Abstract
Introduction
Coronavirus disease 2019 (COVID‐19) and acquired immune deficiency syndrome (AIDS) are two viral diseases for which there are currently no definitive treatments. Nowadays, because of the health system's focus on the COVID‐19 epidemic, the control of human immunodeficiency virus (HIV) has received less attention. In this review, we will discuss the characteristics of COVID‐19 in HIV‐positive patients.
Material and Methods
Using the PRISMA guideline, the databases of Scopus, PubMed, and Web of Science were searched systematically from January 1, 2019 to February 24, 2021. The following keywords were used: “Human Immunodeficiency Virus,” “acquired immune deficiency syndrome,” “HIV,” “AIDS,” “COVID‐19,” “severe acute respiratory syndrome coronavirus 2,” “novel coronavirus,” “SARS‐CoV‐2,” “nCoV disease,” “SARS2,” and “2019‐nCoV disease.”
Results
Twenty‐one percent of studies were conducted in the USA (n = 13), 16% in China (n = 10), and 13% in Italy (n = 8), respectively. The majority of the patients were men (74.3%). Tenofovir disoproxil fumarate was used in 47.4% of patients, emtricitabine in 58.4%, and lamivudine in 34.8% to treat HIV. Symptoms of HIV patients with COVID‐19 included coughing (81.3%), fever (62.8%), and dyspnea (60%). Hydroxychloroquine (39.34%) and azithromycin (36.58%) were the common treatment options for COVID‐19. The total death rate in HIV‐positive patients with COVID‐19 was about 9%.
Conclusion
In the current systematic review, we demonstrated that HIV‐positive patients co‐infected with COVID‐19 have high comorbidity of hypertension and diabetes mellitus. HIV/COVID‐19 co‐infection might have negatively influenced the HIV treatment and diagnosis, which indicates the need to regularly screen HIV patients in the COVID‐19 pandemic.
Keywords: co‐infection, COVID‐19, HIV, review
Outcomes of patients with HIV and COVID‐19 co‐infection.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel coronavirus, was emerged in December 2019 and COVID‐19, as its associated infection was declared as an epidemic in Wuhan, China, which turned into a pandemic in March 2020. 1 Until August 12, 2021, more than 204 million confirmed positive cases and 4.3 million deaths have been reported worldwide. 2
In case of new and emerging diseases, comorbidities are of special interest. It is estimated that 22% of the world's population has at least one disease that increases the risk of severe coronavirus disease 2019 (COVID‐19). 3 According to the Centers for Disease Control and Prevention (CDC) report, people living with human immunodeficiency virus syndrome (PLWH) may be at increased risks for COVID‐19‐related complications and death. 2 Concerns about the increased risks of severe COVID‐19 may be related to the immunosuppressive nature of HIV syndrome which makes people more susceptible to infections. 4 There is increasing evidence that PLWH with a low CD4+ T‐cell count and those who do not receive antiretroviral therapy (ART) are at a higher risk of severe COVID‐19 symptoms, 5 , 6 even though patients with low CD4+ T‐cell count may be more protected against cytokine storming. 7 One study has highlighted the possible protective effects of lymphopenia among PLWH against COVID‐19. 8 Another study has shown that patients with low CD4+ T‐cell counts have a longer course of COVID‐19 and a lower antibody levels. 9 Other risk factors such as age, sex, lung, and kidney diseases might affect the severity of COVID‐19 among PLWH. 10
Another factor that may affect the severity of COVID‐19 is the use of ART in PLWHs. ART was proposed in 2003 as a protective factor against SARS. 11 It can be assumed that long‐term ART may increase the risk of COVID‐19 severity,however, due to the small number of the studied cases, variable reports and insufficient data on PLWH co‐infected with COVID‐19, no certain conclusions have been obtained so far. 12
Therefore, this systematic review was conducted to gather up the current information regarding various risk factors such as age and immune status among PLWH co‐infected with COVID‐19, as well as different antiretroviral therapies and their impacts on the disease outcome among these patients.
2. METHODS
The current study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 13
2.1. Information source and search strategies
The databases Scopus, MEDLINE (via PubMed), and Web of Science were systematically searched from January 1, 2019 to February 24, 2021 to retrieve case series and case reports published in English. The search terms included “Human Immunodeficiency Virus,” “acquired immune deficiency syndrome,” “HIV,” “AIDS,” “COVID‐19,” “severe acute respiratory syndrome coronavirus 2,” “novel coronavirus,” “SARS‐CoV‐2,” “nCoV disease,” “SARS2,” and “2019‐nCoV disease.”
2.2. Study selection
The case series and case reports reporting COVID‐19 among HIV‐positive patients were included. Other types of articles, including review articles, editorials, letters to editor, and guidelines, were excluded from the analysis. Moreover, duplicate publications, articles reported in languages other than English, and papers with insufficient data or available only in the abstract form were also excluded. Two different steps were taken by the authors to check the eligibility of all the potentially related articles. First, two independent authors screened the titles and abstracts and eliminated duplicate papers. Next, full text of the papers that met the inclusion criteria was reviewed.
2.3. Data extraction
The extracted data included the first author's name, country of the study, publication time, number of HIV/COVID‐19 co‐infected patients, HIV diagnosis methods, treatments used for the HIV infection, CD4 lymphocyte count, median duration of the HIV infection, SARS‐CoV‐2 diagnosis methods, clinical manifestations, comorbidities, therapeutic options for COVID‐19, and outcomes. Two authors independently applied the inclusion criteria to the potentially relevant articles, and disagreements between the two authors were resolved by a third author.
2.4. Quality assessment
The case reports/case series appraisal checklist supplied by the Joanna Briggs Institute (JBI) was used to evaluate the quality of the studies. 14
3. RESULTS
As shown in Figure 1, the initial search in the databases resulted in 4502 articles. After removing duplicates, 3599 papers remained, of which 3444 were excluded based on irrelevant titles and abstracts, followed by 155 articles retrieved for detailed full‐text evaluation. Following the full‐text evaluation, 65 articles (29 case reports and 36 case series) fulfilled the inclusion criteria and were considered for further analysis. Tables 1 and 2 show the participants’ characteristics, clinical manifestation, comorbidities, and treatment regimens obtained from the articles included in this review. Also, the demographics, clinical presentations, and the outcomes of COVID‐19 treatment among HIV‐infected individuals are summarized in Table 3. Twenty‐one percent of the studies were conducted in the USA (13 studies), 16% in China (10 studies), and 13% in Italy (8 studies). The average age of the patients was 47.9 years (ranged from 19 to 86 years). The majorities of cases were males (74.3%) and in the age range of 31–59 years (87.8%). Most of the patients had antiretroviral therapy. Tenofovir disoproxil fumarate (TDF) was used in 47.4% of the patients, followed by emtricitabine (FTC) (58.4%) and lamivudine (3TC) (34.8%) as the treatment agents for HIV‐positive participants. The most common clinical manifestations among the HIV‐positive patients with COVID‐19 were coughing (81.3%), fever (62.8%), and dyspnea (60%). The detailed clinical risk factors of individuals are shown in Table 3. Among the treatment options for COVID‐19, hydroxychloroquine (HCQ) (39.34%) and azithromycin (AZM) (36.58%) were the most commonly administered agents. Furthermore, hypertension (77%) and diabetes mellitus (20.7%) were among the most frequent comorbidities reported. The total death rate in HIV‐positive patients with COVID‐19 was about 9%.
FIGURE 1.
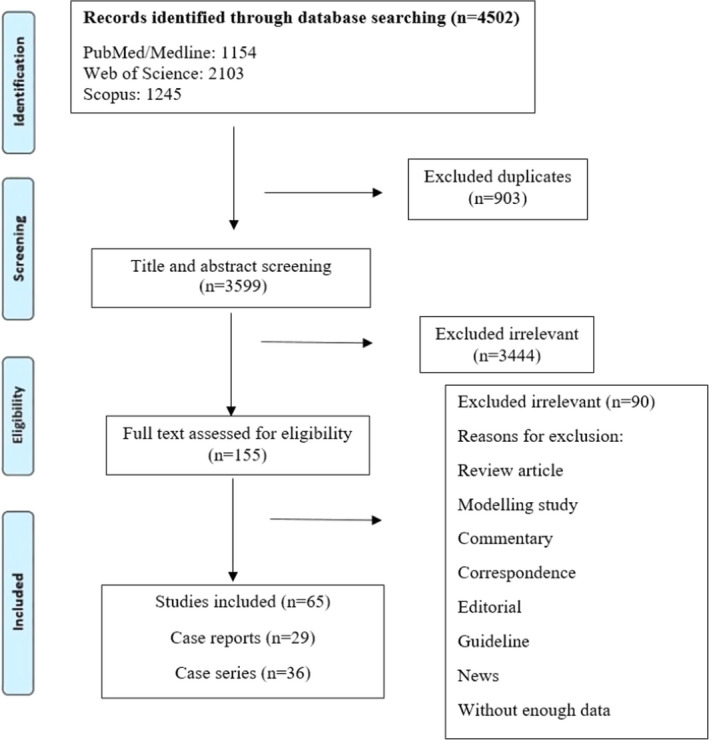
Flow diagram detailing review process and study selection
TABLE 1.
Characteristics of the case report studies
| First author | Country | Published time | Median age (years) | Male/female | HIV treatment | Median duration of HIV infection (years) | CD4 count (Cells/mm3) | SARS‐CoV‐2 diagnosis method | COVID−19 treatment | Clinical manifestations | Other comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alharthy (53) | Saudi Arabia | Nov 2020 | 40 | F | LPV/r, TMP, SMX | NR | 350 |
PCR, Chest CT |
NR | Fever, Cough, Myalgias, Dyspnea | HSV‐2 | Death |
| Giambenedetto (54) | Italy | Jun 2020 | 75 | M | RPV, FTC, TAF, DRV, COBI | 23 | 434 |
PCR, Chest CT |
HCQ, AZI, PI, LMWH | Fever, Diarrhea, Cough, Dyspnea | HTN, HBV | Recovery |
| Bertolini (55) | Argentina | Aug 2020 | 43 | M | TDF, FTC, DTG | NR | 163 |
Chest CT RT‐PCR |
LPV/r, HCQ |
Cough, Dyspnea, Fever Night Sweats, Abdominal pain, Diarrhea |
Disseminated histoplasmosis | Recovery |
| Basso (56) | Southern Brazil | Oct 2020 | 43 | F | TDF, 3TC, ATV/r | 21 | 353 |
Chest CT RT‐PCR |
NR | Disorientation, Cough, Dyspnea, Fever | Disseminated histoplasmosis | Recovery |
| Bessa (57) | Brazil | Oct 2020 | 56 | M | TDF, 3TC, EFV | NR | 1,163 |
Chest CT RT‐PCR |
CRO, CLR | Dyspnea, Asthenia | Ischemic stroke, DM | Recovery |
| Elhadi (58) | Libya | Aug 2020 | 86 | F | ZDV | NR | NR |
RT‐PCR Chest CT |
AMX Clavulanate Pred Mucolytic syrup |
Cough, Fever | T2DM | Death |
| Nakamoto (59) | Japan | Jan 2021 | 28 | M | NR | NR | 194 |
RT‐PCR Chest CT |
HCQ | NR | HBV | NR |
| Khaba (60) | South Africa | Sep 2020 | 19 | M | NR | NR | 17 |
RT‐PCR Chest CT |
CRO, TMP‐SMZ, HCQ, AZI, Enoxaparin | Weakness, Fatigue, Cough, SOB | NR | Death |
| Chiappe‐Gonzalez (61) | South America | Aug 2020 | 38 | M | TDF‐DF, FTC, ATV/r | NR | 438 |
RT‐PCR Chest CT |
Corticosteroids | Weakness, Sore throat, Cough, Dyspnea, Diarrhea | Intra ventricular cryptococcoma | Death |
| Cipolat (62) | Brazil | Aug 2020 | 63 | F | TDF, 3TC, DTG | 15 | 426 |
RT‐PCR Chest CT |
AMX/clavulanate, HCQ, AZI | Myalgia, Inappetence, Nausea, Abdominal pain, Diarrhea, Hyposmia, Hypogeusia, Cough, Dyspnea | NR | Recovery |
| Foster (63) | US | Sep 2020 | 40 | M | LPV, RTV | NR | NR | Chest CT | HCQ, AZI | Fatigue, Cough, Dyspnea, Myalgias, Fever, Chills | NR | Recovery |
| Mahmood (64) | USA | Jul 2020 | 54 | M | FTC‐TDF, Nucleotide reverse transcriptase inhibitor, DTG, Integrase Inhibitor | 29 | 266 |
RT‐PCR Chest CT |
HCQ, Cefuroxime | Fever, Myalgia, Cough, Dyspnea | CHD, CABG, T2DM, KSL, VAD | Recovery |
| Menghua (65) | China | Jul 2020 | 49 | F | EFV, 3TC | 8 | 224 |
RT‐PCR Chest CT |
CRO, Interferon, Atomization, Ribavirin, Abidol |
Fatigue, Fever Pharyngeal pain, Chills |
NR | Recovery |
| Zhao (66) | China | Oct 2020 | 38 | 1 M | 3TC, TDF, EFV, LPV, RTV | 4 | 275 |
RT‐PCR Chest CT |
NR | Fever, Muscle aches | HCV | Recovery |
| Baluku (67) | Uganda | Nov 2020 | 34 | F | TDF, 3TC, EFV | 5 | 965 | RT‐PCR | HCQ, AZI, Paracetamol | Headache, Chest pain, Diarrhea, Anorexia, Fatigue | NR | Recovery |
| Tian (68) | China | Jul 2020 | 24 | M | LPV/r, TDF, 3TC, EFV | 2 | 552 |
RT‐PCR Chest CT |
Ibuprofen, Cefotaxime, Cephalosporin | Fever | NR | Recovery |
| Pujari (69) | India | Jul 2020 | 57 | M | TAF, FTC, DTG, TMP‐SMX | NR | 19 |
RT‐PCR Chest CT |
TDF, 3TC, EFV | Fever, Cough | MTB, HTN, Anemia | Recovery |
| Choy (70) | Singapore | Dec 2020 | 48 | F | NR | NR | 20 |
RT‐PCR Chest CT |
Remdesivir, Corticosteroids |
Cough, SOB, Diarrhea |
PJP Salmonella enteritidis |
Recovery |
| Muller (71) | Austria | May 2020 | 55 | M |
FTC, TDF Alafenamide, RPV |
35 | 820 |
RT‐PCR Chest CT |
Ampicillin/Sulbactam | Fatigue, Fever, Cough, Tachycardia | HCV, liver Transplantation, Liver cirrhosis, HCC | Recovery |
| Martins (72) | Portugal | Jan 2021 | 34 | 1 M | TAF | NR | 77 |
RT‐PCR Chest CT |
Cotrimoxazole, lvx Prednisolone |
Fever, Cough, Bloody sputum, Anterior chest pain, Dyspnea. | Asthenia, Anorexia, Dysphagia, MSSA, PJP | Recovery |
| Bouare (73) | Morocco | Jul 2020 | 32 | F | NR | NR | 32 |
RT‐PCR Chest CT |
Chloroquine, AZI, Rifampin | Fever, Cough, Headache, Myalgia | MTB | NR |
| d’Ettorre G (74) | Italy | July 2020 | 52 | 1F | DRV,COBI | NR | 242 |
RT‐PCR Chest CT |
HCQ | Fever | NR | Recovery |
| Sue (75) | China | 30 Jan 2020 | 32 | M | ZDV, 3TC, EFV | 12 years | 294 | Chest CT | Piperacillin/TazObactam, lvx, OSE, LPV/r, Abidol | Fever, Dizziness, Cough | NR | Recovery |
| Chen J (76) | China | Oct 2020 | 24 | 1 M | TDF, 3TC, Favirenz | 2 years | NR |
RT‐PCR Chest CT |
LPV/r | Fever, Cough | NR | Recovery |
| Tabrizian P (77) | USA | Nov 2020 | 57 | 1F | Atazanavir, FTC‐TDF, Alafenamide, RTV | 24 | NR |
RT‐PCR Antibody testing Chest CT |
NR | Dyspnea, Cough, Malaise | HCV, HCC | Recovery |
| Farinacci (78) | Italy | Apr 2021 | 59 | 1 M | NR | 30 years | 10 |
RT‐PCR Chest CT |
HCQ, Enoxaparin | Fever, Dyspnea | NR | Death |
| Ji‐Yeon Kim (79) | Korea | Sep 2020 | 29 | 1 M | Genvoya, EVG, COBI, FTC, TDF | NR | 555 |
RT‐PCR Chest CT |
HCQ | Sore throat, Cough, loss of taste and smell, Chill, Myalgia, Rhinorrhea | NR | Recovery |
| Coleman H (80) | UK | Apr 2020 | 55 | 1 M | FTC/TDF, Disoproxil, RAL | NR | 422 |
RT‐PCR Chest CT |
NR | Fever, Cough, Hypoxia | PJP, Asthma | Recovery |
| Parker (81) | South Africa | Jun 2020 | 41 | 1 M | TDF, FTC, EFV | 4 years | 78 | Chest CT | NR | Fever, Cough, Myalgia, Diarrhea, Dyspnea | PCP, TB | Recovery |
Abbreviations: LPV/r, lopinavir/ritonavir; TMP, Trimethoprim; SMX, Sulfamethoxazole; TMP‐SMZ, Trimethoprim/sulfamethoxazole; RPV, Rilpivirine; FTC, Emtricitabine; TAF, Tenofovir alafenamide; DRV, darunavir; COBI, Cobicistat; TDF, tenofovir disoproxil fumarate; DTG, Dolutegravir; 3TC, Lamivudine; ATV/r, atazanavir/ritonavir; EFV, Efavirenz; ZDV, Zidovudine; LPV, Lopinavir; RTV, Ritonavir; EFV, Efavirenz; RAL, Raltegravir; HCQ, Hydroxychloroquine; AZI, Azithromycin; LMWH, low molecular weight heparin; CRO, Ceftriaxone; CLR, Clarithromycin; HSV‐2, Herpes simplex virus‐2; HTN, Hypertension; HBV, Hepatitis B; T2DM, Type 2 diabetes; CHD, Coronary heart disease; CABG, coronary artery bypass grafting; KS, Kaposi's sarcoma; LVAD, A left ventricular assist device; PJP or PCP, Pneumocystis jirovecii pneumonia; MSSA, Methicillin‐susceptible Staphylococcus aureus.
TABLE 2.
Characteristics of the case series studies
| First author | Country | Published time | Number of co‐infected patients | Median age (years) | Male/female | HIV treatment | Median duration of HIV infection (years) | CD4 count (Cells/mm3) | SARS‐CoV‐2 diagnosis method | COVID‐19 treatment | Clinical manifestations | Other comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bartilotti‐Matos (82) | UK | Feb 2021 | 2 | 44.5 | 2 M | 2 FTC, 2 DTG, 2 TDF, 2TMP‐SMZ | NR | 122.5 |
PCR Chest CT |
NR | 1 Cough, 1 Dyspnea, 1 Diarrhea | 1 HZV, 1 Weight loss, 1 Oral candidiasis, 1 Pernicious anemia | 2 Recovery |
| Isernia (83) | France | Sep 2020 | 30 | 53.7 | 19 M/11F |
7 ABC, 8 3TC, 3 RAL, 5 DRV 5 RTV, 12 TAF 15 FTC, 7 EVG 7 COBI, 9 DTG 7 RPV, 2 NVP 2 BIC, 1 ATV 7 TDF, 2 DOR 1 MVC, 1 ZDV |
NR | 500 |
PCR Chest CT |
2 HCQ, 1 TCZ, 5 Dexamethasone | NR | 11 CVD, 11 HTN, 9 DM, 7 Obesity, 5 CKD |
2 Death 28 Recovery |
| Toombs (84) | UK | Jan 2021 | 3 | 55 | 2 M/1F | 1 RAL, 3TC, 1 ABC, 1 TVD, 1DTG, 1 NVP | 12 | 50/mm3 to 890/mm3 | Chest CT | NR |
3 Dyspnea, 1 Cough 2 Fevers, 1 anorexia, 1 Headaches |
2 T2DM, 2 HTN, 1 G6PD, 1 Stroke, 1 Obesity |
2 Recovery 1 Death |
| Li (85) | China | 2 | 30.5 | 2 M | NR | NR | NR |
RT‐PCR Chest CT |
1 Abidor, 1 (TCZ/Abidor) | 2 Intermittent, 1 Fever, 2 Chest pain, 2 Dyspnea, 2 Cough, 1 Fatigue, 1 Poor appetite, 1 Dizziness | NR | 2 Recovery | |
| Madge (86) | London | Aug 2020 | 18 | 63 | 14 M/4F | 6 PI, 7TVD, 4ABC/3TC, 11 Integrase strand transfer Inhibitor | 19.5 | 439 |
RT‐PCR Chest CT |
NR | NR | 3 CVD, 7 DM, 4 CKD, 8 HTN, 4 CHD, 4 COPD, 1 Breast cancer |
3 Death 15 Recovery |
| Ridgway (30) | USA | Aug 2020 | 5 | 48 | 1 M/4F |
1ABC, 1DTG, 1 3 TC, 2 BIC, 4 FTC, 3 TAF, 1 EVG, 1COBI, 2 RTV, 2 DRV, 1 TDF, 1 RAL |
NR | 257.5 |
RT‐PCR Chest CT |
2 HCQ | 5 Cough, 4 Fever, 1 SOB, 2 Headache, 2 Myalgias, 3 Diarrhea, 1 yellow sputum, 3 Diarrhea, 2 Nausea, 2 Vomiting, 1 Dehydration, 2 Chills | 1 Predominantly cardiac symptoms, 1 T2DM, 1 Obstructive sleep apnea, 1 Hyperlipidemia, 2 HTN, 3 Obesity, 1 MTB, | 5 Recovery |
| Guo (87) | China | Jun 2020 | 14 | 56 | 13 M/1F |
7AZT, 5TDF, 11 3TC, 6 EFV, 1 RPV, 4 NVP, 1 Lpv/r, 1TAF, 1 FTC, 1 EVG/c |
NR | 141 to 817 |
RT‐PCR Chest CT |
NR |
10 Fever, 7 Cough, 7 Dyspnea, 11 Fatigue, 8 Blood pressure |
5 HTN, 1 COPD, 1 DM, 1 Lymphoma, 1 AF, 1 Cerebral Infarction, 1 KS, 1 Bronchiectasia, 1 MTB, 1 Anemia |
12 Recovery 2 Death |
| Sandes‐Freitas (88) | Brazil | Jan 2021 | 8 | 53.9 | 6 M/2F | 5 DTG, 8 3TC, 3 DRV, 3 RTV, 7 ABC, 1 EFV | NR | 535 | NR | 2 ST, 2 HCQ, 4 AZI, 3 OSE, 3 Heparin, 4 ATB, 1 Prophylactic enoxaparin | Fever, Dyspnea | 6 DM, 7 HTN, 1 HCV, 1 COPD, 3 CAD, 1 PKD |
3 Death 5 Recovery |
| Benkovic (89) | New York | Nov 2020 | 4 | 59.8 | 4 M |
4 FTC, 4 TAF, 1 ABC, 2 DTG, 1 MVC, 1 ETR, 1 EVG, 1 COBI |
23.8 | 794 |
RT‐PCR Chest CT |
NR | 3 Fever, 3 Fatigue, 2 Cough, 1 Diarrhea | 3 Hyperlipidemia, 3 HTN, 1 HCV, 1 T2DM, 1 AF | 4 Recovery |
| Chowdary (90) | UK | Oct 2020 | 2 | 45 | 1 M/1F | 2 ABC, 2 3TC, 2 RAL, 2 TMP‐SMZ | 14 | 384 |
RT‐PCR Chest CT |
1 Doxycycline 1 Coamoxiclav |
1 Myalgia, 1 Reduced appetite, 1 Weight loss, 1 Anosmia, 1 ageusia 1 Bloody diarrhea, 1 Vomiting, 1 Cough, 1 SOB, 1 Headache, 1 Malaise |
2 KTR, 1 HTN, 1 RAO, 1 Asthma, 1 G6PD, 1 Monoclonal gammopathy | 2 Recovery |
| Byrd (91) | US | Jun 2020 | 27 | 49 | 20 M/7F | 3 ABC, 3 DTG, 2 3TC, 7 EVG/c, 2 3FTC, 22 TAF, 2 EFV, 1 TDF, 14 DTG, 1 RPV, 1 DVR/c | 12 | 87 to 1441 |
RT‐PCR Chest CT |
6 Remdesivir, 6 RDV | 8 SOB, 2 Lethargy, 2 Fever, 4 Headache, 2 Cough, 1 Sore throat, 1 Chest pain, 1 Decreased appetite, 1 Chills, 1 Asthma |
1 Dementia, 1 CVA 3 Obesity, 2 HTN, 1 DM, 1 HLD, 1 Cancer, 1 ESRD, 1 Alcoholism, 1 COPD |
8 Recovery 1 Death |
| Zhang (92) | China | Sep 2020 | 2 | 30.5 | 2 M | NR | NR | NR |
RT‐PCR Chest CT |
2 TCZ | 1 Fatigue, 1 Anorexia, 1 Dizziness, 2 Apparent, 1 Chest tightness, 2 SOB, 1 Fever, 1 Chest pain | NR | NR |
| Pata (93) | USA | Jul 2020 | 3 | 50.3 | 1F/2 M |
1RTV, 1ABC, 2 DTG, 1 COBI, 1 3TC, 1 RPV, 2 darunavir, 1 BIC |
NR | 168 |
RT‐PCR Chest CT |
3 HCQ, 3 AZI, 3 CRO, 1 Tamiflu | 1 Diarrhea, 2 Cough, 2 SOB, 1 Abdominal pain, 1 Headaches, 1 Myalgia, 2 Fever | 1 Asthma, 1 CAD, 1 HTN, 1 Hyperlipidemia, 1 Renal disease |
2 Recovery 1 Death |
| Suwanwongse (94) | USA | Feb 2021 | 5 | 70 | 4 M/1F |
2 FTC, 2 TAF, 1 DRV, 2 COBI, 2 DTG, 1TDF 1 RPV, 1 3TC 1 EVG |
NR | 37 to 1539 |
RT‐PCR Chest CT |
4 Symptomatic 1 Convalescent plasma 1 Sarilumab trial 1HCQ |
1 Rhinorrhea, 1 Abdominal Pain 1 Diarrhea, 1 Vomiting 2 Dyspnea, 2 myalgia, 1 anorexia, 3 Cough, 3 Fever, 1 Headache, 1 Nasal congestion |
3 ARDS, 2 AKI, 2 Asthma, 3 HTN, 1 Alcohol abuse, 2 HCV, 2 DM 1 Hyperlipidemia 1 ESRD, 1 BPH |
4 Recovery 1 Death |
| Rivas (95) | Panama | Aug 2020 | 2 | 41 | 2 M | 1 TDF, 1 3TC, 1 DTG | NR | 213 |
RT‐PCR Chest CT |
2 HCQ, 1 Heparin, 1 Ceftriaxone, 1 AZI | 1 Cough, 2 Dyspnea, 2 Asthenia, 2Adynamia, 1 Loss weight, 1 Fever | 1 MTB | 2 Recovery |
| Calza (28) | Italy | Jan 2021 | 9 | 56.2 | 7 M/2F | 1 LPV/r, 4 DRV, 4 RTV, 4 COBI | 21.4 | 258 | NR | 5 HCQ,3 AZI, 3 Enoxaparin | 7 Fever, 7 Cough, 9 Fatigue, 7 Myalgia, 1 COPD, 7 RTI, 2 Dyspnea | 1 KS, 3 PJP, 2 Interstitial Pneumonia, 6 HTN, 2 DM | 9 Recovery |
| Farias (96) | Brazil | Aug 2020. | 2 | 41 | 2 M | NR | NR | 276 |
RT‐PCR Chest CT |
2 AZI, 2 HCQ 2 Ceftriaxone |
1 Fever, 1 Myalgia, 1 Headache, 2 Cough 1 Hemoptoic sputum 2 Respiratory distress |
2 MTB | 2 Recovery |
| Okoh (97) | USA | NR | 27 | 58 | 15 M/12F |
9 Integrase‐based regimen, 5 NNRTI 4 PI +Integrase, 3 NNRTI +Integrase, 1 PI based |
NR | 551 | NR |
7 HCQ 1 Corticosteroid 8 AZI, 8 CRO 8 Doxycycline |
18 Cough, 17 Fever, 17 Dyspnea, 13 Fatigue, 9 Myalgias, 4 Diarrhea, 4 Nausea, 4 vomiting | 16 HTN, 9 DM, 10 CKD, 6 Dialysis, 3 CHF, 1 CAD | 27 Recovery |
| Harter (98) | Germany | May 2020 | 33 | 48 | 30 M/3F | 31 NRTIs, 20 INSTI, 4 PI, 9 NNRTIs, 16 TAF, 6 TDF, 22 FTC, 9 3TC | 13.9 | 670 | RT‐PCR | 6 COBI, 4 DRV, 1 RTV, 2 DOR, 6 BIC | 25 Cough, 22 Fever, 7 Arthralgia, 7 Myalgia, 7 Headache, 7 Sore throat, 6 Sinusitis, 6 Anosmia | 10 HTN, 6 COPD, 4 DM, 3 CVD, 5 HBV, 2 Renal insufficiency |
30 Recovery 3 Death |
| Swaminathan (99) | USA | Nov 2020 | 6 | 64 | 5 M/1F | 1 RPV, 1 RAL, 2 3TC, 1 ABC, 2 EFV, 2 BIC, 3 TAF, 4 FTC, 1 EVG, 1TDF | NR | 765 |
RT‐PCR Chest CT |
4 HCQ, 2 AZI 1 Corticosteroid |
NR | 3 Active mental health problems, 2 Active substance use(1 Tobacco, 1 Cocaine), 2 COPD, 3 DM, 1 ESRD, 2 CAD, 4 HTN, 1 PVD, 4 Hyperlipidemia |
4 Recovery 2 Death |
| Calza (100) | Italy | Jul 2020 | 26 | 54 | 19 M/7F | 6 PI, 5DRV/COBI, 1DRV/RTV, 16TDF/TAF, 5 RPV, 1 EFV | 16.2 | 566 |
RT‐PCR Chest CT |
13 HCQ, 6 AZI, 6 Enoxaparin |
20 RTI, 6 Interstitial pneumonia (Most) Fever, Cough Fatigue, Myalgia, Tachypnea |
14 HTN, 4 DM, 3 Obesity, 3 Asthma | 26 Recovery |
| Biagio (101) | Italy | Nov 2020 | 69 | 53.5 | 50 M/19F | NR | 13.5 | 590 |
RT‐PCR Chest CT |
HCQ, TCZ, AZI, PI, Heparin, Corticosteroids | NR | 31 HTN, 10 DM, 9 CVD |
62 Recovery 7 Death |
| Stoeckle (21) | USA | Jul 2020 | 30 | 60.5 | 24 M/6F | 6 PI, 4 DRV, 3 COBI, 6 DTG, 1 LPV, 4 RTV, 1 ZDV, 1 RAL, 4 3TC,7 FTC, 1 Atazanavir, 1 BIC, 1 EVG, 2 Abacavir, 2 RPV, 1 ETR, 1 Entecavir | NR | 332 | chest CT | HCQ, Remdesivir, Corticosteroids |
17 Fever, 21 Cough, 20 Dyspnea, 10 Diarrhea, 1 Sputum, 1 Rhinorrhea, 3 Headache, 4 Myalgias, 5 Nausea, 5 vomiting 10 Diarrhea, 2 Ageusia 3 Abdominal pain, 3 Chest pain, 1 Anosmia |
12 HTN, 8 DM, 1 Heart failure, 2 ESRD, 2 CAD, 4 COPD, 3 Asthma, 1 Cirrhosis, 6 HBV, 1 HCV |
28 Recovery 2 Death |
| Przydzial (102) | USA | NR | 2 | 57 | 2 M | NR | NR | 63 |
RT‐PCR chest CT |
1 AZI, 1 Piperacillin tazobactam 1 Zosyn, 1 Bactrim |
Cough, Fevers, Myalgias Dyspnea Nausea/emesis |
HTN, Hyperlipidemia | 2 Recovery |
| Akyala (24) | North Central Nigeria | Sep 2020 | 4 | 29.5 | 4F | 2 Abacavir, 2 TAF, 2 3TC, 1 FTC, 1 Alafenamide | 4.7 | 254 | RT‐PCR |
1 Norfloxacin 1 γ‐globulin 1Methylprednisolone |
Malaise, Cough, Fever, Headaches | 1 DM, 1 Asthmatic, 1 Chronic sinusitis, 1 MTB | 4 Recovery |
| Shekhar (27) | USA | Sep 2020 | 5 | 48.8 | 4 M/1F | 5 FTC, 3 BIC, 1 EVG,1 RAL, 5 Tenofovir, 1 COBI | NR | 603 |
RT‐PCR chest CT |
NR | 2 Chills, 1 Fatigue, 2 Fever, 2 Diarrhea, 1 SOB, 2 Cough, 1Chest discomfort, 1 Anosmia, 1 Hypogeusia, 1Abdominal pain, 1 Myalgias | 2 DM, 1 CKD, 1 PAD, 1 HTN, 1 Depression, 1 Alcohol abuse | 5 Recovery |
| Marimuthu (103) | India | Jul 2020 | 6 | 38 | 3 M/3F | 2 ZDV, 6 3TC, 2 ZLN, 4 TDF, 3 EFV, 1 ATV/r | 10.4 | 535 | chest CT | NR | 5 Fever, 2 Cough, 1 Sore throat | 2 HTN, 1 EC | 6 Recovery |
| Pinnetti (104) | Italy | Sep 2020 | 2 | 43.5 | 2 M | 1 TDF, 1 FTC, 1 DTG, 1 EFV, | NR | 127 |
RT‐PCR chest CT |
1 HCQ | NR |
2 Opportunistic infections of the (CNS), 1 HTN 1 Interstitial pneumonia |
2 Recovery |
| Collins (105) | Georgia | Jun 2020 | 20 | 57 | 14 M/6F | 2 NNRTIs, 4 PI, 16 INSTI, | NR | 425 |
RT‐PCR chest CT |
9 Supportive care only, 8 HCQ, 2 AZI, 1 Remdesivir |
18 Cough, 13 Fever, 12 Malaise, 10 Chills, 12 SOB, 6 Diarrhea 4 Chest tightness, 6 Nausea or vomiting, 8 Myalgias, 4 Headache, 3 Sore throat, 2 Anosmia, 2 Ageusia |
14 HTN, 12 Dyslipidemia 9 T2DM, 6 CVD, 6 CLD, 6 Obesity 5 CKD,3 Cancer, 8 Depression, 8 Anxiety |
17 Recovery 3 Death |
| Gudipati (106) | USA | Oct 2020 | 14 | 56.2 | 12 M/2F |
14 TDF, 1 DRV 1 PI/COBI |
NR | 612 | RT‐PCR | 3 Intravenous fluids, 3Corticosteroid, 14 HCQ | 7 Fever, 7 SOB, 10 Cough, 4 Diarrhea, 4 loss of taste and smell | 8 Obesity, 8 HTN, 6 DM, 5 CKD, 2 ESRD |
11 Recovery 3 Death |
| Charre (26) | France | Jul 2020 | 77 | 53 | 52 M/25F | 9 PI, 48 INSTI, 23 NNRTI, 52 TDF/TAF | 15 | 529 | RT‐PCR | NR | NR | NR | NR |
| Cabellos (19) | Spain | Oct 2020 | 63 | 46 | 56 M/7F | 1 PI, 4 INSTI, 4 NNRTIs | 10.8 | 605 | RT‐PCR, IgG‐SARS‐CoV−2, IgM‐SARS‐CoV−2, Chest CT |
10 Corticosteroids 13 HCQ, 4 LPV/RTV |
42 Fever, 42 Cough, 31 Dyspnea, 7 Anosmia, 6 Ageusia, 14 Diarrhea, 9 Headache, 16 Weakness, 15 Myalgia/Arthralgia | 12 HTN, 6 DM, 8 Overweight, 8 CVD, 3 COPD, 2 Renal chronic failure |
61Recovery 2 Death |
| Wu Q (107) | China | Nov 2020 | 2 | 53.5 | 2 M | 1TDF, 1 3TC, 1 EFV | 6 years | NR |
RT‐PCR Chest CT |
1 OSE, 1 CRO 1 Moxifloxacin, 1 Tazobactam 2 Moxifloxacin 1 Ribavirin 1 Umifenovir |
1 Fatigue, 2 Dyspnea, 1 Cough, 2 Myalgia, 1 SOB, 1 Sore throat, 1 Intermittent Diarrhea | 1 MTB, 1 T2DM | 2 Recovery |
| Guo W (108) | China | Aug 2020 | 2 | NR | NR | 1 SMZ/TMP | NR | NR |
Chest CT IgM and IgG |
NR | 2 Fever, 2 Dyspnea | NR | 2 Recovery |
| Ciccullo (109) | Italy | Jan 2020 | 4 | 19 to 43 years | 4 M | 3 FTC, 1 TDF, 1 RGV, 1 3TC, 3 DTG, 2 TAF | NR | 516 |
RT‐PCR chest CT |
NR | 4 Fever, 3 Cough, 3 Asthaenia, 1 Sore throat | NR | 4 Recovery |
| Cucurull‐Canosa J (110) | Spain | Jul 2020 | 12 |
62 (55–80) 51 (37–58) |
7 M/5F | NR | 23.6 (4–35) 21.8 (14–31) | 508 | RT‐PCR | 7 HCQ, 7 AZI, 6 Corticosteroids | 8 Fever, 3 Asthenia, 6 Respiratory symptoms, 1Gastrointestinal symptoms, 1 Headache, 4 Conjunctivitis, 3 Asymptomatic, | 1 Cancer, 4 HTM, 1 DM, 1 COPD |
9 Recovery 3 Death |
Abbreviations: VD, Cardiovascular disease; BPH, Benign prostatic hypertrophy; CRDs, Chronic respiratory diseases; MTB, Mycobacterium tuberculosis infection; AHRF, Acute Hypoxemic Respiratory Failure; AKI, Acute kidney injury; RTIs, Respiratory tract infections; KS, Kaposi's sarcoma; CHF, Congestive heart failure; PJP, Pneumocystis jirovecii pneumonia; PVD, Peripheral vascular disease; CABG, coronary artery bypass grafting; HCC, Hepatocellular carcinoma; RAO, Retinal artery occlusion; EC, Esophageal candidiasis; CKD, Chronic kidney disease; CLD, Chronic lung disease; T2DM, Type 2 diabetes; HSV‐2, Herpes simplex virus‐2; HBV, Hepatitis B virus; G6PD, Glucose‐6‐phosphate dehydrogenase; CHD, Coronary heart disease; SOB, Shortness of breath; OSA, Obstructive sleep apnea; AF, Atrial fibrillation; HCV, Hepatitis C virus; COPD, Chronic obstructive pulmonary disease; CAD, Coronary artery disease; PKD, Polycystic kidney disease; KTR, kidney transplant recipients; HTN or HT, Hypertension; HLD, High‐level design; ESRD, End‐Stage Renal Disease; LVAD, A left ventricular assist device; CABG, Coronary artery bypass grafting; MSSA, Methicillin‐susceptible Staphylococcus aureus; FTC, Emtricitabine; TAF, Tenofovir alafenamide; DTG, Dolutegravir; LPV/r, lopinavir/ritonavir; TMP, Trimethoprim; SMX, Sulfamethoxazole; DRV, darunavir; HCQ, Hydroxychloroquine; TCZ, Tocilizumab; AZI, Azithromycin; TCZ, Tocilizumab; LMWH, low molecular weight heparin; CRO, Ceftriaxone; CLR, Clarithromycin; AMX, Amoxicillin; Pred, Prednisone; Ace, Acetaminophen; TMP‐SMZ, Trimethoprim/sulfamethoxazole; HC, Hydrocortis one; lvx, Levofloxacin; TDF, tenofovir disoproxil fumarate; 3TC, Lamivudine; RPV, Rilpivirine; COBI, Cobicistat; ATV/r, atazanavir/ritonavir; EFV, Efavirenz; RAL, Raltegravir; TVD, Truvada; NVP, Nevirapine; MVC, Maraviroc; ETR, Etravirine; EVG, Elvitegravir; LPV, Lopinavir; RTV, Ritonavir; ZDV, Zidovudine; NRTIs, nucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitors; EVG, Elvitegravir; BIC, Bictegravir.
TABLE 3.
Summary of the case report and case series findings
| n/N (%) | No. of studies that mentioned | |
|---|---|---|
| Sex | ||
| Female | 146/569 (25.66%) | 64 |
| Male | 423/569 (74.34%) | |
| Age | ||
| ≤30 | 5/551 (0.9%) | 62 |
| 30 < n < 60 | 484/551 (87.85%) | |
| ≥60 | 62/551 (11.25%) | |
| CD | ||
| HIV Treatment | ||
| FTC | 111/190 (58.42%) | 26 |
| DTG | 42/129 (32.55%) | 17 |
| TDF | 128/270 (47.41%) | 31 |
| TMP‐SMZ | 6/7 (85.7%) | 4 |
| Azithromycin (AZ) | 7/14 (50%) | 1 |
| ABC | 28/106 (26.41%) | 10 |
| 3TC | 71/204 (34.8%) | 24 |
| RAL | 7/76 (9.21%) | 5 |
| DRV | 26/127 (20.47%) | 8 |
| RTV | 21/113 (18.58%) | 9 |
| TAF | 138/238 (57.98%) | 15 |
| EVG | 21/126 (16.66%) | 9 |
| COBI | 29/134 (21.64%) | 13 |
| RPV | 21/143 (14.68%) | 10 |
| NVP | 7/47 (14.89%) | 3 |
| BIC | 11/79 (13.92%) | 6 |
| ATV | 4/38 (10.53%) | 4 |
| TDF | 114/270 (42.22%) | 31 |
| DOR | 2/30 (6.66%) | 1 |
| MVC | 2/34 (5.88%) | 1 |
| ZDV | 7/123 (5.69%) | 6 |
| TVD | 8/21 (38.09%) | 2 |
| PI | 36/317 (11.35%) | 9 |
| Integrase strand transfer inhibitor | 11/18 (61.11%) | 1 |
| EFV | 24/98 (24.48%) | 15 |
| RPV | 21/143 14.89%) | 10 |
| Lpv/r | 4/25 (16%) | 4 |
| ETR | 2/70 (2.85%) | 2 |
| DTG | 56/129 (43.41%) | 17 |
| DVR/c | 1/27 (3.70%) | 1 |
| Darunavir | 2/3 (66.66%) | 1 |
| Integrase‐based regimen | 17/82 (20.73%) | 2 |
| NNRTI | 46/220 (20.90%) | 5 |
| NRTIs | 31/33 (93.93%) | 1 |
| INSTI | 88/193 (45.59%) | 1 |
| Atazanavir | 2/31 (6.45%) | 2 |
| Abacavir | 4/34 (11.76%) | 1 |
| Entecavir | 1/30 (3.33%) | 1 |
| Tenofovir | 5/5 (100%) | 1 |
| ZLN | 2/6 (33.33%) | 1 |
| ATV/r | 3/8 (37.5%) | 3 |
| Nucleoside and nucleotide reverse transcriptase inhibitor | 1/1 (100%) | 1 |
| Integrase inhibitor | 8/28 (28.57%) | 2 |
| Alafenamide | 3/6 (50%) | 3 |
| Favirenz | 1/1 (100%) | 1 |
| Genvoya | 1/1 (100%) | 1 |
| Clinical manifestations | ||
| Cough | 192/236 (81.35%) | 35 |
| Dyspnea | 95/158 (60.12%) | 22 |
| Diarrhea | 52/185 (28.11%) | 16 |
| Fever | 203/323 (62.84%) | 44 |
| Anorexia | 4/11 (36.36%) | 4 |
| Headache | 4/8 (50%) | 4 |
| Chest pain | 9/63 (14.28%) | 6 |
| Fatigue | 45/70 (64.28%) | 13 |
| Poor appetite | 1/2 (50%) | 1 |
| Dizziness | 3/5 (60%) | 3 |
| SOB | 35/82 (42.68%) | 11 |
| Myalgias | 26/89 (29.21%) | 7 |
| Sputum | 5/40 (12.5%) | 5 |
| Nausea | 18/83 (21.69%) | 5 |
| Vomiting | 19/89 (21.35%) | 6 |
| Dehydration | 1/5 (20%) | 1 |
| Chills | 17/59 (28.81%) | 6 |
| Blood pressure | 8/14 (57.14%) | 1 |
| Weight loss | 2/4 (50%) | 2 |
| Anosmia | 18/143 (12.59%) | 6 |
| Ageusia | 11/115 (9.56%) | 4 |
| Lethargy | 2/27 (7.41%) | 1 |
| Sore throat | 16/94 (17.02%) | 8 |
| Abdominal pain | 7/40 (17.50%) | 5 |
| Rhinorrhea | 3/36 (8.33%) | 3 |
| Nasal congestion | 1/5 (20%) | 1 |
| RTI | 7/9 (77.77%) | 1 |
| COPD | 1/9 (11.11%) | 1 |
| Arthralgia | 22/96 (22.92%) | 1 |
| Myalgia | 66/213 (30.98%) | 20 |
| Sinusitis | 6/33 (18.18%) | 1 |
| Chest discomfort | 1/5 (20%) | 1 |
| Hypogeusia | 2/6 (33.33%) | 2 |
| Loss of taste and smell | 5/15 (33.33%) | 2 |
| Weakness | 18/65 (27.69%) | 3 |
| Respiratory symptoms | 6/12 (50%) | 1 |
| Gastrointestinal symptoms | 6/12 (50%) | 1 |
| Conjunctivitis | 4/12 (33.33%) | 1 |
| Night Sweats | 1/1 (100%) | 1 |
| Disorientation | 1/1 (100%) | 1 |
| Inappetence | 1/1 (100%) | 1 |
| Hyposmia | 1/1 (100%) | 1 |
| Hypogeusia | 2/6 (33.33%) | 2 |
| Tachycardia | 1/1 (100%) | 1 |
| COVID‐19 Treatment | ||
| HCQ | 96/244 (39.34%) | 26 |
| TCZ | 4/34 (11.76%) | 3 |
| Abidor | 2/2 (100%) | 1 |
| ST | 2/8 (25%) | 1 |
| AZI | 45/123 (36.58%) | 17 |
| OSE | 5/11 (45.45%) | 3 |
| Heparin | 4/10 (40%) | 2 |
| ATB | 4/8 (50%) | 1 |
| Enoxaparin | 12/45 (26.66%) | 5 |
| Doxycycline | 9/29 (31.03%) | 2 |
| Co‐amoxiclav | 1/2 (50%) | 1 |
| Remdesivir | 9/78 (11.54%) | 4 |
| RDV | 6/27 (22.22%) | 1 |
| TCZ | 4/34 (11.76%) | 3 |
| CRO | 15/35 (42.86%) | 6 |
| Tamiflu | 1/3 (33.33%) | 1 |
| Symptomatic | 4/5 (80%) | 1 |
| Convalescent plasma | 1/5 (20%) | 1 |
| Sarilumab trial | 1/5 (20%) | 1 |
| Ceftriaxone | 3/4 (75%) | 2 |
| Corticosteroids | 23/122 (18.85%) | 5 |
| Doxycycline | 9/29 (31.03%) | 2 |
| COBI | 6/33 (18.18%) | 1 |
| DRV | 4/33 (12.12%) | 1 |
| RTV | 5/96 (5.21%) | 2 |
| DOR | 2/33 (6.06%) | 1 |
| Convalescent Plasma | 1/5 (20%) | 1 |
| Cotrimoxazole | 1/1 (100%) | 1 |
| CD | ||
| ≤50 | 5/510 (0.98%) | 53 |
| 50 < n < 200 | 13/510 (2.55%) | |
| ≥200 | 492/510 (96.47%) | |
| Comorbidities | ||
| HZV | 1/2 (50%) | 1 |
| Weight loss | 2/4 (50%) | 2 |
| Oral candidiasis | 1/2 (50%) | 1 |
| Anemia | 1/2 (50%) | 1 |
| CVD | 40/233 (17.17%) | 6 |
| HTN | 178/231 (77.06%) | 26 |
| DM | 83/401 (20.70%) | 19 |
| Obesity | 31/125 (24.8%) | 7 |
| CKD | 30/114 (26.31%) | 6 |
| T2DM | 15/36 (41.66%) | 7 |
| G6PD | 2/5 (40%) | 1 |
| Stroke | 2/4 (50%) | 2 |
| CHD | 5/19 (26.32%) | 2 |
| COPD | 23/211 (10.90%) | 9 |
| Cancer | 6/77 (7.79%) | 4 |
| Predominantly cardiac symptoms | 1/5 (20%) | 1 |
| Obstructive sleep apnea | 1/5 (20%) | 1 |
| Hyperlipidemia | 10/23 (43.48%) | 5 |
| MTB | 9/31 (29.03%) | 8 |
| Lymphoma | 1/14 (7.14%) | 1 |
| AF | 2/18 (11.11%) | 2 |
| Cerebral Infarction | 1/14 (7.14%) | 1 |
| KS | 2/23 (8.69%) | 2 |
| Bronchiectasia | 1/14 (7.14%) | 1 |
| HCV | 8/50 (16%) | 7 |
| CAD | 9/47 (19.14%) | 5 |
| PKD | 1/8 (12.5%) | 1 |
| KTR | 2/2 (100%) | 1 |
| RAO | 1/2 (50%) | 1 |
| Asthma | 12/71 (16.90%) | 7 |
| Monoclonal gammopathy | 1/2 (50%) | 1 |
| Dementia | 1/27 (3.70%) | 1 |
| CVA | 1/27 (3.70%) | 1 |
| HLD | 1/27 (3.70%) | 1 |
| ESRD | 7/82 (8.54%) | 5 |
| Alcoholism | 1/27 (3.70%) | 1 |
| Renal disease | 1/3 (33.33%) | 1 |
| ARDS | 3/5 (60%) | 1 |
| AKI | 2/5 (40%) | 1 |
| BPH | 1/5 (20%) | 1 |
| PJP | 6/12 (50%) | 4 |
| Interstitial Pneumonia | 3/11 (27.27%) | 2 |
| Dialysis | 6/27 (22.22%) | 1 |
| CHF | 3/27 (11.11%) | 1 |
| HBV | 13/65 (20%) | 4 |
| HCV | 7/50 (14%) | 7 |
| Active mental health problems | 3/6 (50%) | 1 |
| Active substance use | 2/6 (33.33%) | 1 |
| PVD | 1/6 (16.66%) | 1 |
| Cirrhosis | 2/31 (6.45%) | 2 |
| Depression | 9/25 (36%) | 2 |
| Chronic sinusitis | 1/4 (25%) | 1 |
| PAD | 1/5 (20%) | 1 |
| EC | 1/6 (16.66%) | 1 |
| Opportunistic infections of CNS | 2/2 (100%) | 1 |
| Dyslipidemia | 12/20 (60%) | 1 |
| CLD | 6/20 (30%) | 1 |
| Overweight | 8/63 (12.70%) | 1 |
| HSV‐2 | 1/1 (100%) | 1 |
| Disseminated histoplasmosis | 2/2 (100%) | 2 |
| Intra ventricular cryptococcoma | 1/1 (100%) | 1 |
| CABG | 1/1 (100%) | 1 |
| liver transplantation | 1/1 (100%) | 1 |
| HCC | 2/2 (100%) | 2 |
| Asthenia | 6/15 (40%) | 3 |
| Anorexia | 4/11 (36.36%) | 4 |
| Dysphagia | 1/1 (100%) | 1 |
| MSSA | 1/1 (100%) | 1 |
| VAD | 1/1 (100%) | 1 |
| PCP | 1/1 (100%) | 1 |
| KSL | 1/1 (100%) | 1 |
| Outcome | ||
| Discharge | 444/488 (90.98%) | 61 |
| Death | 44/488 (9.02%) |
Abbreviations: VD, Cardiovascular disease; BPH, Benign prostatic hypertrophy; CRDs, Chronic respiratory diseases; MTB, Mycobacterium tuberculosis infection; AHRF, Acute Hypoxemic Respiratory Failure; AKI, Acute kidney injury; RTIs, Respiratory tract infections; KS, Kaposi's sarcoma; CHF, Congestive heart failure; PJP, Pneumocystis jirovecii pneumonia; PVD, Peripheral vascular disease; CABG, coronary artery bypass grafting; HCC, Hepatocellular carcinoma; RAO, Retinal artery occlusion; EC, Esophageal candidiasis; CKD, Chronic kidney disease; CLD, Chronic lung disease; T2DM, Type 2 diabetes; HSV‐2, Herpes simplex virus‐2; HBV, Hepatitis B virus; G6PD, Glucose‐6‐phosphate dehydrogenase; CHD, Coronary heart disease; SOB, Shortness of breath; OSA, Obstructive sleep apnea; AF, Atrial fibrillation; HCV, Hepatitis C virus; COPD, Chronic obstructive pulmonary disease; CAD, Coronary artery disease; PKD, Polycystic kidney disease; KTR, kidney transplant recipients; HTN or HT, Hypertension; HLD, High‐level design; ESRD, End‐Stage Renal Disease; LVAD, A left ventricular assist device; CABG, Coronary artery bypass grafting; MSSA, Methicillin‐susceptible Staphylococcus aureus; FTC, Emtricitabine; TAF, Tenofovir alafenamide; DTG, Dolutegravir; LPV/r, lopinavir/ritonavir; TMP, Trimethoprim; SMX, Sulfamethoxazole; DRV, darunavir; HCQ, Hydroxychloroquine; TCZ, Tocilizumab; AZI, Azithromycin; TCZ, Tocilizumab; LMWH, low molecular weight heparin; CRO, Ceftriaxone; CLR, Clarithromycin; AMX, Amoxicillin; Pred, Prednisone; Ace, Acetaminophen; TMP‐SMZ, Trimethoprim/sulfamethoxazole; HC, Hydrocortis one; lvx, Levofloxacin; TDF, tenofovir disoproxil fumarate; 3TC, Lamivudine; RPV, Rilpivirine; COBI, Cobicistat; ATV/r, atazanavir/ritonavir; EFV, Efavirenz; RAL, Raltegravir; TVD, Truvada; NVP, Nevirapine; MVC, Maraviroc; ETR, Etravirine; EVG, Elvitegravir; LPV, Lopinavir; RTV, Ritonavir; ZDV, Zidovudine; NRTIs, nucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitors; EVG, Elvitegravir; BIC, Bictegravir.
Although several extensive studies have reported the clinical characteristics of COVID‐19 and its treatment outcomes among the general population, only sparse data are available regarding the COVID‐19 status among HIV‐positive patients. 15 , 16 Thirty‐six case series have so far been conducted on HIV patients co‐infected with COVID‐19. In these studies, most of the participants have been on ART, have had a CD4 T‐cell count ranging from 63 to 1,441 cells/mm3, and have had a mild‐to‐moderate COVID‐19 disease, with symptoms like fatigue, cough, and fever. 17 , 18 , 19 In 2 articles, however, it was reported that most cases had severe COVID‐19 associating with high mortality rates. 20 , 21 According to Table 2, COVID‐19 was diagnosed by either PCR/RT‐PCR (5 studies) (24, 26, 98, 106, and 110) or chest CT scan (3 studies) (84, 21, and 103). For the clinical diagnosis of COVID‐19, 2 studies used PCR and chest CT scans along with IgG and IgM antibody titers. 22 , 23 The rest of the articles included both PCR/RT‐PCR and chest CT scans and 3 not reported the criteria of diagnosis.
Table 1 summarizes the demographics, clinical presentation, and treatment outcome of HIV‐infected individuals with COVID‐19 from case reports. Twenty‐nine papers were analyzed for HIV patients with COVID‐19. In total, 65.5% of the patients surveyed were men with an age range of 19 to 75 years. Two studies have shown that old age (≥75 years), male sex, and comorbidities such as cardiac disease (CD), chronic kidney disease (CKD), and HCV infection are major risk factors of hospital admission and critical condition (17, 18). CD4 count in case reports ranged from 10 to 1,163 cells/mm3. About half of the cases had a CD4 count higher than 350 cells/mm3. In 2 cases, the COVID‐19 was diagnosed based on a Chest CT scan (75, 81). Baluku et al. confirmed the COVID‐19 by real‐time PCR (67). Tabrizian et al. used the COVID‐19 IgM and IgG serology test in addition to real‐time and chest CT scans (77). Unfortunately, despite the diagnosis of COVID‐19 and taking related treatment, 5 cases have died due to a large number of comorbidities (53, 58, 60, 61, and 78).
4. DISCUSSION
On March 11, 2020, the World Health Organization (WHO) labeled the new coronavirus outbreak as a pandemic. According to the latest data from Johns Hopkins University in September 12, 2021, more than 223 million COVID‐19‐positive individuals, with more than 4.6 million deaths, have been reported worldwide. 24 , 25 Nearly 40 million people are currently living with HIV around the world. 26 Compromised immune responses are the main risk factors predisposing PLWH to severe forms of COVID‐19 and higher death rates. 27 This review provides valuable information regarding various risk factors such as age and immune status among PLWH co‐infected with COVID‐19, as well as different antiretroviral therapies and their impacts on the disease outcome among these patients.
The transmission routes of HIV and SARS‐CoV‐2 are not the same; however, COVID‐19 infection can increase the transmission risk of the HIV infection. Both infections may initially present with influenza‐like symptoms such as fever, cough, and difficulty in breathing, albeit with different severities. 3 In our study, the most prevalent clinical manifestations of COVID‐19 among PLWH were coughing (81.35%) and fever (62.84%), respectively. A longer duration of fever and lung recovery was observed in these patients as compared to COVID‐19 patients without HIV. This might be due to a delayed SARS‐CoV‐2‐specific antibody response caused by HIV infection, which can lead to a delay in the recovery of lung lesions. 28 This delayed SARS‐CoV‐2‐specific antibody response may be due to the reduction of CD4+ T lymphocyte counts during HIV infection, as reported by numerous studies in the USA (20%), China (15.63%), and Italy (12.5%).
Sentongo et al, in a systematic review and meta‐analysis study, showed that the prevalence of HIV‐co‐infected COVID‐19 patients was significantly higher in the USA compared with Spain but was not significantly higher than that in China. 29 Despite a high prevalence of PLWH and COVID‐19‐positive cases in Africa, only scarce data are available on the possible COVID‐19/ HIV‐co‐infected individuals, 30 which is probably due to poor hospital records and lack of data publication in this continent.
In this study, as in other reports, most of the COVID‐19/HIV‐co‐infected patients were males (74.34%), which might be related to various behavioral, social, and biological differences between the two genders. 31 , 32
Among the 64 COVID‐19/HIV‐co‐infected patients with available age information, the mean age was 47.9 years which was similar to that reported by other studies 27 , 32 and was 2 decades younger than the mean age of the hospitalized COVID‐19 patients without HIV.
In this study, HIV‐positive patients co‐infected with COVID‐19 had high comorbidity of hypertension (77%) and diabetes mellitus (20.7%). Vizcarra et al reported a higher proportion of comorbidities among COVID‐19/ HIV‐co‐infected patients compared with COVID‐19 patients without HIV. 33
A 9% death and 91% discharge rates have been reported among 61 COVID‐19/ HIV‐co‐infected cases for whom the information on the co‐infection outcome is available. A high discharge rate among these patients may be related to a theory that mentions PLWH are less predisposed to severe COVID‐19 infections. 34 Ucciferri et al showed that a hyperimmune response and the subsequent cytokine storm play major roles in the pathogenicity and severity of COVID‐19 infection. 35 Therefore, the compromised immune status of HIV‐positive individuals can explain their milder COVID‐19 symptoms, as well as a lower morbidity and mortality rates among these individuals, as compared to the HIV‐negative COVID‐19 patients.
Makoti et al noted that the respiratory load of SARS‐CoV‐2 was low in people with HIV due to the viral interference phenomenon that leads to the interruption of SARS‐CoV‐2 replication in cells already infected with HIV. 36 In overall, there are not yet sufficient data to support or refuse any of the two hypotheses mentioned above.
The death rates among COVID‐19/HIV‐co‐infected individuals vary across different countries and regions. For example, death rates have been reported as 13.9% in the UK, 4.3% in Italy, and 3.6% in Spain, respectively. 31 These discrepancies may be due to differences in the type of study, number of people participating in the study, characteristics of the healthcare services, and the patients’ demographic characteristics.
In this review, the majority of HIV patients with COVID‐19 co‐infection were on ART therapy. The most common antiviral drugs used in different studies for HIV‐positive patients were nucleoside reverse transcriptase inhibitors, including FTC (58.42%), TDF (47.41%), and 3TC (34.8%), respectively. These drugs have high antiviral potency, good tolerability, and low risk of severe adverse reactions associated with mitochondrial toxicity. 37 , 38 Hydroxychloroquine (39.24%) and azithromycin (36.58%) have been prescribed as common drugs for COVID‐19 treatment in different studies. Hydroxychloroquine (an analog of chloroquine) has been associated with a decrease in the viral load among COVID‐19 patients and has shown a comparable effect with azithromycin. 39 There are evidences that suggest ART therapy alleviates COVID‐19 severity through immune reconstitution, but these results are not yet proven. 10 , 40 , 41 In one study, most COVID‐19‐infected patients were on ART, and the median CD4 count was 752, with 96.47% having a CD4 count over 200, and only 3.53% having a CD4 count below 200. Many studies have shown an association between a low CD4 count and an increased COVID‐19 death rate among people with HIV, 42 , 43 which might be the reason for the 9% prevalence of death in these patients.
Co‐infection of COVID‐19 with other pathogens is one of the most important medical concerns in today's COVID‐19 pandemic days, resulting in complicated diagnosis, treatment, and disease prognosis. HIV/COVID‐19 co‐infection might have negatively influenced the HIV treatment through different means like applying social restrictions and quarantines, closure of pharmaceutical factories, and employment of health workers to care for COVID‐19 patients. Moreover, HIV/COVID‐19 co‐infection has also a serious negative impact on the 90‐90‐90 UNAIDS (the Joint United Nations Program on HIV and AIDS (UNAIDS)) strategy, which aims to end the AIDS epidemic. 44
There are several limitations to this study, including (1) the small number of studies included (2) inclusion of only case reports and case series with small sample size and individual patient‐level data, which make it hard to generalize the data of the present study, and (3) lack of control subjects in the included studies which can insert bias into the results of the present study. The studies included in this review are observational studies which describe only a detailed description of disease occurrence in a single person or a group of individuals who all have a particular disease or condition. Therefore, these studies cannot generate information regarding rates, ratios, incidences, or prevalence of the disease conditions.
5. CONCLUSION
There are many reports of co‐infections associated with COVID‐19. In this review, it has been reported that most HIV/COVID‐19‐co‐infected patients, reported so far, have a high comorbidity of hypertension and diabetes mellitus and have ages above 47.9 years. HIV can increase the severity, morbidity, and mortality rates of COVID‐19 infection, but this theory has not been proven yet. On the contrary, HIV/COVID‐19 co‐infection can probably disrupt HIV treatment and diagnosis. Further studies are needed to assess the impacts of HIV infection in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Heidary M, Asadi A, Noorbakhsh N, et al. COVID‐19 in HIV‐positive patients: A systematic review of case reports and case series. J Clin Lab Anal. 2022;36:e24308. doi: 10.1002/jcla.24308
Funding information
This study was supported by a grant from Behbahan Faculty of Medical Sciences [grant number 400019]
DATA AVAILABILITY STATEMENT
All relevant data are included in the manuscript.
REFERENCES
- 1. Evans N, Martinez E, Petrosillo N, et al. SARS‐CoV‐2 and human immunodeficiency virus: pathogen pincer attack. HIV/AIDS (Auckland, NZ). 2021;13:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silveira MM, Moreira G, Mendonça M. DNA vaccines against COVID‐19: perspectives and challenges. Life Sci. 2021;267:118919. doi: 10.1016/j.lfs.2020.118919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark A, Jit M, Warren‐Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID‐19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health. 2020;8(8):e1003‐e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang CC, Crane M, Zhou J, et al. HIV and co‐infections. Immunol Rev. 2013;254(1):114‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang J, Xie N, Hu X, et al. Epidemiological, virological and serological features of coronavirus disease 2019 (COVID‐19) cases in people living with human immunodeficiency virus in Wuhan: a population‐based cohort study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tesoriero JM, Swain C‐AE, Pierce JL, et al. Elevated COVID‐19 outcomes among persons living with diagnosed HIV infection in New York State: results from a population‐level match of HIV, COVID‐19, and hospitalization databases. MedRxiv. 2020. [Google Scholar]
- 7. Guo W, Ming F, Dong Y, et al. A survey for COVID‐19 among HIV/AIDS patients in two districts of Wuhan, China. SSRN Electron J. 2020. doi: 10.2139/ssrn.3550029 [DOI] [Google Scholar]
- 8. Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS‐CoV‐2 infection? When less is better. J Med Virol. 2020;92(10):1777‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharov KS. HIV/SARS‐CoV‐2 co‐infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int J Infect Dis. 2021;102:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID‐19 in HIV‐positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173(7):536‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen XP, Cao Y. Consideration of highly active antiretroviral therapy in the prevention and treatment of severe acute respiratory syndrome. Clin Infect Dis. 2004;38(7):1030‐1032. [DOI] [PubMed] [Google Scholar]
- 12. Costenaro P, Minotti C, Barbieri E, Giaquinto C, Donà D. SARS‐CoV‐2 infection in people living with HIV: a systematic review. Rev Med Virol. 2021;31(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 14. Aromataris E, Munn Z. JBI Manual for Evidence Synthesis. JBI. 2020. [Google Scholar]
- 15. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benkovic S, Kim M, Sin E. 4 cases: HIV and SARS‐CoV‐2 co‐infection in patients from Long Island, New York. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calza L, Bon I, Borderi M, et al. COVID‐19 outcomes in patients with uncontrolled HIV‐1 infection. J Acqu Immu Defic Syndr. 2021;86(1):e15‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shekhar R, Barton A, Sheikh AB, Upadhyay S, Salas NM. Coronavirus disease of 2019 in patients with well‐controlled human immunodeficiency virus on antiretroviral therapy. J Acqu Immu Defic Syndr. 2020;85(1):e1‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowdary P, Shetty S, Booth J, Khurram MA, Yaqoob M, Mohamed IH. Experience of SARS‐CoV‐2 infection in two kidney transplant recipients living with HIV‐1 infection. Transpl Infect Dis. 2021;23(2):e13500. [DOI] [PubMed] [Google Scholar]
- 21. Ridgway JP, Farley B, Benoit J‐L, et al. A case series of five people living with HIV hospitalized with COVID‐19 in Chicago, Illinois. AIDS Patient Care STDs. 2020;34(8):331‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabello A, Zamarro B, Nistal S, et al. COVID‐19 in people living with HIV: a multicenter case‐series study. Int J Infect Dis. 2021;102:310‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W, Ming F, Feng Y, et al. Patterns of HIV and SARS‐CoV‐2 co‐infection in Wuhan, China. J Int AIDS Soc. 2020;23(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins J. COVID‐19 Map‐Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center; 2020;1. [Google Scholar]
- 26. HIV, W . AIDS Data and Statistics. 2019.
- 27. Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID‐19 in HIV‐infected individuals: a systematic review and meta‐analysis. Sci Rep. 2021;11(1):6283. doi: 10.1038/s41598-021-85359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang R, Gui X, Zhang Y, Xiong Y, Gao S, Ke H. Clinical characteristics of COVID‐19 patients with HIV coinfection in Wuhan, China. Expert Rev Respir Med. 2021;15(3):403‐409. doi: 10.1080/17476348.2021.1836965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID‐19 in HIV‐infected individuals: a systematic review and meta‐analysis. Sci Rep. 2021b;11(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanwugu ON, Adadi P. HIV/SARS‐CoV‐2 coinfection: a global perspective. J Med Virol. 2021;93(2):726‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morani Z, Patel S, Ghosh S, et al. COVID‐19 in HIV: a review of published case reports. SN Comp Clin Med. 2020;1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vizcarra P, Pérez‐Elías MJ, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals: a single‐centre, prospective cohort. Lancet HIV. 2020;7(8):e554‐e564. doi: 10.1016/s2352-3018(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. SeyedAlinaghi S, Karimi A, MohsseniPour M, et al. The clinical outcomes of COVID‐19 in HIV‐positive patients: a systematic review of current evidence. Immun Inflamm Dis. 2021;9(4):1160‐1185. doi: 10.1002/iid3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ucciferri C, Auricchio A, Di Nicola M, et al. Canakinumab in a subgroup of patients with COVID‐19. Lancet Rheumatol. 2020;2(8):e457‐e458. doi: 10.1016/s2665-9913(20)30167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Makoti P, Fielding BC. HIV and human coronavirus coinfections: a historical perspective. Viruses. 2020;12(9):937. doi: 10.3390/v12090937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeJesus E, Herrera G, Teofilo E, et al. Abacavir versus zidovudine combined with lamivudine and efavirenz, for the treatment of antiretroviral‐naive HIV‐infected adults. Clin Infect Dis. 2004;39(7):1038‐1046. doi: 10.1086/424009 [DOI] [PubMed] [Google Scholar]
- 38. Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251‐260. doi: 10.1056/NEJMoa051871 [DOI] [PubMed] [Google Scholar]
- 39. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID‐19 in HIV‐positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173(7):536‐541. doi: 10.7326/m20-3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suwanwongse K, Shabarek N. Clinical features and outcome of HIV/SARS‐CoV‐2 coinfected patients in The Bronx, New York City. J Med Virol. 2020;92(11):2387‐2389. doi: 10.1002/jmv.26077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffmann C, Casado JL, Härter G, et al. Immune deficiency is a risk factor for severe COVID‐19 in people living with HIV. HIV Med. 2021;22(5):372‐378. doi: 10.1111/hiv.13037 [DOI] [PubMed] [Google Scholar]
- 43. Zhang H, Wu T. CD4+T, CD8+T counts and severe COVID‐19: a meta‐analysis. J Infect. 2020;81(3):e82‐e84. doi: 10.1016/j.jinf.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun S, Hou J, Chen Y, Lu Y, Brown L, Operario D. Challenges to HIV care and psychological health during the COVID‐19 pandemic among people living with HIV in China. AIDS Behav. 2020;24(10):2764‐2765. doi: 10.1007/s10461-020-02903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the manuscript.


