MZ, Duman TT, Atak BM, Savli H. Diabetic regulation of subjects with type 2 diabetes mellitus is associated with serum vitamin D levels. Rev Assoc Med Bras. 2019;65(1):51‐55. [DOI] [PubMed] [Google Scholar]
- 6. Aktas G, Kocak MZ, Bilgin S, Atak BM, Duman TT, Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098‐1102. [DOI] [PubMed] [Google Scholar]
- 7. Aktas G, Alcelik A, Ozlu T, et al. Association between omentin levels and insulin resistance in pregnancy. Exp Clin Endocrinol Diabet. 2014;122(3):163‐166. [DOI] [PubMed] [Google Scholar]
- 8. Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet‐to‐lymphocyte ratio in hemograms. Rev Assoc Med Bras. 2019;65(1):38‐42. [DOI] [PubMed] [Google Scholar]
- 9. Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin‐4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50(3):e13206. [DOI] [PubMed] [Google Scholar]
- 10. Bilgin S, Kurtkulagi O, Atak Tel BM, et al. Does C‐reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabet. 2021;15(6):1071‐1074. [DOI] [PubMed] [Google Scholar]
- 11. Kutlutürk Karagöz I, Allahverdiyev A, Bağırova M, Abamor E, Dinparvar S. Current approaches in treatment of diabetic retinopathy and future perspectives. J Ocular Pharmacol Therapeut. 2020;36(7):487‐496. [DOI] [PubMed] [Google Scholar]
- 12. Lechner J, O'Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7‐14. [DOI] [PubMed] [Google Scholar]
- 13. Tan GS, Cheung N, Simó R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabet Endocrinol. 2017;5(2):143‐155. [DOI] [PubMed] [Google Scholar]
- 14. Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage‐to‐albumin ratio is associated with all‐cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. 2020;148:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Y, Liu Y, Ling X, et al. The neutrophil percentage‐to‐albumin ratio as a new predictor of all‐cause mortality in patients with cardiogenic shock. Biomed Res Int. 2020;2020:7458451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang B, Li D, Cheng B, Ying B, Gong Y. The neutrophil percentage‐to‐albumin ratio is associated with all‐cause mortality in critically ill patients with acute kidney injury. Biomed Res Int. 2020;2020:5687672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tingle SJ, Severs GR, Goodfellow M, Moir JA, White SA. NARCA: A novel prognostic scoring system using neutrophil‐albumin ratio and Ca19‐9 to predict overall survival in palliative pancreatic cancer. J Surg Oncol. 2018;118(4):680‐686. [DOI] [PubMed] [Google Scholar]
- 18. Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21(9):1687‐1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park I, Kim M, Choe K, et al. Neutrophils disturb pulmonary microcirculation in sepsis‐induced acute lung injury. Eur Resp J. 2019;53(3):1800786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil‐to‐lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127(7–8):262‐265. [DOI] [PubMed] [Google Scholar]
- 21. Basaran E, Aktas G, Taslamacıoğlu Duman T, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Family Med Prim Care Rev. 2020;22:107‐110. [Google Scholar]
- 22. Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E, Savli H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19(1):1602‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahin S, Sarikaya S, Alcelik A, et al. Neutrophil to lymphocyte ratio is a useful predictor of atrial fibrillation in patients with diabetes mellitus. Acta Medica Mediterranea. 2013;29:847‐851. [Google Scholar]
- 24. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil‐to‐lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras. 2017;63(12):1065‐1068. [DOI] [PubMed] [Google Scholar]
- 25. Aktas G. Hematological predictors of novel Coronavirus infection. Rev Assoc Med Bras. 2021;67(Suppl 1):1‐2. [DOI] [PubMed] [Google Scholar]
- 26. Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62‐70. [DOI] [PubMed] [Google Scholar]
- 27. Ha CE, Bhagavan NV. Novel insights into the pleiotropic effects of human serum albumin in health and disease. Biochem Biophys Acta. 2013;1830(12):5486‐5493. [DOI] [PubMed] [Google Scholar]
- 28. Agoritsas T, Merglen A, Shah ND, O'Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: Users’ guides to the medical literature. JAMA. 2017;317(7):748‐759. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Wu T, Tian X, Lyu P, Wang J, Cao Y. High Neutrophil percentage‐to‐albumin ratio can predict occurrence of stroke‐associated infection. Front Neurol. 2021;12:705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui H, Ding X, Li W, Chen H, Li H. The neutrophil percentage to albumin ratio as a new predictor of in‐hospital mortality in patients with ST‐segment elevation myocardial infarction. Med Sci Monitor. 2019;25:7845‐7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tawfik B, Mokdad AA, Patel PM, Li HC, Huerta S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879‐883. [DOI] [PubMed] [Google Scholar]
- 32. Atlı H, Onalan E, Yakar B, Duzenci D, Dönder E. Predictive value of inflammatory and hematological data in diabetic and non‐diabetic retinopathy. Eur Rev Med Pharmacol Sci. 2022;26(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 33. Crespo‐Garcia S, Reichhart N, Kociok N, Skosyrski S, Joussen AM. Anti‐Inflammatory Role of Netrin‐4 in Diabetic Retinopathy. Int J Mol Sci. 2021;22(9):4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Proteome analysis of retinal glia cells‐related inflammatory cytokines in the aqueous humour of diabetic patients. Acta Ophthalmol. 2016;94(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 35. Xiao H, Xin W, Sun LM, Li SS, Zhang T, Ding XY. Comprehensive proteomic profiling of aqueous humor proteins in proliferative diabetic retinopathy. Transl Vision Sci Technol. 2021;10(6):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lessieur EM, Liu H, Saadane A, et al. Neutrophil‐Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2021;62(13):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu H, Hwang DK, Song X, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol. 2017;2017:9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rübsam A, Parikh S, Fort PE. Role of Inflammation in Diabetic Retinopathy. Int J Mol Sci. 2018;19(4):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochemia Medica. 2020;30(3):30502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ninomiya H, Katakami N, Osonoi T, et al. Association between new onset diabetic retinopathy and monocyte chemoattractant protein‐1 (MCP‐1) polymorphism in Japanese type 2 diabetes. Diabetes Res Clin Pract. 2015;108(3):e35‐37. [DOI] [PubMed] [Google Scholar]
- 41. Cardona SM, Mendiola AS, Yang YC, Adkins SL, Torres V, Cardona AE. Disruption of fractalkine signaling leads to microglial activation and neuronal damage in the diabetic retina. ASN Neuro. 2015;7(5):1759091415608204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vallejo S, Palacios E, Romacho T, Villalobos L, Peiró C, Sánchez‐Ferrer CF. The interleukin‐1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin‐induced diabetic rats. Cardiovasc Diabetol. 2014;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the present research were obtained from publicly accessible sources. These data, according to the authors, could be accessible at the following URL: https://www.cdc.gov/nchs/nhanes/.


