Abstract
Background
Mycoplasma hominis is the smallest prokaryotic microorganism with no cell wall, high pleomorphism, and slower reproduction than bacteria. It is difficult for clinical technicians to find M. hominis through the negative Gram staining of specimens. Therefore, it is likely to miss detection in routine clinical smear etiological examination. M. hominis is generally considered to be a common colonizing bacterium in urogenital tract with low pathogenicity, and it is usually difficult to invade submucosal tissue and enter the bloodstream.
Methods
The abscesses of the patient were examined histopathologically, and the pus in the abscesses was extracted for etiological examination. MALDI‐TOF MS was used to identify and confirmed the pathogens in the specimens. The commercial Mycoplasma isolation, culture, and drug sensitivity kit was used to determine antibiotic susceptibility.
Results
No pathogens were found after pathological and smear microscopic examination of the puncture fluid from the sacrococcygeal and pelvic abscesses. Until 48 h later, small, translucent, and gray‐white colonies were observed in the blood plate culture results. The laboratory physician ultimately determined that the pathogen was M. hominis by MALDI‐TOF MS.
Conclusion
We report a case of extra‐urogenital cystic abscesses infected by M. hominis, in order to improve clinicians’ comprehensive understanding of the pathogenicity of Mycoplasma. In addition, the clinical laboratory technician should pay attention to the role of Wright–Giemsa staining of puncture fluid smear in the preliminary detection and the application of MALDI‐TOF MS in identification of uncommon pathogenic microorganisms.
Keywords: matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF MS), Mycoplasma hominis (M. hominis MH), puncture fluid, sacrococcygeal and pelvic abscesses
It is generally considered that M. hominis is a low virulence opportunistic pathogen and rarely invades tissue. We report a case of extra‐urogenital cystic abscesses infected by M. hominis, in order to improve clinicians' comprehensive understanding of the pathogenicity of Mycoplasma. No pathogens were found after pathological and smear microscopic examination of the puncture fluid from the sacrococcygeal and pelvic abscesses. Until 48 hours later, small, translucent and gray white colonies were observed in the blood plate culture results. The laboratory physician ultimately determined that the pathogen was M. hominis by MALDI‐TOF MS.
1. INTRODUCTION
Mycoplasma hominis (M. hominis) is a common colonization bacterium in the urogenital tract, it can be universally isolated from sexually mature women, approximately 21% to 53% of asymptomatic women are colonized with M. hominis, and the colonization rate of male urethra can be as high as 20%. 1 , 2 Under certain conditions, M. hominis can cause urogenital tract infections such as pelvic inflammation and cervicitis, but its pathogenicity is weak. Generally, it only causes genital tract mucosal surface infection and does not invade tissues and blood. 3 , 4 In this case, the special clinical manifestations of extra‐urogenital cystic abscesses caused by M. hominis infection were identified by flight mass spectrometry rather than routine Gram staining and colony morphology.
2. CASE PRESENTATION
2.1. Patient and basic clinical information
A 70‐year‐old man with more than half a year's history of an oval cystic abscess in sacrococcygeal region presented to the outpatient department with increasingly unbearable pain and fidgety inconvenience. High‐resolution computed tomography (HRCT) of the sacrococcygeal vertebrae showed two connected oval cystic lesions involving in subcutaneous tissue and pelvic cavity (Figure 1). The surgeon performed resection of sacrococcygeal and pelvic abscesses at the request of the patient. The incision was about 7 cm long along the long axis of the cyst, the skin and subcutaneous tissue were cut in turn, the surrounding adhesive tissue was separated along the surface of the cyst, the cyst was completely stripped, the root was ligated with No. 4 silk thread, continued to separate downward, the pelvic abscess of about 40 × 30 mm was stripped, the subcutaneous fascia was free, sutured intermittently, and the incision was closed layer by layer.
FIGURE 1.
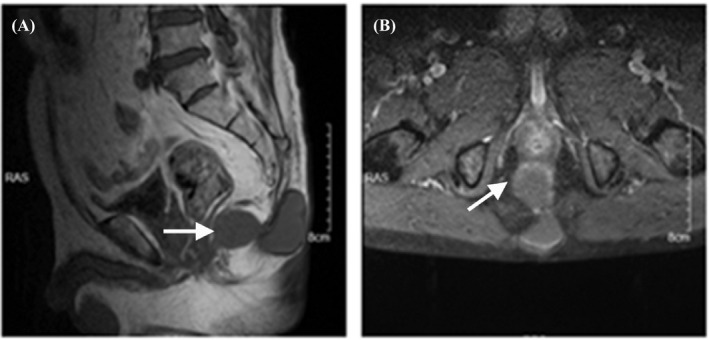
High‐resolution computed tomography (HRCT) of the sacrococcygeal vertebrae showed oval cystic lesions involving in subcutaneous tissue and pelvic cavity. Figure A and Figure B represented the location and size of the abscess under the lateral position and frontal position, respectively. There was an oval cystic lesion near the fifth cone of sacrum and subcutaneous tissue, respectively, and the two lesions were connected. The size of the larger abscess was about 50 × 30 mm, and the size of the smaller abscess was about 40 × 30 mm. White arrow indicated the abscesses
2.2. Routine smear etiological examination of histopathological and Gram staining
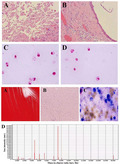
Both histopathological examination and Gram staining results of the specimens revealed numerous polymorphonuclear leukocytes with no visible pathogens (Figure 2). Pinpoint‐sized translucent and gray‐white colonies were observed on blood agar following 48 h of incubation under 5% CO2 at 37 °C (Figure 3A). Microorganisms dyed lavender of different shapes were observed by Wright–Giemsa staining under a 100× oil microscope (Figure 3B). VITEK 2‐compact automatic bacterial detection and analysis system (BioMérieux), one of the most commonly used identification methods of bacteria and Candida in clinic, was failed in this detection.
FIGURE 2.
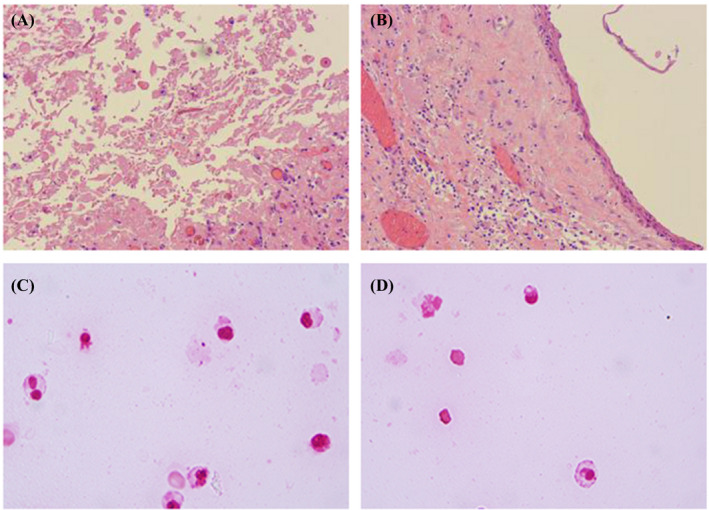
Initial pathogen‐negative results from histopathological examination and Gram staining of abscess tissue and puncture fluid. Figure A and Figure B represented histopathological findings of pelvic and sacrococcygeal abscesses, respectively. The appearance of pelvic abscess was grayish red, the size was 40 × 30 × 14 mm, the section was cystic, the capsule wall thickness was 1 mm, the fibrous capsule wall tissue was accompanied by multifocal lymphocytes infiltration, part of the capsule wall tissue was covered with squamous epithelial cells, the inner wall of the capsule was smooth, and no obvious vegetations were found. The appearance of the sacrococcygeal abscess was grayish red, with the size of 52 × 32 × 25 mm and a cystic appearance in the section, and a brick‐red body in the capsule. The capsule wall thickness was 5 mm, locally calcified. Some cystic wall tissues were covered with squamous epithelial cells, and a small amount of keratinocytes and foam cells could be seen. Figure C and Figure D represented Gram staining results of pelvic and sacrococcygeal abscess, respectively. Both Gram staining results revealed numerous polymorphonuclear leukocytes with no visible pathogens
FIGURE 3.
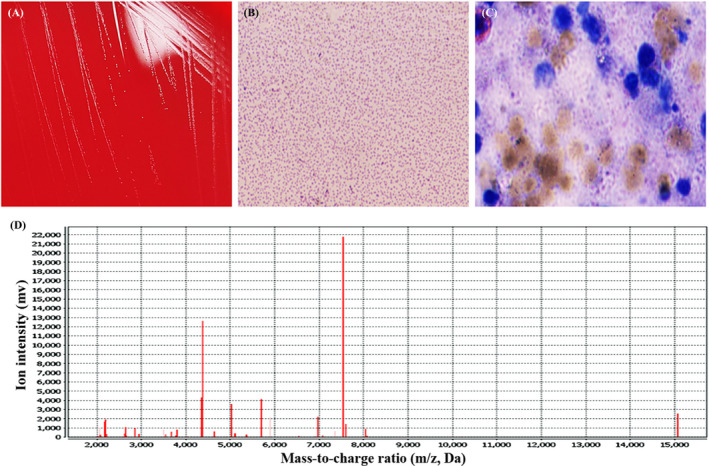
Positive etiological analysis images of colony morphology, Wright–Giemsa staining, and MALDI‐TOF MS detection. Pinpoint‐sized translucent and gray‐white colonies, later identified as M. hominis, on blood agar following 48 h of incubation under 5% CO2 at 37 °C (Figure A). The colonies on the blood agar plate were stained with Wright–Giemsa staining and small round or rod‐shaped bacteria dyed lavender could be observed under a 100× oil microscope (Figure B). We re‐stained the puncture fluid of the abscess, and Wright–Giemsa staining was used. The results showed that the pleomorphic leukocytes were surrounded by microorganisms of different shapes dyed lavender, with a diameter of about 1–3 mm (100× oil lens and 10× eyepiece) (Figure C). Matrix‐assisted laser desorption ionization–time of flight mass spectrometry (MALDI‐TOF MS) (BioMérieux) was used to identify and confirmed that the pathogen was M. hominis with the confidence of 99. 9% (database VITEK MS IVD 3.0 and SARAMIS V4.14) (Figure D)
2.3. MALDI‐TOF MS identification and Wright–Giemsa staining results
MALDI‐TOF MS was used to identify and confirmed that the pathogen was M. hominis with the confidence of 99. 9% (database VITEK MS IVD 3.0 and SARAMIS V4.14) (Figure 3D). Wright–Giemsa staining results of the puncture fluid of the abscesses showed that the existence of the microorganisms of different shapes was confirmed again (Figure 3C).
2.4. Antimicrobial susceptibility of the isolated strain
The commercial Mycoplasma (Ureaplasma urealyticum / M. hominis) isolation, culture, and drug sensitivity kit (Zhuhai DL) was used to determine antibiotic susceptibility by using the Clinical and Laboratory Standards Institute (CLSI) breakpoints. 5 The isolate was susceptible to doxycycline, erythromycin, minocycline, and josamycin, while resistant to ciprofloxacin, clindamycin, tetracycline, levofloxacin, ofloxacin, roxithromycin, sparfloxacin, and azithromycin (Table 1). After 3 days of anti‐infection treatment, the patient's condition improved significantly and was discharged after expert evaluation.
TABLE 1.
Antibiotic sensitivity test results of the isolate from the puncture fluid of the abscesses of the patient
| Antibiotics | Quantitative results (μg/ml) | Sensitivity | Methodology | Break point range of antibiotic sensitivity |
|---|---|---|---|---|
| Ciprofloxacin | ≥4 | Resistant | MIC | 0.25–1 |
| Clindamycin | ≥8 | Resistant | MIC | Derived from the results |
| Doxycycline | ≤4 | Susceptible | MIC | 4–6 |
| Erythromycin | ≤1 | Susceptible | MIC | Derived from the results |
| Tetracycline | ≥16 | Resistant | MIC | 4–6 |
| Levofloxacin | ≥8 | Resistant | MIC | Derived from the results |
| Minocycline | ≤4 | Susceptible | MIC | 4–6 |
| Ofloxacin | ≥8 | Resistant | MIC | 2–8 |
| Roxithromycin | ≥8 | Resistant | MIC | Derived from the results |
| Sparfloxacin | ≥8 | Resistant | MIC | 0.5–2 |
| Azithromycin | ≥8 | Resistant | MIC | 2–8 |
| Josamycin | ≤2 | Susceptible | MIC | Derived from the results |
3. DISCUSSION
Urogenital inflammation caused by M. hominis infection mainly includes urinary tract infection, cervicitis, pelvic inflammation, vaginitis, chorioamnionitis, and premature abortion and infertility related to Mycoplasma infection. These diseases have been widely reported and are often limited to the surface of urogenital mucosa. 6 , 7 , 8 , 9 It is generally considered that M. hominis is a low virulence opportunistic pathogen. 10 , 11 Therefore, invasive M. hominis infection outside the urogenital tract is possible to be ignored by clinicians. What is more worrying is that M. hominis is gram‐negative due to its lack of cell wall and has no response to all antibiotics targeting cell wall synthesis. 12 This special biological characteristic makes it can not only escape the routine etiological examination such as Gram staining, but also escape the clinician's empirical anti‐infection treatment. The growth rate of Mycoplasma on the blood agar medium routinely used for pathogen proliferation is slower than that of common bacteria, it usually takes 48 h or even longer, and the colony morphology is not typical. 13 , 14 Undetected report or untimely pathogen identification may cause an aggravation of the inflammation and lead to poor outcomes of complications and prolonged hospital stays.
At present, the clinical diagnosis of invasive M. hominis infection outside the urogenital tract is facing great challenges. In this case, clinicians did not tend to consider it as M. hominis infection at first, and they chose the etiological screening of puncture fluid rather than targeted Mycoplasma identification. From the results of histopathological examination and Gram staining, we intuitively found that a large number of leukocytes such as lymphocytes and granulocytes were distributed in the visual field, but there was a lack of visible microorganisms. Therefore, when we encountered such a contradictory phenomenon, the increased number of leukocytes indicated that there was inflammation in this region with a fierce struggle to resist the invasion of bacteria, but there was no colored microorganism, and the infection possibility of pathogenic bacteria with negative conventional staining such as M. hominis should be considered. In this case, the pathogen of the abscesses was identified as M. hominis by MALDI‐TOF MS. MALDI‐TOF MS technology is a new soft ionization organic mass spectrometry developed in recent years. 15 , 16 By detecting the characteristic protein peaks of the pathogenic microorganisms, the colonies can be identified and classified directly in a few minutes. At present, the flight mass spectrometry database contains 14 main Mycoplasmas causing human diseases, such as M. hominis and M. pneumoniae. This method has the advantages of simple and fast operation, relatively low cost, accurate identification results, and high repeatability, and significantly reduces the cost of consumables and the time of identification and diagnosis. When the traditional colony morphology identification and VITEK 2‐compact automatic bacterial detection and analysis system cannot be determined in clinical laboratory, MALDI‐TOF MS is a favored alternative method.
Extra‐urogenital tract infection caused by invasive M. hominis should be paid attention to. In recent years, for patients with low immune function or defects, M. hominis can penetrate the mucosa, invade the vascular walls, and spread to all tissues and organs of the body. 17 , 18 , 19 Surgical incision infection, postoperative blood flow infection, post cardiopulmonary transplantation infection, and other cases have been reported from time to time. 20 , 21 , 22 In addition, infants whose immune function is not yet fully developed often have serious clinical manifestations by infecting M. hominis in utero or through vertical transmission. 23 The latest research found that M. hominis is the main respiratory pathogens in severe novel coronavirus pneumonia in the investigation of SARS‐CoV‐2‐related microbial dysbiosis and various antibiotic‐resistant respiratory pathogens in hospitalized COVID‐19 patients, including 8 mildly and 15 severely ill patients in Guangdong province, China. 24
4. CONCLUSION
Although M. hominis as a colonization of urogenital tract is common in the human body, attention should be paid to the possibility of invasive M. hominis infection when patients have chronic diseases such as trauma, diabetes and infectious diseases such as HIV infection, and other immunodeficiency or deficiency conditions, such as tumor and chemotherapy, organ transplant, and immunosuppressive usage. Because the selection of antibiotics for the treatment of M. hominis infection is highly targeted, timely and accurate etiological results are of great positive significance to prevent antibiotic resistance, shorten the course of disease, and reduce the cost of treatment.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
FS collects the patient clinical information. JWZ, YZZ, and HYL analyzed the data. YMG drawn the manuscript. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Written and informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
ACKNOWLEDGMENTS
We thank all members of the microbiology laboratory of Zhejiang Provincial People's Hospital for their help in the collection of clinical data and thank the patient for his cooperation.
Su F, Zhang J, Zhu Y, Lv H, Ge Y. Identification of sacrococcygeal and pelvic abscesses infected with invasive Mycoplasma hominis by MALDI‐TOF MS. J Clin Lab Anal. 2022;36:e24329. doi: 10.1002/jcla.24329
Fang Su and Junwu Zhang made the same contribution to this article.
REFERENCES
- 1. Waites KB, Xiao L, Paralanov V, et al. Molecular methods for the detection of Mycoplasma and ureaplasma infections in humans: a paper from the 2011 William Beaumont Hospital Symposium on molecular pathology. J Mol Diagn. 2012;14(5):437‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horner P, Donders G, Cusini M, et al. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?‐a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32(11):1845‐1851. [DOI] [PubMed] [Google Scholar]
- 3. Shao L, Wu X, Gao S, et al. Epidemiological investigation and antimicrobial susceptibility analysis of Ureaplasma and Mycoplasma hominis in a teaching hospital in Shenyang, China. J Infect Chemother. 2021;27(8):1212‐1216. [DOI] [PubMed] [Google Scholar]
- 4. Stabler S, Faure E, Duployez C, et al. The brief case: Mycoplasma hominis Extragenital abscess. J Clin Microbiol. 2021;59(4):e02343‐e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute . Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas; Approved Guideline; CLSI document M43‐A. Clinical and Laboratory Standards Institute: 2011. [PubMed] [Google Scholar]
- 6. Agger WA, Siddiqui D, Lovrich SD, et al. Epidemiologic factors and urogenital infections associated with preterm birth in a midwestern U.S. population. Obstet Gynecol. 2014;124(5):969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vouga M, Greub G, Prod'hom G, et al. Treatment of genital mycoplasma in colonized pregnant women in late pregnancy is associated with a lower rate of premature labour and neonatal complications. Clin Microbiol Infect. 2014;20(10):1074‐1079. [DOI] [PubMed] [Google Scholar]
- 8. Paira DA, Molina G, Tissera AD, et al. Results from a large cross‐sectional study assessing Chlamydia trachomatis, Ureaplasma spp. and Mycoplasma hominis urogenital infections in patients with primary infertility. Sci Rep. 2021;11(1):13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plummer EL, Vodstrcil LA, Bodiyabadu K, et al. Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women? Clin Infect Dis. 2021;73(4):659‐668. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Wang Y, Zhu J, et al. A sensitive and rapid assay for Mycoplasma hominis detection based on recombinase polymerase amplification. Clin Lab. 2021;67(4):1‐2. [DOI] [PubMed] [Google Scholar]
- 11. Saadat S, Karami P, Jafari M, et al. The silent presence of Mycoplasma hominis in patients with prostate cancer. Pathog Dis. 2020;78(7):37. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Ren D, Li H, et al. Periprosthetic joint infection caused by Mycoplasma hominis, diagnosed using metagenomic sequencing. Int J Gen Med. 2021;14(1):7003‐7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardot Martin E, Dolidon S, Lesprit P, et al. A strain uncoloured by Gram staining in a pleural fluid. Clin Microbiol Infect. 2021;S1198–743X(21):467‐475. [DOI] [PubMed] [Google Scholar]
- 14. Spiller OB. Emerging pathogenic respiratory Mycoplasma hominis infections in lung transplant patients: time to reassesses it's role as a pathogen? EBioMedicine. 2017;19(1):8‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gallo R, Tamborini AL, Di Bella H, et al. Actinotignum schaalii: reporte de dos casos de bacteriemias en Argentina [Actinotignum schaalii: Report of two cases of bacteremia in Argentina]. Rev Argent Microbiol. 2021;S0325–7541(21):97‐103. [DOI] [PubMed] [Google Scholar]
- 16. Sekercioglu AO, Cekin Y, Ogunc D, et al. Matrix‐assisted laser desorption‐ionization time‐of‐flight mass spectrometry (MALDI‐ TOF MS) for early identification of septic patients. Clin Lab. 2017;63(4):839‐844. [DOI] [PubMed] [Google Scholar]
- 17. Smibert OC, Wilson HL, Asma S, et al. Donor‐derived Mycoplasma hominis and an apparent cluster of M. hominis cases in solid organ transplant recipients. Clin Infect Dis. 2017;65(9):1504‐1508. [DOI] [PubMed] [Google Scholar]
- 18. Sampath R, Patel R, Cunningham SA, et al. Cardiothoracic transplant recipient Mycoplasma hominis: an uncommon infection with probable donor transmission. EBioMedicine. 2017;19(1):84‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer RD, Clough W. Extragenital Mycoplasma hominis infections in adults: emphasis on immunosuppression. Clin Infect Dis. 1993;17(1):S243‐249. [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Huang Z, Fang X, et al. Diagnosis and treatment of Mycoplasmal septic arthritis: a systematic review. Int Orthop. 2020;44(2):199‐213. [DOI] [PubMed] [Google Scholar]
- 21. Hinić V, Seth‐Smith HMB, Damm S, et al. Unexpected Mycoplasma hominis infection in two renal transplant recipients traced back to the same donor by whole‐genome sequencing. Eur J Clin Microbiol Infect Dis. 2021;40(5):1097‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vecchio M, Koutsokera A, Touilloux B, et al. Bronchial anastomosis dehiscence and stenosis caused by donor‐transmitted Mycoplasma hominis infection in a lung transplant recipient: Case report and literature review. Transpl Infect Dis. 2021;23(2):e13475. [DOI] [PubMed] [Google Scholar]
- 23. Wildenbeest JG, Said I, Jaeger B, et al. Neonate with Mycoplasma hominis meningoencephalitis given moxifloxacin. Lancet Infect Dis. 2016;16(11):e261‐e266. [DOI] [PubMed] [Google Scholar]
- 24. Zhong H, Wang Y, Shi Z, et al. Characterization of respiratory microbial dysbiosis in hospitalized COVID‐19 patients. Cell Discov. 2021;7(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]


