Abstract
Background
The study aimed at evaluating the prognostic utility of the prognostic nutritional index (PNI) for patients with sepsis.
Methods
Data in the present study were obtained from the Multiparameter Intelligent Monitoring in Intensive Care Database III. The calculation for PNI was as follows: serum albumin concentration (g/L) +0.005 × total lymphocyte count. 30‐day mortality was considered as the primary outcome, while 90‐day mortality and one‐year mortality were the secondary outcomes. Cox proportional risk models and propensity score matching (PSM) analyses were used to analyze the association between PNI and clinical outcomes in patients with sepsis. To assess the predictive value of PNI for 30‐day mortality, receiver operator characteristic (ROC) curve analysis was performed.
Results
A total of 2669 patients were in the study. After the confounding factors were adjusted, PNI ≥ 29.3 was identified as an independent predictive prognostic factor for the 30‐day all‐cause mortality (hazard ratio [HR]: 0.65; 95% confidence interval [CI]: 0.56–0.76; p < 0.00001). Moreover, PSM analysis further validated the prognostic predictive value of PNI for patients with sepsis. The AUC of the PNI was 0.6436 (95% CI: 0.6204–0.6625) which was significantly high than the AUC of NLR (0.5962, 95% CI: 0.5717–0.6206) (p = 0.0031), the RDW (0.5878, 95% CI: 0.5629–0.6127) (p < 0.0001), and PLR (0.4979, 95% CI: 0.4722–0.5235) (p < 0.0001).
Conclusion
The findings suggested that PNI was also a significant risk factor for sepsis.
Keywords: intensive care unit, mortality, prognostic nutritional index, sepsis
The findings suggested that PNI, a reproducible and readily available indicator for predicting the clinical outcomes of sepsis patients, was also a significant risk factor for sepsis.
1. INTRODUCTION
Sepsis is caused by an aberrant regulatory host response toward an infection. It is linked to acute organ dysfunction and associated with high mortality. 1 , 2 Despite advances in care and treatment, sepsis remains the costliest disease to treat, with over $20 billion (5.2%) being spent annually in the United States. 3 The number of hospitalizations for sepsis cases in the United States even exceeds those for stroke and myocardial infarctions. 4 According to the statistics, 5 the incidence of sepsis is 500 per 100,000 cases and is on the rise. Globally, there are over 31 million sepsis cases; among them, 19.4 million cases are of severe sepsis associated with a mortality of 25%. 4 , 6 However, given the limitations, including the timely identification of patients having sepsis, it poses a challenge for clinical management of the patients. Therefore, early detection of patients with severe sepsis would play an important role in improving their prognoses. 7 , 8
Many preclinical and clinical studies show that the interactions between host‐derived molecules and foreign molecule productions stimulated by pathogenic microorganisms through the pathogen recognition receptors expressed on immune cells, thereby causing an unbalanced activation of innate immunity, are based on sepsis. 9 , 10 , 11 Furthermore, the crosstalk between systemic inflammatory mechanisms is important in the pathogenesis of sepsis. 12 , 13 As immune dysregulation worsens continually, oxidative stress also intensifies, eventually culminating into a cascade of redox‐related cellular damage, impaired mitochondrial function, and exacerbated inflammation. Thus, patients with sepsis who are at risk of immune deterioration should be identified and treated before the onset of organ dysfunction. 14 Therefore, the identification of immune‐related predictors in sepsis has great applicability for improving the diagnosis, evaluation, and treatment strategies for sepsis‐related complications. 15
Neutrophil/lymphocyte ratio 16 , 17 (NLR), red cell distribution width 17 , 18 (RDW), platelet‐to‐lymphocyte ratio(PLR), and monocyte/high‐density lipoprotein cholesterol ratio 19 (MHR) are simple markers of inflammation; these have been validated for their long‐term prognostic predictive abilities in sepsis patients. Prognostic nutritional index (PNI) is a comprehensive and novel biomarker of inflammation based on albumin levels and lymphocytes. 20 , 21 , 22 The PNI was initially used for prognostic assessment of patients with cancer 22 , 23 ; however, at present, it is thought to better reflect the inflammatory status and nutritional status of patients. 24 , 25 The role of PNI in the prognoses of patients with sepsis remains unclear. Thus, we reasonably hypothesized that a high PNI would indicate higher mortality in sepsis patients, given their enhanced levels of inflammation and poorer nutritional statuses. A retrospective cohort study was performed which aimed to assess the link between PNI and patient prognoses in sepsis. We also adjusted the parameters for the potential confounding factors to determine the role of PNI in predicting the mortality of patients with sepsis.
2. METHODS
2.1. Source of data
The data in the present study were obtained from the MIMIC‐III database (version 1.4) 26 , 27 and included the definitive health records of more than 50,000 critically ill patients, who were admitted between 2001 and 2012, to the Beth Israel Deaconess Medical Center (Boston, MA, USA). The design of the database was approved by the Institutional Review Board of the Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA). To protect the privacy of the included patients, their information data were entirely deleted.
2.2. Criteria for inclusion
The database contained the records of a total of 58,976 ICU patients diagnosed with sepsis according to the International Classification of Diseases (ICD‐9) codes 99591 and 99592. Inclusion criteria were as follows: all patients aged >16 years at the time of their first admission and hospitalization for more than two days. Patients with the following criteria were excluded from this study: (1) missing PNI data; (2) missing data representing more than 10% of total data; (3) diagnosis of liver diseases (chronic hepatitis C, chronic viral hepatitis B, liver cirrhosis, and/or autoimmune hepatitis).
2.3. Data extraction
Data extraction was performed using the SQL. The patient information included their gender, age, race, heart rate, respiratory rate, temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean pressure, SPO2, and multiple comorbidities included coronary heart disease (CHD), pneumonia, acute respiratory distress syndrome (ARDS), and stroke. Laboratory parameters included lymphocyte count, albumin, and WBC count. The severity of illness was measured using sequential organ failure assessment 28 , 29 and simplified acute physiology score II. 30 Variables with missing values ≥40% were excluded directly. Follow‐up duration was measured from the first day of admission up to the event of death. Clinical outcomes were the values of 30‐day mortality, 90‐day mortality, and one‐year mortality. Patient information was recorded within 24 h of their admission to the ICU. PNI calculation was as follows: serum albumin concentration (g/L) +0.005 × total lymphocyte count. 31
2.4. Statistical analysis
According to their PNI values, the baseline patient characteristics were divided into three groups. Continuous variables were expressed as mean ± standard deviation (mean ± SD); categorical data were expressed in frequencies. In addition, continuous variables were tested for significance using the Kruskal–Wallis test; the chi‐squared test or Fisher's exact test was used for categorical variables. The relationship between PNI and patient mortality was ascertained based on the Cox proportional risk model and expressed as the HR and corresponding 95% CI value. In addition, multivariate analysis was used to control for the corresponding confounding factors; in model I, the confounding factors, including age, gender, and race, were adjusted, while in model II, confounding factors, including age, sex, race, diastolic blood pressure, heart rate, respiratory rate, temperature, SpO2, heart failure, anion gap, platelet, and serum chloride, were adjusted. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive power of SOFA, SOFA + PNI, PNI, NLR, PLR, and RDW for mortality in patients with sepsis. PSM analysis was performed to avoid potential bias due to the differences in baseline characteristics. Moreover, the PSM analysis was performed at a 1:1 ratio; the standard caliper width was set at 0.01, and the two‐tailed p value < 0.05 was considered statistically significant. The R software (version: 4.01; the R Foundation) was used to perform all statistical analyses.
3. RESULTS
3.1. Patient characteristics
A total of 2669 patients with sepsis were retrospectively enrolled, including 1483 males and 1186 females, with a mean age of 66.2 ± 16.5 years. According to the absence or presence of 30‐day mortality, the patients were classified into survival and mortality groups; data for a total of 719 patients in the mortality group and 1950 in the survival group were analyzed. The patient baseline information is listed in Table 1. The patients in the 30‐day mortality group were older and had significantly higher SIRI scores, sequential organ failure assessment (SOFA) scores, SAPS II scores, and PNI as compared to those in the survival group. Vital signs, including SPO2, body temperature, SBP, DBP, and MBP, were lower in the mortality group, while the mean heart rate in the mortality group was greater than that in the survival group. Laboratory indicators were used for the assessment of organ functions for both groups of patients. The results demonstrated that patients in the mortality group exhibited poorer liver and kidney functions than those in the survival group.
TABLE 1.
Baseline characteristics of the study population
| Characteristics | Total | Survival | Mortality | p Value |
|---|---|---|---|---|
| N | 2669 | 1950 | 719 | |
| Age, years | 66.2 ± 16.5 | 65.1 ± 16.9 | 69.4 ± 14.9 | <0.001 |
| Sex, n (%) | 0.236 | |||
| Male | 1483 (55.6%) | 1070 (54.9%) | 413 (57.4%) | |
| Female | 1186 (44.4%) | 880 (45.1%) | 306 (42.6%) | |
| Ethnicity, n (%) | 0.006 | |||
| White | 1964 (73.6%) | 1440 (73.8%) | 524 (72.9%) | |
| Black | 271 (10.2%) | 214 (11.0%) | 57 (7.9%) | |
| Other | 434 (16.3%) | 296 (15.2%) | 138 (19.2%) | |
| Vital signs | ||||
| SBP, mmHg | 109.0 ± 14.0 | 110.5 ± 14.0 | 105.1 ± 13.1 | <0.001 |
| DBP, mmHg | 57.1 ± 9.6 | 58.0 ± 9.5 | 54.4 ± 9.3 | <0.001 |
| MAP, mmHg | 72.1 ± 9.6 | 73.0 ± 9.5 | 69.7 ± 9.5 | <0.001 |
| Heart rate, beats/min | 92.7 ± 17.8 | 92.1 ± 17.5 | 94.5 ± 18.4 | <0.001 |
| Respiratory rate, t/min | 21.4 ± 4.7 | 21.2 ± 4.6 | 22.1 ± 4.8 | <0.001 |
| Temperature, ℃ | 36.8 ± 0.8 | 36.9 ± 0.8 | 36.6 ± 0.9 | <0.001 |
| SpO2, % | 96.5 ± 4.0 | 96.9 ± 2.7 | 95.3 ± 6.2 | <0.001 |
| Comorbidities | ||||
| Heart failure, n (%) | 541 (20.3%) | 413 (21.2%) | 128 (17.8%) | 0.054 |
| CHD, n (%) | 477 (17.9%) | 349 (17.9%) | 128 (17.8%) | 0.955 |
| Stroke, n (%) | 136 (5.1%) | 89 (4.6%) | 47 (6.5%) | 0.040 |
| ARDS, n (%) | 64 (2.4%) | 48 (2.5%) | 16 (2.2%) | 0.723 |
| Pneumonia, n (%) | 1124 (42.1%) | 800 (41.0%) | 324 (45.1%) | 0.061 |
| Laboratory parameters | ||||
| PNI | 34.3 ± 24.9 | 34.4 ± 9.1 | 34.1 ± 45.7 | <0.001 |
| Albumin, g/dL | 2.8 ± 0.7 | 2.9 ± 0.7 | 2.7 ± 0.7 | <0.001 |
| WBC, 109/L | 15.1 ± 12.8 | 14.7 ± 12.3 | 15.9 ± 14.2 | 0.087 |
| Platelet, 109/L | 228.1 ± 152.2 | 233.9 ± 152.6 | 212.3 ± 150. | <0.001 |
| Lymphocyte, % | 9.9 ± 11.6 | 10.0 ± 11.4 | 9.7 ± 12.2 | 0.017 |
| Creatinine, mg/dl | 2.3 ± 2.0 | 2.2 ± 2.0 | 2.7 ± 1.9 | <0.001 |
| BUN, mg/dl | 42.7 ± 29.0 | 39.3 ± 27.8 | 52.1 ± 30.3 | <0.001 |
| Serum chloride, mg/dl | 108.8 ± 7.5 | 109.0 ± 7.3 | 108.2 ± 8.1 | 0.005 |
| Serum sodium, mg/dl | 140.5 ± 6.1 | 140.5 ± 5.9 | 140.3 ± 6.7 | 0.262 |
| Anion gap, mg/dl | 18.7 ± 5.6 | 17.9 ± 4.9 | 20.7 ± 6.6 | <0.001 |
| Lactate, mol/L | 3.9 ± 3.3 | 3.4 ± 2.5 | 5.4 ± 4.5 | <0.001 |
| Scoring systems | ||||
| SAPSII score | 47.3 ± 16.4 | 43.5 ± 14.9 | 57.4 ± 15.7 | <0.001 |
| SOFA score | 7.5 ± 4.0 | 6.8 ± 3.6 | 9.6 ± 4.2 | <0.001 |
| Length of stay in ICU | 7.3 ± 9.1 | 7.8 ± 10.0 | 5.8 ± 5.8 | <0.001 |
| Length of stay in hospital | 14.1 ± 15.4 | 16.2 ± 17.0 | 8.4 ± 7.2 | <0.001 |
Abbreviations: ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; CHD, coronary heart disease; DBP, diastolic blood pressure; ICU, intensive care unit; MAP, mean arterial pressure; PNI, prognostic nutritional index; SAPSII, simplified acute physiology score II; SBP, systolic blood pressure; SOFA, sequential organ failure assessment; WBC, white blood cell.
3.2. Relationship between PNI and all‐cause mortality in sepsis patients
Following adjustments for the potential confounding variables, we constructed various models to evaluate the independent impacts of PNI on the all‐cause mortality in patients with sepsis. As shown in Table 2, the HR and 95% CI values and the univariate analysis suggested that PNI ≥29.3 was a predictive prognostic factor for 30‐day all‐cause mortality (HR: 0.58; 95% CI: 0.50–0.67; p < 0.00001). After adjustments for other confounding factors, gender, age, race, temperature, DBP, heart rate, respiratory rate, SpO2, HF, AG, platelet count, and serum chloride, the results showed that PNI ≥ 29.3 was an independent predictive prognostic factor for 30‐day all‐cause mortality (HR: 0.65; 95% CI: 0.56–0.76; p < 0.00001).
TABLE 2.
HR (95% CIs) for all‐cause mortality across groups of PNI
| PNI | Model 1 b | Model 2 c | Model 3 d | Model 4 * | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | |
| 30‐day all‐cause mortality | ||||||||
| <29.3 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| ≥29.3 | 0.58 (0.50, 0.67) | <0.0001 | 0.56 (0.48, 0.65) | <0.0001 | 0.65 (0.56, 0.76) | <0.0001 | 0.68 (0.55, 0.85) | <0.0001 |
| 90‐day all‐cause mortality | ||||||||
| <29.3 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| ≥29.3 | 0.59 (0.52, 0.67) | <0.0001 | 0.57 (0.50, 0.65) | <0.0001 | 0.61 (0.56, 0.73) | <0.0001 | 0.67 (0.54, 0.82) | <0.0001 |
| One‐Year all‐cause mortality | ||||||||
| <29.3 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| ≥29.3 | 0.66 (0.59, 0.74) | <0.0001 | 0.63 (0.56, 0.71) | <0.0001 | 0.69 (0.61, 0.78) | <0.0001 | 0.72 (0.59, 0.88) | <0.0001 |
Models 1, 2, and 3 were derived from Cox proportional hazards regression models.
Model 1 covariates were adjusted for nothing.
Model 2 covariates were adjusted for age, sex, and race.
Model 3 covariates were adjusted for age, sex, race, diastolic blood pressure, heart rate, respiratory rate, temperature, SpO2, heart failure anion gap, platelet, and serum chloride.
After PSM.
Similar trends were observed for 90‐day all‐cause mortality and 1‐year all‐cause mortality among the patients with sepsis. PNI ≥ 29.3 was also an independent prognostic factor for 90‐day all‐cause mortality and 1‐year all‐cause mortality among the patients with sepsis (HR: 0.61; 95% CI: 0.56–0.73; p < 0.00001, HR: 0.69; 95% CI: 0.91–0.78; p < 0.00001).
3.3. Propensity score matching analysis
The link between mortality and PNI in patients with sepsis was further assessed through PSM analysis. Baseline characteristics of patients with different PNI values did not differ significantly (Table 3). Cox regression analysis showed that a high PNI (PNI ≥ 29.3) was independently prognostic factor for mortality in sepsis patients (HR: 0.68; 95% CI: 0.55–0.85; p < 0.0001).
TABLE 3.
Characteristics of patients before and after PSM
| Before propensity score | After propensity score | |||
|---|---|---|---|---|
| <29.3 | ≥29.3 | <29.3 | ≥29.3 | |
| N | 850 | 1819 | 785 | 785 |
| Age, years | 65.4 ± 16.0 | 66.6 ± 16.7 | 65.3 ± 15.9 | 65.8 ± 17.0 |
| Sex, n (%) | ||||
| Male | 454 (53.4) | 1029 (56.6) | 421 (53.6) | 410 (52.2) |
| Female | 396 (46.6) | 790 (43.4) | 364 (46.4) | 375 (47.8) |
| Ethnicity, n (%) | ||||
| White | 636 (74.8) | 1328 (73.0) | 583 (74.3) | 581 (74) |
| Black | 78 (9.2) | 193 (10.6) | 74 (9.4) | 72 (9.2) |
| Other | 136 (16.0) | 298 (16.4) | 128 (16.3) | 132 (16.8) |
| Vital signs | ||||
| SBP, mmHg | 106.1 ± 12.8 | 110.4 ± 14.3a | 106.5 ± 12.8 | 106.9 ± 12.0 |
| DBP, mmHg | 55.6 ± 9.0 | 57.7 ± 9.8a | 55.9 ± 9.0 | 55.9 ± 8.9 |
| MAP, mmHg | 70.6 ± 9.0 | 72.8 ± 9.8a | 70.9 ± 8.9 | 70.84 ± 8.79 |
| Heart rate, beats/min | 95.4 ± 18.4 | 91.5 ± 17.4a | 94.6 ± 18.2 | 93.9 ± 17.7 |
| Respiratory rate, t/min | 21.6 ± 4.8 | 21.3 ± 4.6 | 21.6± 4.8 | 21.5 ± 4.8 |
| Temperature, ℃ | 36.7 ± 0.8 | 36.9 ± 0.8a | 36.8 ± 0.8 | 36.7 ± 0.8 |
| SpO2, % | 96.2 ± 5.0 | 96.6 ± 3.5 | 96.2 ± 4.9 | 96.6 ± 3.4 |
| Comorbidities | ||||
| Heart failure, n (%) | 131 (15.4) | 410 (22.5)a | 122 (15.5) | 132 (16.8) |
| CHD, n (%) | 113 (13.3) | 364 (20.0)a | 110 (14) | 117 (14.9) |
| Stroke, n (%) | 39 (4.6) | 97 (5.3) | 37 (4.7) | 32 (4.1) |
| ARDS, n (%) | 16 (1.9) | 48 (2.6) | 16 (2) | 24 (3.1) |
| Pneumonia, n (%) | 313 (36.8) | 811 (44.6)a | 291 (37.1) | 307 (39.1) |
| Laboratory parameters | ||||
| PNI | 25.1 ± 3.5 | 38.7 ± 29.1a | 25.1 ± 3.4 | 39.1 ± 41.9a |
| Albumin, g/dl | 2.2 ± 0.4 | 3.2 ± 0.6a | 2.20 ± 0.4 | 3.06 ± 0.5a |
| WBC, 109/L | 13.9 ± 10.4 | 15.6 ± 13.8a | 13.8 ± 10.3 | 16.2 ± 17.5 |
| Platelet, 109/L | 214.8 ± 159.8 | 234.3 ± 148.1a | 213.7 ± 157.7 | 215.0 ± 138.6 |
| Lymphocyte, % | 8.2 ± 11.7 | 10.7 ± 11.5a | 8.0 ± 11.6 | 10.9 ± 12.3a |
| Creatinine, mg/dl | 2.1 ± 1.6 | 2.4 ± 2.1 | 2.1 ± 1.6 | 2.3 ± 1.9 |
| BUN, mg/dl | 44.3 ± 30.0 | 42.0 ± 28.5a | 44.1 ± 30.5 | 44.1 ± 29.7 |
| Serum chloride, mg/dl | 109.8 ± 7.4 | 108.3 ± 7.6a | 109.8± 7.1 | 109.1 ± 7.78 |
| Serum sodium, mg/dl | 140.0 ± 6.1 | 140.7 ± 6.1a | 140.1 ± 5.9 | 140.9 ± 6.6 |
| Anion gap, mg/dl | 17.9 ± 5.3 | 19.0 ± 5.6a | 17.94 ± 5.40 | 18.08 ± 4.75 |
| Scoring systems | ||||
| SAPSII score | 50.8 ± 16.6 | 45.6 ± 16.0a | 49.8 ± 16.2 | 49.8 ± 16.2 |
| SOFA score | 8.4 ± 4.1 | 7.1 ± 3.9a | 8.2 ± 4.0 | 8.2 ± 4.2 |
| Length of stay in ICU | 8.1 ± 9.8 | 6.9 ± 8.8a | 8.1 ± 9.7 | 6.9 ± 8.8 |
| Length of stay in hospital | 14.7 ± 14.6 | 13.8 ± 15.7a | 14.9 ± 14.7 | 14.5 ± 17.4 |
| 30‐day mortality | 308 (36.2) | 411 (22.6) | 272 (34.6) | 209 (26.6)a |
| 90‐day mortality | 377 (44.4) | 531 (29.2)a | 340 (43.3) | 265 (33.8)a |
| One‐year mortality | 438 (51.5) | 711 (39.1)a | 397 (50.6) | 334 (42.5)a |
Abbreviations: ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; CHD, coronary heart disease; DBP, diastolic blood pressure; ICU, intensive care unit; MAP, mean arterial pressure; PNI, prognostic nutritional index; SAPSII, simplified acute physiology score II; SBP, systolic blood pressure; SOFA, sequential organ failure assessment; WBC, white blood cell.
p < 0.05.
3.4. ROC curve analysis for 30‐Day Mortality
To assess the predictive value of PNI for 30‐day mortality, ROC curve analysis was performed (Figure 1). Table 4 displays the results of the area under curve (AUC) with 95% CI in ROC analysis. The AUC of the PNI was 0.6436(95% CI: 0.6204–0.6625) which was significantly high than the AUC of NLR (0.5962, 95% CI: 0.5717–0.6206) (p = 0.0031), the RDW (0.5878, 95% CI: 0.5629–0.6127) (p < 0.0001), and PLR (0.4979, 95% CI: 0.4722–0.5235) (p < 0.0001). The AUC of the SOFA + PNI was 0.7140 (95% CI: 0.6921–0.7360), which was significantly high than the AUC of SOFA (0.6933, 95% CI: 0.6708–0.7158) (p < 0.0001).
FIGURE 1.
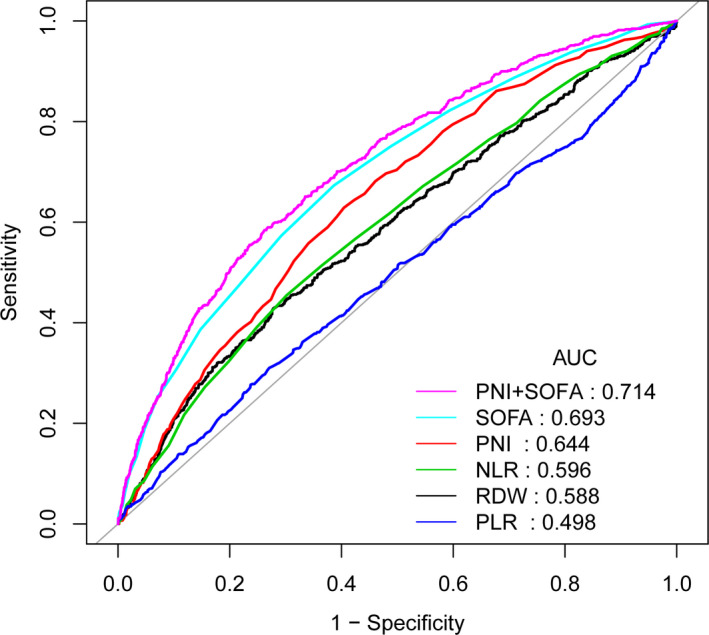
ROC analyses for the prediction of 30‐day mortality. ROC, receiver operating characteristic curve; AUC, area under the curve; AUC, area under the curve; CI, confidence interval; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; RDW, red blood cell distribution width; PNI, Prognostic nutrition index; and SOFA, sequential organ failure assessment
TABLE 4.
Receiver operating curve (ROC) for 30‐day mortality
| AUC | 95%CI low | 95%CI up | |
|---|---|---|---|
| NLR | 0.5962 | 0.5717 | 0.6206 |
| PLR | 0.4979 | 0.4722 | 0.5235 |
| RDW | 0.5962 | 0.5717 | 0.6206 |
| PNI | 0.6436 | 0.6204 | 0.6668 |
| SOFA | 0.6933 | 0.6708 | 0.7158 |
| SOFA + PNI | 0.7140 | 0.6921 | 0.7360 |
Abbreviations: AUC, area under the curve; CI, confidence interval; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutrition index; RDW, red blood cell distribution width; and SOFA, sequential organ failure assessment.
4. DISCUSSION
The present study, to our knowledge, is the first to demonstrate a strong association between PNI and mortality in sepsis patients. We found that the PNI of deceased sepsis patients was significantly greater as compared to those of surviving patients. Another important finding was that low PNI was an independent risk factor of high mortality in patients with sepsis.
Sepsis, caused by infection, is a syndrome of the systemic inflammatory response 1 , 32 ; it is usually evaluated by measuring the body temperature, peripheral blood leukocyte count, neutrophil percentage, C‐reaction protein (CRP) levels, 33 and organ function. However, the sensitivity and specificity of these indicators remain poor; our findings further confirmed their low value in evaluating the severity of sepsis and failure to identify patients with potential poor prognoses. 34 , 35
Nutritional status is closely linked to the prognosis of patients. 36 , 37 Inadequate nutrition leads to poorer survival outcomes in patients with sepsis. 38 , 39 PNI was firstly established by Onodera et al., in 1984. 23 It is a viable tool to ascertain the relationship between the immune‐nutritional state and prognosis of a patient, which has been widely used in the abovementioned evaluation of acute heart failure, 40 esophageal cancer, 41 and lymphoma. 42 The abovementioned study did not consider the confounding factors, which may lead to a mis assessment of the prognostic value of PNI. Our findings showed PNI was an independent risk factor for predicting mortality in sepsis patients. PSM analysis was performed to adjust for patient clinical parameters, which reduced the interference of the confounding factors with the survival outcomes. PNI levels include albumin levels and lymphocyte counts, wherein a low PNI implies hypoalbuminemia and lymphocytopenia. Patients with low PNI may have lower albumin levels, indicative of malnutrition and impaired capacity for protein synthesis. Furthermore, lymphocytes play an important role in the host immune responses, as they fight against the occurrence and progression of sepsis.
However, there are certain limitations to our study as follows: (1) due to the retrospective observational study design, causality could not be determined; therefore, prospective studies are needed in the future to address this issue; (2) PNI is a readily available tool in clinical practice; however, the loss of albumin and lymphocyte count in the database is common, which may cause a selection bias.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENT
Thanks for Feihong Hu help and support.
Wu H, Zhou C, Kong W, Zhang Y, Pan D. Prognostic nutrition index is associated with the all‐cause mortality in sepsis patients: A retrospective cohort study. J Clin Lab Anal. 2022;36:e24297. doi: 10.1002/jcla.24297
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCE
- 1. Fortini A, Faraone A, Meini S, Bettucchi M, Longo B, Valoriani B & Forni S. Validity of "Sepsis‐3" criteria in identifying patients with community‐onset sepsis in Internal Medicine wards; a prospective, multicenter study. European Journal of Internal Medicine. 2021;85:92‐97. doi: 10.1016/j.ejim.2020.12.025 [DOI] [PubMed] [Google Scholar]
- 2. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644‐1655. [DOI] [PubMed] [Google Scholar]
- 3. Shorr AF, Zilberberg MD. Sepsis and septic shock: evolving evidence, evolving paradigms. Seminars in Respiratory and Critical Care Medicine. 2022;43(01):39‐45. doi: 10.1055/s-0041-1740975 [DOI] [PubMed] [Google Scholar]
- 4. Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng B, Xie G, Yao SL, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35(11):2538‐2546. [DOI] [PubMed] [Google Scholar]
- 6. Sands KE. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA, J Am Med Assoc. 1997;278(3):234. [PubMed] [Google Scholar]
- 7. Cao W, Yun Q, Chang C & Ji Y. Family support and social support associated with national essential public health services utilization among older migrants in china: a gender perspective. International Journal of Environmental Research and Public Health. 2022;19(3):1610. doi: 10.3390/ijerph19031610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie J, Li H, Chen L, et al. A novel pyroptosis‐related lncRNA signature for predicting the prognosis of skin cutaneous melanoma. Int J Gen Med. 2021;14:6517‐6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weighardt H, Holzmann BJI. Role of Toll‐like receptor responses for sepsis pathogenesis. Immunobiology. 2007;212(9–10):715‐722. [DOI] [PubMed] [Google Scholar]
- 10. Gong Y, Li D, Cheng B, Ying B & Wang B. Increased neutrophil percentage‐to‐albumin ratio is associated with all‐cause mortality in patients with severe sepsis or septic shock. Epidemiology and Infection. 2020;148. doi: 10.1017/s0950268820000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115(6):457. [DOI] [PubMed] [Google Scholar]
- 12. Diosa‐Toro MA, Fabián A, María T & Paula A Cells with immunoregulatory properties and their impact in the pathogenesis of sepsis. 2011;28(6):572‐578. [PubMed]
- 13. Szabo G, Romics L, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002;6(4):1045‐1066. [DOI] [PubMed] [Google Scholar]
- 14. Xu J‐Y, Chen Q‐H, Liu S‐Qiao, et al. The effect of early goal‐directed therapy on outcome in adult severe sepsis and septic shock patients. Anesthesia & Analgesia. 2016;123(2):371‐381. doi: 10.1213/ane.0000000000001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin J, Tan B, Li Y, Feng H & Chen Y. Sepsis‐Exacerbated brain dysfunction after intracerebral hemorrhage. Frontiers in Cellular Neuroscience. 2022;15. doi: 10.3389/fncel.2021.819182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun H, Que J, Peng Y, et al. The neutrophil‐lymphocyte ratio: A promising predictor of mortality in coronary care unit patients — A cohort study. Int Immunopharmacol. 2019;74:105692. [DOI] [PubMed] [Google Scholar]
- 17. Tartar A, Balin SJ. Geriatric urinary tract infections: The value of laboratory parameters in estimating the need for bacteremia and Intensive Care Unit. Pak J Med Sci 2019;35(1):215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang W, Zou Z, Zhao S, et al. Erythrocyte transfusion limits the role of elevated red cell distribution width on predicting cardiac surgery associated acute kidney injury. Cardiol J. 2021;28(2):255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Yao R, Liu S, Zhang Y, Yao Y, Tian Y‐P. Efficiency of monocyte/high‐density lipoprotein cholesterol ratio combined with neutrophil/lymphocyte ratio in predicting 28‐day mortality in patients with sepsis. Front Med. 2021;8:741015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. 이봉화, 전시열, Treatment 이JAoS, Research. Prognostic Nutritional Index. 1988;34. [Google Scholar]
- 21. Ekremcengiz S, Cetinkaya E, Altin S, et al. Nutritional and prognostic significance of sick euthyroid syndrome in non‐small cell lung cancer patients. Intern Med. 2008;47(4):211‐216. [DOI] [PubMed] [Google Scholar]
- 22. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional Index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440‐443. [DOI] [PubMed] [Google Scholar]
- 23. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85(9):1001‐1005. [PubMed] [Google Scholar]
- 24. Wang A, He Z, Cong P, et al. Controlling Nutritional Status (CONUT) Score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: A single‐center retrospective study. Front Oncol. 2021;10:593452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kos FT, Hocazade C, Kos M, Uncu D, Zengin N. Assessment of prognostic value of "Neutrophil to Lymphocyte Ratio" and "Prognostic Nutritional Index" as a sytemic inflammatory marker in non‐small cell lung cancer. Asian Pac J Cancer Prev 2015;16(9):3997‐4002. [DOI] [PubMed] [Google Scholar]
- 26. Johnson A, Pollard TJ & Shen L et al. MIMIC‐III, a freely accessible critical care database. 2016;3:Article Number 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Wang J, Wu S, Ni Q, Chen P. Association between the neutrophil percentage‐to‐albumin ratio and outcomes in cardiac intensive care unit patients. Int J Gen Med. 2021;14:4933‐4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prasad P, Fang M, Abe‐Jones Y, Calfee C, Matthay M, Kangelaris KN. Time to recognition of sepsis in the emergency department using electronic health record data. Crit Care Med. 2020;48(2):200‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herwanto V, Shetty A, Nalos M, et al. Accuracy of quick sequential organ failure assessment score to predict sepsis mortality in 121 studies including 1,716,017 individuals: a systematic review and meta‐analysis. Critical Care Explorations. 2019;1(9):e0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amirabadizadeh A, Nakhaee S, Jahani F, et al. Prognostic indicators in critically ill poisoned patients: development of a risk‐prediction nomogram. Drug Metabol Personal Therapy. 2020;35:4. [DOI] [PubMed] [Google Scholar]
- 31. Kelly MP, Kight MA, Migliore V. A prognostic nutrition index: does one exist in hemodialysis patients? J Ren Nutr. 1993;3(1):10‐22. [Google Scholar]
- 32. Kumaraswamy SB, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care. 2012;16(2):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makhoul IR, Yacoub A, Smolkin T, Sujov P, Kassis I, Sprecher H. Values of C‐reactive protein, procalcitonin, and Staphylococcus ‐specific PCR in neonatal late‐onset sepsis. Acta Paediatr. 2010;95(10):1218‐1223. [DOI] [PubMed] [Google Scholar]
- 34. Houle JE, Farnsworth KD, Rossberg AG, Reid DG. Assessing the sensitivity and specificity of fish community indicators to management action. Can J Fish Aquat Sci 2012;69(6):1065‐1079. [Google Scholar]
- 35. Qiu Y, Li H, Xie J, Qiao X, Wu J. Identification of ABCC5 among ATP‐binding cassette transporter family as a new biomarker for hepatocellular carcinoma based on bioinformatics. Analysis. 2021;14:7235‐7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson MK, Mogensen KM, Casey JD, Mckane CK, Christopher KB. Nutritional status and mortality in the critically Ill. Crit Care Med 2015;43(12):87‐100. [DOI] [PubMed] [Google Scholar]
- 37. Gage BF, Birman‐Deych E, Radford MJ, Nilasena DS, Binder EF. Risk of osteoporotic fracture in elderly patients taking warfarin. Arch Intern Med. 2006;166(2):241‐246. [DOI] [PubMed] [Google Scholar]
- 38. Cohen J, Chin DN. Nutrition and sepsis. World Rev Nutr Diet 2013;105:116. [DOI] [PubMed] [Google Scholar]
- 39. Renko M, Valkonen P, Tapiainen T, et al. Xylitol‐supplemented nutrition enhances bacterial killing and prolongs survival of rats in experimental pneumococcal sepsis. BMC Microbiol. 2008;8(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Takeishi Y. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. 2017;5:e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakatani M, Migita K, Matsumoto S, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus 2017;30(8):1‐7. [DOI] [PubMed] [Google Scholar]
- 42. Raffetti E, Donato F, Castelnuovo F, et al. The prognostic role of systemic inflammatory markers on HIV‐infected patients with non‐Hodgkin lymphoma, a multicenter cohort study. J Transl Med. 2015;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


