Abstract
Pathogens can affect host cells in various ways, and the same effect can be found in the Treponema pallidum acting on the endothelium of host vessels, and the mechanism is often complex and multiple. Based on the existing T. pallidum of a cognitive framework, the first concerns involving T. pallidum or the bacteria protein directly acted on vascular endothelial cells of the host, the second concerns mainly involved in the process of T. pallidum infection in vivo blood lipid change, secretion of cytokines and the interactions between immune cells indirectly. Through both direct and indirect influence, this study explores the role of host by T. pallidum infect in the process of the vascular endothelium.
Keywords: endothelial cells, membrane protein, serum lipoprotein, syphilis, Treponema pallidum
Role of Treponema pallidum in endothelial cell phenotype and functions and their direct or indirect targets. Treponema pallidum is involved in, (A) serum lipoprotein concentration, (B) cytokines in the inflammatory response to syphilis, (C) vascular endothelial cell activation by cytokines interact with chemokines, (D) binding between pathogens and lipoproteins of the intima, (E) interactions between bacteria, the immune cells, and the endothelial cells
1. INTRODUCTION
Treponema pallidum belongs to the Spirochaetaceae (spirochetes), ranging from 6 to 15 µm in length, with a diameter of 0.2 µm. It can cause syphilis, a sexually transmitted disease with multiple stages and development that seriously endangers human health, causing 6.3 million new infections each year. 1 Treponema pallidum directly across the mucous membranes of the host or broken skin caused by sexual activity. From a series of previous bioinformatics analyses related to genomics of T. pallidum, it can be seen that T. pallidum lacks a complete biosynthetic pathway, 2 , 3 which may not satisfy its own needs for survival in the host, so T. pallidum obtains important or essential macromolecules during the infection of host cells. At the same time, the barrier function of the vascular endothelium of the host is destroyed, allowing it to enter the blood and then spread to the distal tissues and organs through flow of blood. 4 Therefore, T. pallidum attaching to and altering host cells (such as endothelial cells) and extracellular matrix are a key initial steps for infection.
Treponema pallidum bind to vascular endothelial receptors (unfortunately, the specific receptors are still unknown) by the bacteria adhesion protein (adhesins such as Tp0751, Tp0136, and Tp0155.) (Table 1) to penetrate tissue barriers, such as the intima of vascular endothelium. 5 , 6 , 7 It has also been determined that TP0326/Tp92 is a T. pallidum outer membrane protein, and it is presumed that Tp92 also helps T. pallidum attach to host endothelial cells according to the function of homologous protein for mediating cell adhesion. 8 Treponema pallidum then spreads throughout the whole body utilizing blood circulation through tissue and vascular endothelial barriers (e.g., blood–brain barrier and placental barrier), causing multiple organ and tissue infections. 9 However, it is not clear how T. pallidum benefits from invading deep tissue of the gut and musculoskeletal, T. pallidum reaches the distant skin and mucous membranes increasing the chances of subsequent transmission. Treponema pallidum can affect vascular endothelial function and even cause vascular injury during adhesion and invasion. Clinically, the typical histopathological features of syphilis include vascular injury 10 and abnormal endothelial cells. 11 There have been somebreakthroughs in vitro cell culture of T. pallidum in recent years. 12 , 13 , 14 Inability to independently culture and genetically manipulate T. pallidum subsp. pallidum, the causative agent of this disease, has hindered our understanding of the molecular pathogenesis of pathogens. 15
TABLE 1.
Treponema pallidum affects endothelial cells
| Mycoprotein | Effectors | Effect |
|---|---|---|
| Tp0574/Tp47 | ICAM‐1 | Vascular endothelial cell activation |
| RhoA | Increase endothelial permeability | |
| Tp0435/Tp17 | VE‐cadherin, F‐actin | Disruption of endothelial cell‐to‐cell Connections. |
| ICAM‐1, E‐selectin | Endothelial adhesion | |
| Tp0965 | ICAM‐1, E‐selectin, MCP‐1 | Increase endothelial permeability |
| Tp92 | TNF‐α, IL‐1β, IL‐6, IL‐8 | Damage endothelial cells |
| Tp1038(TpF1) | IL‐10, TGF‐β, IL‐8 | |
| Tp0751 | TNF‐α, IL‐1β, IL‐6 | |
| Tp0155,Tp0483 | Bind fibronectin | Adhesion invasion |
| Tp0751 | Fn, ECM proteins | |
| Tp0136 | Bind fibronectin and laminin |
Endothelial cells are highly adaptive because it is constantly aware of changes in the local extracellular environment, such as transient bacteremia, minor trauma, inflammation, or other stresses. 16 As the immunity in the first line prevents infection, endothelial cells, invaded by bacteria information, give host feedback in a specific manner. Especially, systemic infection of bacteria can change the physiological state of the blood vessels. This change can be presented in several ways, including the death of endothelial cells by directly inducing, weakening for the endothelial cells internal cytoskeleton, undermining the connection between the endothelial cells, or indirectly regulating endothelial function by influencing the immune cells. 17 This article prefers to focus on exploring the relationship between T. pallidum and host endothelial cells, expound and summarize the impact of T. pallidum on endothelial function, and explore the relationship between the impact and the relationship between pathogenesis of T. pallidum, the diagnosis and treatment of syphilis in the existing cognitive background.
2. DIRECT EFFECTS OF T. PALLIDUM TO ENDOTHELIAL CELLS
Early research has shown that 18 vascular tissue cells mainly utilized mucopolysaccharide as a support structure to grow to meet demand. The first step in the interaction between T. pallidum and endothelial cells is adhesion, and adhesion protein (such as Tp0751, Tp0136, and Tp0155) can mediate Tp adhesion to human endothelial cells derived from large blood vessels and microvessels. 5 , 6 , 7 , 19 Treponema pallidum in the host for the ongoing metabolism process can use its head end surface of mucopolysaccharide enzyme decomposition glycosaminoglycan, destroy the close connection between the endothelial cells, into perivascular tissue, and further continue to destroy the blood vessels surrounding tissues polysaccharide. The integrity of the support structure makes blood vessel inward or outward, leading to extensive lesions around the blood vessels and blood vessels, thus influencing tissue. 20 In addition, obstructive endarteritis, periarteritis, and necrosis are further developed, and eventually extensive lesions of blood vessels and perivascular tissues are generated, leading to dysfunction of tissues and organs. 21
In vitro experiments have also found T. pallidum to pass through the endothelial cell monolayer without changing the tight connection and found at the intercellular connection. 22 Some patients with syphilis, such as neurosyphilis, did not find that the blood–brain barrier was damaged, so it was speculated that T. pallidum might cross the barrier by regulating the molecular phenotypic changes in host endothelial cells instead of destroying endothelial cells. Multiple proteins of T. pallidum are involved in influence for vascular endothelial cells of the body, and these include Human Dermal Microvascular Endothelial Cells (HDMEC), Human Aortic Endothelial Cells (HAEC), Human Umbilical Vein Endothelial Cells (HUVEC), and Human Brain Microvascular Endothelial Cells (HBMEC). 22 , 23 , 24 , 25 , 26 Table 1 summarizes the results of multiple studies on the effect of T. pallidum expressing proteins on endothelial cells in vitro. Tp0574/Tp47 can not only promote the expression of intercellular cell adhesion molecule‐1 (ICAM‐1) and participate in the activation of vascular endothelial cells, 23 but also induce the expression of RhoA (a small GTPase protein and its immediate downstream target, Rho kinase [ROCK], control a wide variety of signal transduction pathways) in vascular endothelial cells, activate RhoA/ROCK signal transduction pathway, and induce cytoskeletal recombination, loose intercellular connections, and crevice, resulting in increased vascular endothelial permeability 24 ; the present study also provides evidence that TP47 causes an imbalance of matrix metalloproteinase (MMP)/tissue inhibitors of matrix metalloproteinase (TIMP) by increasing the expression and activity of MMP‐1 and MMP‐10 and prompts angiogenesis through Akt/mTOR/S6 signaling in HUVECs. 25
Tp0435/Tp17 can regulate the expression of ve‐cadherin (cadherin) membrane and F‐actin rearrangement of human vascular endothelial cells, change the connection between endothelial cells, and improve the permeability of vascular endothelial barrier. 26 Tp0965 can stimulate the expression mRNA and protein of ICAM‐1, E‐selectin, and monocyte chemotactic protein‐1 (MCP‐1) of HUVEC, and decrease the Claudin‐1 expression level of the tight junction protein, leading to increased vascular endothelial cell permeability, which further assists T. pallidum in crossing the vascular endothelium. 22 Recent research results have highlighted that Tp0965 induced Chemerin in endothelial cell dysfunction, which may be involved in the immune pathogenesis of vascular inflammation of syphilis. 27
3. INDIRECT EFFECTS OF T. PALLIDUM ON ENDOTHELIAL CELLS
3.1. Factor 1: T. pallidum infection associated with serum lipoprotein concentrations
The causal relationship between the occurrence and development of syphilis and lipid metabolism is not clear. However, the study 28 showed that the serum concentrations of high‐density lipoprotein cholesterol (HDL‐C) and apolipoprotein A‐I (ApoA‐I) were decreased in patients with syphilis compared with those in the syphilitic negative control group, while the concentration of apolipoprotein B (ApoB) was significantly increased in patients with syphilis. The main function of HDL‐C is to remove excess cholesterol from the blood and cells. ApoA‐I is one of the main components of HDL‐C, accounting for about 50% of the protein content of HDL‐C. Since ApoA‐I with rare in other lipoproteins, serum ApoA‐I can represent the basic level of HDL‐C. HDL‐C is involved in endothelial cell protection through a variety of pathways, 29 , 30 such as endothelial nitric oxide production, 31 endothelial cell apoptosis, 32 and endothelial repair after vascular injury. 33 Like low‐density lipoprotein (LDL), the harmful ApoB of cholesterol‐rich and triglyceride‐rich remains is retained and modified in the arterial wall, leading to vascular damage. Combined with the present study, 34 the concentration of serum lipoprotein ApoB may be promoting a basic element of vascular endothelial injury. The analysis shows a direct link between ApoB concentrations and low‐density lipoprotein (sd‐LDL) concentrations. The risk of cardiovascular disease is the best predictor of lipoprotein ApoB. 35 These caused lipid composition, are widely recognized as vascular injury's pathophysiology. 36 , 37 For this reason, T. pallidum may be thought to affect the vascular endothelium by altering lipid composition, even causing vascular damage.
3.2. The second factor: the development of syphilis and the secretion of vascular endothelial cytokines
When the pathogen acts on the vascular endothelium, endothelial cells are activated and releases inflammatory mediators (TNF‐α and IL‐1), chemokines (MCP‐1 and IL‐8), adhesion molecules (ICAM‐1 and vascular cell adhesion molecule‐1, VCAM‐1), clotting factors (PAI‐1), thrombomodulin (TM), etc. 38 Studies on T. pallidum protein stimulating endothelial cells in clinical practice and in vitro 22 , 23 , 24 , 25 , 26 , 39 also agree that T. pallidum can induce the secretion of endothelial cells cytokines through a variety of pathways and even affect the vascular structure and function.
In clinical cases, microvascular density and VEGFA expression in dermal lesions of patients with secondary syphilis were significantly higher than normal skin tissues. 39 Histological sections also showed significant increases in the expressions of Vascular Endothelial Growth Factor A(VEGFA) and ICAM‐1 in the derm‐mucosa of syphilis patients. 40 The levels of ICAM‐1, VCAM‐1, and IL‐8 were also increased in the rabbit model of syphilis infection. 41 On the contrary, outer membrane protein Tp92 of T. pallidum can induce further elevated levels of the endothelial cell adhesion molecule ICAM‐1 and TNF‐α through Chemerin. 42 Tp1038/TpF1 has a growth factor‐like activity that induces IL‐8 secretion by activating the CREB/NF‐κB signaling pathway. 43 Tp0326/Tp92 can promote the secretion of pro‐inflammatory cytokines TNF‐α, IL‐1, and IL‐6 in HMEC‐1 cells and promote the secretion of IL‐8 in HMEC‐1 cells and macrophages through the MyD88/NF‐κB pathway. 44
During inflammation, the endothelial cells’ cell phenotype (Figure 1) is activated by major mediators of TNF‐α and IL‐1. 45 Moreover, endothelial cell activation induces increased vascular permeability and increases the expression of pro‐inflammatory cytokines, chemokines, and adhesion molecules. 46 , 47 , 48 Chemokines, such as MCP‐1 and IL‐8, led to endothelial cells’ inflammatory response by activating the classic NF‐κB pathway. 49 NF‐κB also regulates cell adhesion molecules, such as ICAM‐1, VCAM‐1, and E‐selectin. Resting expression of adhesion molecules and VEGF, VCAM‐1, and ICAM‐1 is very low, but T. pallidum infection leading to endothelial cell activation speculated that involved in the inflammatory factors and endothelial cell adhesion, vessel wall and inflammatory cells infiltration and inflammatory factor accumulation within the immune reaction, the amplification of the inflammatory response can be further activation of endothelial cells, increase the function of endothelial cell damage and damage. 50 , 51
FIGURE 1.
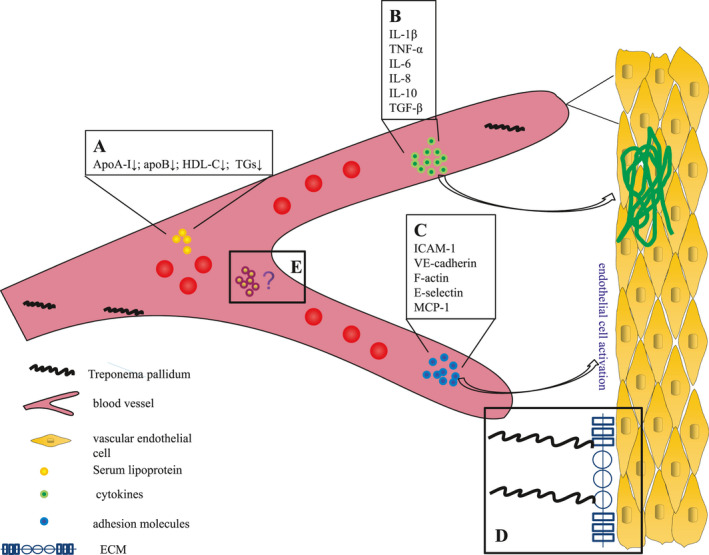
Role of Treponema pallidum in endothelial cell phenotype and functions and their direct or indirect targets. Treponema pallidum is involved in, (A) serum lipoprotein concentration, (B) cytokines in the inflammatory response to syphilis, (C) vascular endothelial cell activation by cytokines interact with chemokines, (D) binding between pathogens and lipoproteins of the intima, (E) interactions between bacteria, the immune cells, and the endothelial cells
3.3. Third factor: T. pallidum, the interaction between immune cells and endothelial cells
Many studies have shown a close relationship between T. pallidum infection and endothelial cells and immune cells, and the response of endothelial cells by T. pallidum infection is related to immunity. For example, endothelial cells showed higher adhesion to leukocytes and monocytes by upregulating ICAM‐1, VCAM‐1, and differentiated cluster (CD)11/CD18. 52 Chemokines released by endothelial cells can recruit large numbers of inflammatory cells to the site of infection, triggering a series of events that lead to an inflammatory response.
Early detection of inactivated spirochetes or non‐pathogenic spirochetes can promote increased adherence of lymphocytes and monocytes to HUVEC. 53 Treponema pallidum reduces leukocyte migration and endothelial permeability across the endothelium, possibly related to immune escape of T. pallidum. 54 Studies have shown that T. pallidum regulates the adhesion, permeability, and cytokine secretion of HUVEC in vitro by macrophages exosomes, thereby inhibiting the common inflammatory response of the host and prolonging the survival of the spirochete. 55 For example, two exosomes containing miR‐216a‐5p and miRNA‐223‐3p, respectively, can suppress the inflammatory response induced by rTp17, miR‐223‐3p is significantly negatively correlated with NLRP3, caspase‐1, and IL‐1β, miRNA‐223‐3p is significantly negatively correlated with IL‐1β, IL‐6, and TNF‐α, 56 , 57 and exosomal miR‐146a‐5p is efficiently transported into endothelial cells, reducing monocyte transendothelial migration and endothelial permeability by targeting junctional adhesion molecule C (JAM‐C). 58 Treponema pallidum‐infected macrophages secrete cytokines including ICAM‐1, VCAM‐1, VEGF, and IL‐8. Increased secretion of cytokines and chemokines plays a key role in leukocyte–endothelial interaction, promoting endothelial migration of monocytes, macrophages, dendritic cells, B cells, natural killer cells, and T cells. 59 These changes may play an important role in the pathogenesis of syphilis. Tp47 can up‐regulate the expression of adhesion molecules ICAM‐1, VCAM‐1, and E‐selectin in HDMEC and promote the adhesion of HDMEC to peripheral blood T cells. 60 Tp17 also significantly up‐regulated the levels of ICAM‐1 and E‐selectin in vascular endothelial cells, promoted the secretion of MCP‐1 and inflammatory cytokines TNF‐α, enhanced the chemotaxis of HUVEC to monocytes and macrophages, and increased the adhesion rate of THP‐1 cells to HUVEC. 61 In addition, patients with tertiary syphilis have TpF1‐specific T cells activated by TpF1, which can stimulate HUVEC to secrete IL‐8 and CCL‐20. 62
Our previous study 63 showed that Tp92 of T. pallidum could induce THP‐1 cells to secrete chemokines of the CXC family, such as IL‐8, CXCL2, and IL‐8/CXCL86, which are also produced by endothelial cells and inflammatory cells and are involved in angiogenesis. 44 In turn, angiogenesis promotes the metabolism of the proliferative site, allowing more nutrients and energy to flow to the site, which may provide T. pallidum with material support for survival, exacerbating the process.
4. CLINICAL SIGNIFICANCE AND FUTURE DIRECTION
To sum up, T. pallidum infection affects host vascular endothelial cells in various ways, all of which may be closely related, for example, the interaction between lipids and inflammation, the coordination between cytokines and chemokines, and adhesion molecules. These direct and indirect influences may be related to the histopathological characteristics typical of clinical syphilis: vascular injury and endothelial cell abnormalit. 64 Histological lesions of patients with tertiary syphilis were related to severe arterial wall damage. 65 The treatment of syphilis is currently dominated by antibiotics. 66 The patients with third‐stage syphilis have not been adequately treated, and the more common causes of death are aneurysms and thrombosis on atherosclerotic plaques. 67 However, no studies on T. pallidum and/or mycoprotein have confirmed this complex synergistic interaction.
The response of endothelial cells to invasive pathogens is an important predictor of disease severity, and the detection of endothelial function‐related indicators can be used to screen for high‐risk factors of cardiovascular events. 68 , 69 Although the effect of spirochete on endothelial cells has only been partially revealed, the significance for T. pallidum infection in the pathology of host organs such as blood vessels could be profound. However, changes in vascular endothelial function were not routinely monitored in syphilis patients. Endothelial function testing is used as a marker for early intervention, and it may guide clinical drug therapy and determine whether further symptomatic treatment is needed. Perhaps, we could further explore new therapies for T. pallidum infections, for example, by preventing T. pallidum from attaching to the endothelium in the first place, or by preventing the endothelium from responding unnaturally to the invading pathogen.
Pathogens can affect the vascular endothelium of the host through a variety of pathways, 69 , 70 , 71 , 72 with different mechanisms. We note that Treponema denticola is a pathogen belonging to the genus T. pallidum, which can cause periodontal disease (PD). At present, there is grade A evidence supporting the independent correlation between the occurrence of PD and cardiovascular disease, and the occurrence mechanism is related to the change in serum lipoprotein concentration 72 , 73 and the increase in vascular endothelial permeability. 73 , 74 Many clinical and basic studies 72 , 73 , 74 have demonstrated that these two elements can affect the vascular endothelium. Therefore, summarizing the available evidence and the study results, 74 , 75 it may be reasonable to conclude that T. pallidum‐induced vascular injury has a compound cause or possible pathway involving direct and indirect interactions. Treponema pallidum lacks a tricarboxylic acid cycle pathway and oxidative phosphorylation component compared with other bacterial pathogens, requiring it to rely on the host for survival. 75 , 76 The serum metabolites of syphilis patients showed that the metabolites involved fatty acid biosynthesis, bile acid biosynthesis, ABC transporter, glycerolipid metabolism, choline metabolism, etc. These metabolite changes indicate that T. pallidum affects the normal metabolic activity of host cells. 76 , 77 In addition, T. pallidum may have pathogenic enzymes. For example, Tp34 may play a role in the metal ion homeostasis of the bacteria, 77 , 78 and RpoE may play a role in the bacteria's avoidance of the harmful environment of the host. 78 Nevertheless, whether or not these factors help T. pallidum to affect endothelial cell metabolism or damage it needs further testing.
Therefore, the effect of T. pallidum on endothelial cells is a complex, multistage, and multipathway process. To further enhance clinical and basic research related to syphilis, it is necessary to elevate the effect of T. pallidum on endothelial cells to a causal relationship rather than a simple association. Treponema pallidum cannot be cultured in vitro, hindering the research process. In the current study, continuous growth in vitro can be achieved by co‐cultivating Sf1Ep cottontail rabbit epithelial cells in a special tissue culture medium (T. pallidum culture medium 2, or TpCM‐2) under microaerobic conditions (1.5% O2 and 5% CO2). 13 The parameters affecting the continuous in vitro culture of T. pallidum are also listed. 14 The above may help understand how T. pallidum infection causes vascular damage. Causation requires treatment to reduce the risk of complications or be used as a targeted diagnostic indicator of course and efficacy, which requires an effective evaluation of the risk of T. pallidum of causing vascular damage to the body during infection. We believe that this is a challenge of great clinical value, and its solution has broad implications for the effective diagnosis and treatment of syphilis.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Bibo Xie and Tie Zhao made substantial contributions to the conception or design of the work, the acquisition, and analysis; Sisi Zhao and Jie Zhou drafted the work or revised it critically for important intellectual content. All the authors approved the version to be published, and Feijun Zhao agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from all participants.
Xie B, Zhao T, Zhao S, Zhou J, Zhao F. Possible effects of Treponema pallidum infection on human vascular endothelial cells. J Clin Lab Anal. 2022;36:e24318. doi: 10.1002/jcla.24318
Bibo Xie and Tie Zhao contributed equally to this work.
Funding information
This study is funded by the National Natural Science Foundation of China (no. 81971980), Major Scientific and Technological Projects for collaborative prevention and control of birth defects in Hunan Province (no. 2019SK1010)
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Rowley J, et al. WHO Bulletin, 2019.
- 2. Das R, Hegyi H, Gerstein M. Genome analyses of spirochetes: a study of the protein structures, functions and metabolic pathways in Treponema pallidum and Borrelia burgdorferi . J Mol Microbiol Biotechnol. 2000;2(4):387–392. [PubMed] [Google Scholar]
- 3. Porcella SF, Schwan TG. Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms. J Clin Invest. 2001;107(6):651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lidar M, Lipschitz N, Langevitz P, Shoenfeld Y. The infectious etiology of vasculitis. Autoimmunity. 2009;42(5):432–438. [DOI] [PubMed] [Google Scholar]
- 5. Djokic V, Giacani L, Parveen N. Analysis of host cell binding specificity mediated by the Tp0136 adhesin of the syphilis agent Treponema pallidum subsp. pallidum. PLoS Negl Trop Dis. 2019;13(5):e0007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lithgow KV, Church B, Gomez A, et al. Identification of the neuroinvasive pathogen host target, LamR, as an endothelial receptor for the Treponema pallidum adhesin Tp0751. mSphere. 2020;5(2):e00195–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cameron CE, Brown EL, Kuroiwa JM, Schnapp LM, Brouwer NL. Treponema pallidum fibronectin‐binding proteins. J Bacteriol. 2004;186(20):7019–7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181(4):1401–1413. [DOI] [PubMed] [Google Scholar]
- 9. Barnett R. Syphilis. Lancet. 2018;391(10129):1471. [DOI] [PubMed] [Google Scholar]
- 10. Flamm A, Parikh K, Xie Q, Kwon EJ, Elston DM. Histologic features of secondary syphilis: A multicenter retrospective review. J Am Acad Dermatol. 2015;73(6):1025–1030. [DOI] [PubMed] [Google Scholar]
- 11. Riley BS, Oppenheimer‐Marks N, Radolf JD, Norgard MV. Virulent Treponema pallidum promotes adhesion of leukocytes to human vascular endothelial cells. Infect Immun. 1994;62(10):4622–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edmondson DG, Hu B, Norris SJ. Long‐term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. MBio. 2018;9(3):e01153‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edmondson DG, Norris SJ. In vitro cultivation of the syphilis spirochete Treponema pallidum . Curr Protoc. 2021;1(2):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edmondson DG, DeLay BD, Kowis LE, Norris SJ. Parameters affecting continuous in vitro culture of Treponema pallidum strains. MBio. 2021;12(1):e03536‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smolak A, Rowley J, Nagelkerke N, et al. Trends and predictors of syphilis prevalence in the general population: global pooled analyses of 1103 prevalence measures including 136 million syphilis tests. Clin Infect Dis. 2018;66(8):1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemichez E, Lecuit M, Nassif X, Bourdoulous S. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol. 2010;8(2):93–104. [DOI] [PubMed] [Google Scholar]
- 17. Lubkin A, Torres VJ. Bacteria and endothelial cells: a toxic relationship. Curr Opin Microbiol. 2017;35:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzgerald TJ, Johnson RC. Surface mucopolysaccharides of Treponema pallidum . Infect Immun. 1979;24(1):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parker ML, Houston S, Pětrošová H, et al. The structure of Treponema pallidum Tp0751 (Pallilysin) reveals a non‐canonical lipocalin fold that mediates adhesion to extracellular matrix components and interactions with host cells. PLoS Pathog. 2016;12(9):e1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Sluis JJ, van Dijk G, Boer M, Stolz E, van Joost T. Mucopolysaccharides in suspensions of Treponema pallidum extracted from infected rabbit testes. Genitourin Med. 1985;61(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12(2):187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang RL, Zhang JP, Wang QQ. Recombinant Treponema pallidum protein Tp0965 activates endothelial cells and increases the permeability of endothelial cell monolayer. PLoS One. 2014;9(12):e115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee KH, Choi HJ, Lee MG, Lee JB. Virulent Treponema pallidum 47 kDa antigen regulates the expression of cell adhesion molecules and binding of T‐lymphocytes to cultured human dermal microvascular endothelial cells. Yonsei Med J. 2000;41(5):623–633. [DOI] [PubMed] [Google Scholar]
- 24. Zhang RL, Wang QQ. Treponema pallidum membrane protein Tpp47 regulates the permeability of vascular endothelial cells via the RhoA/ROCK signal pathway: an experimental study. Chinese J Dermatol. 2016;49(1):21–25. [Google Scholar]
- 25. Gao ZX, Luo X, Liu LL, Lin LR, Tong ML, Yang TC. Recombinant Treponema pallidum protein Tp47 induces angiogenesis by modulating the matrix metalloproteinase/tissue inhibitor of metalloproteinase balance in endothelial cells. J Eur Acad Dermatol Venereol. 2019;33(10):1958–1970. [DOI] [PubMed] [Google Scholar]
- 26. Zhang RL, Wang QQ, Zhang JP, Yang LJ. Tp17 membrane protein of Treponema pallidum activates endothelial cells in vitro. Int Immunopharmacol. 2015;25(2):538–544. [DOI] [PubMed] [Google Scholar]
- 27. Zhang RL, Wang QQ, Yang LJ. Chemerin induced by Treponema pallidum predicted membrane protein Tp0965 mediates the activation of endothelial cell via MAPK signaling pathway. J Cell Biochem. 2019;120(12):19621–19634. [DOI] [PubMed] [Google Scholar]
- 28. Jiang Y, Zhang YF, Liu M, et al. Syphilitic dementia and lipid metabolism. Eur J Neurol. 2016;23(10):1541–1547. [DOI] [PubMed] [Google Scholar]
- 29. Riwanto M, Landmesser U. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. J Lipid Res. 2013;54(12):3227–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell S, Genest J. HDL‐C: clinical equipoise and vascular endothelial function. Expert Rev Cardiovasc Ther. 2013;11(3):343–353. [DOI] [PubMed] [Google Scholar]
- 31. Yuhanna IS, Zhu Y, Cox BE, et al. High‐density lipoprotein binding to scavenger receptor‐BI activates endothelial nitric oxide synthase. Nat Med. 2001;7(7):853–857. [DOI] [PubMed] [Google Scholar]
- 32. Nofer JR, Levkau B, Wolinska I, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL‐associated lysosphingolipids. J Biol Chem. 2001;276(37):34480–34485. [DOI] [PubMed] [Google Scholar]
- 33. Seetharam D, Mineo C, Gormley AK, et al. High‐density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor‐B type I. Circ Res. 2006;98(1):63–72. [DOI] [PubMed] [Google Scholar]
- 34. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd‐Jones DM. Discordance between apolipoprotein B and LDL‐cholesterol in young adults predicts coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2016;67(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walldius G, Jungner I. The ApoB/ApoA‐I ratio‐A new predictor of fatal stroke, myocardial infarction and other ischaemic disease‐stronger than LDL and lipid ratios. Atherosclerosis Suppl. 2006;7(3):468. [Google Scholar]
- 36. Chukkapalli SS, Easwaran M, Rivera‐Kweh MF, et al. Sequential colonization of periodontal pathogens in induction of periodontal disease and atherosclerosis in LDLRnull mice. Pathog Dis. 2017;75(1):ftx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahotupa M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic Res. 2017;51(4):439–447. [DOI] [PubMed] [Google Scholar]
- 38. Hippenstiel S, Suttorp N. Interaction of pathogens with the endothelium. Thromb Haemost. 2003;89(1):18–24. [PubMed] [Google Scholar]
- 39. Macaron NC, Cohen C, Chen SC, Arbiser JL. Cutaneous lesions of secondary syphilis are highly angiogenic. J Am Acad Dermatol. 2003;48(6):878–881. [DOI] [PubMed] [Google Scholar]
- 40. Pires FR, da Silva PJ, Natal RF, et al. Clinicopathologic features, microvessel density, and immunohistochemical expression of ICAM‐1 and VEGF in 15 cases of secondary syphilis with oral manifestations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):274–281. [DOI] [PubMed] [Google Scholar]
- 41. Xu BF, Wang QQ, Zhang JP, Hu WL, Zhang RL. Treponema pallidum induces the activation of endothelial cells via macrophage‐derived exosomes. Arch Dermatol Res. 2019;311(2):121–130. [DOI] [PubMed] [Google Scholar]
- 42. Zhang RL, Wang QQ. The Treponema pallidum outer membrane protein Tp92 activates endothelial cells via the chemerin/CMKLR1 pathway. Int J Med Microbiol. 2020;310(3): 151416. [DOI] [PubMed] [Google Scholar]
- 43. Hazlett KR, Rusnak F, Kehres DG, et al. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc‐dependent transcriptional repressor, and a semi‐autonomously expressed phosphoglycerate mutase. J Biol Chem. 2003;278(23):20687–20694. [DOI] [PubMed] [Google Scholar]
- 44. Luo X, Zhang X, Zhao T, et al. A preliminary study on the proinflammatory mechanisms of Treponema pallidum outer membrane protein Tp92 in human macrophages and HMEC‐1 cells. Microb Pathog. 2017;110:176–183. [DOI] [PubMed] [Google Scholar]
- 45. Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50(4):537–544. [DOI] [PubMed] [Google Scholar]
- 46. Denk A, Goebeler M, Schmid S, et al. Activation of NF‐kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276(30):28451–28458. [DOI] [PubMed] [Google Scholar]
- 47. Oppenheimer‐Marks N, Lipsky PE. Adhesion molecules as targets for the treatment of autoimmune diseases. Clin Immunol Immunopathol. 1996;79(3):203–210. [DOI] [PubMed] [Google Scholar]
- 48. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. [DOI] [PubMed] [Google Scholar]
- 49. Kempe S, Kestler H, Lasar A, Wirth T. NF‐kappaB controls the global pro‐inflammatory response in endothelial cells: evidence for the regulation of a pro‐atherogenic program. Nucleic Acids Res. 2005;33(16):5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. El‐Ashmawy HM, Selim FO, Hosny TAM, Almassry HN. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract. 2019;150:57–63. [DOI] [PubMed] [Google Scholar]
- 51. Milani AT, Khadem‐Ansari MH, Rasmi Y. Effects of thyroid‐stimulating hormone on adhesion molecules and pro‐inflammatory cytokines secretion in human umbilical vein endothelial cells. Res Pharm Sci. 2018;13(6):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beekhuizen H, van de Gevel JS, Olsson B, van Benten IJ, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158(2):774–782. [PubMed] [Google Scholar]
- 53. Thomas DD, Navab M, Haake DA, Fogelman AM, Miller JN, Lovett MA. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988;85(10):3608–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu W, Xu B, Zhang J, et al. Exosomal miR‐146a‐5p from Treponema pallidum‐stimulated macrophages reduces endothelial cells permeability and monocyte transendothelial migration by targeting JAM‐C. Exp Cell Res. 2020;388(1): 111823. [DOI] [PubMed] [Google Scholar]
- 55. Xu BF, Wang QQ, Zhang JP, Hu WL, Zhang RL. Treponema pallidum induces the activation of endothelial cells via macrophage‐derived exosomes. Arch Dermatol Res. 2019;311(2):121–130. [DOI] [PubMed] [Google Scholar]
- 56. Long FQ, Kou CX, Li K, Wu J, Wang QQ. MiR‐223‐3p inhibits rTp17‐induced inflammasome activation and pyroptosis by targeting NLRP3. J Cell Mol Med. 2020;24(24):14405–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peng RR, Shang SX, Zhao LS, Long FQ. MiR‐216a‐5p‐containing exosomes suppress rTp17‐induced inflammatory response by targeting TLR4. Biosci Rep. 2019;39(8):BSR20190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu W, Xu B, Zhang J, et al. Exosomal miR‐146a‐5p from Treponema pallidum‐stimulated macrophages reduces endothelial cells permeability and monocyte transendothelial migration by targeting JAM‐C. Exp Cell Res. 2020;388(1): 111823. [DOI] [PubMed] [Google Scholar]
- 59. Xu BF, Wang QQ, Zhang JP, Hu WL, Zhang RL. Treponema pallidum induces the activation of endothelial cells via macrophage‐derived exosomes. Arch Dermatol Res. 2019;311(2):121–130. [DOI] [PubMed] [Google Scholar]
- 60. Zhang RL, Wang QQ. Treponema pallidum membrane protein Tpp47 promotes the adhesion ability of vascular endothelial cells in vitro: an experimental study. Chin J Dermatol. 2014;47(5):328–332. [Google Scholar]
- 61. Zhang RL, Wang QQ, Zhang JP, Yang LJ. Tp17 membrane protein of Treponema pallidum activates endothelial cells in vitro. Int Immunopharmacol. 2015;25(2):538–544. [DOI] [PubMed] [Google Scholar]
- 62. Pozzobon T, Facchinello N, Bossi F, et al. Treponema pallidum (syphilis) antigen TpF1 induces angiogenesis through the activation of the IL‐8 pathway. Sci Rep. 2016;5(6):18785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luo X, Zhang X, Gan L, et al. The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL‐8 secretion. J Cell Mol Med. 2018;22(12):6039–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu N, Li N, Hu L, et al. Immunogenicity and immunoreactivity of Tp0821 recombinant protein from Treponema pallidum . Mol Med Rep. 2017;16(1):851–856. [DOI] [PubMed] [Google Scholar]
- 65. Brautigam CA, Deka RK, Liu WZ, Tomchick DR, Norgard MV. Functional clues from the crystal structure of an orphan periplasmic ligand‐binding protein from Treponema pallidum . Protein Sci. 2017;26(4):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Buchan CA, Bravi A, Seely AJ. Variability analysis and the diagnosis, management, and treatment of sepsis. Curr Infect Dis Rep. 2012;14(5):512–521. [DOI] [PubMed] [Google Scholar]
- 67. Koizumi Y, Watabe T, Ota Y, Nakayama SI, Asai N, Hagihara M, Yamagishi Y, Suematsu H, Tsuzuki T, Takayasu M, Ohnishi M, Mikamo H. Cerebral syphilitic gumma can arise within months of reinfection: a case of histologically proven Treponema pallidum strain type 14b/f infection with human immunodeficiency virus positivity. Sex Transm Dis. 2018;45(2):e1‐e4. [DOI] [PubMed] [Google Scholar]
- 68. Kerrigan SW. McDonnell C Dysregulation of the endothelium following Staphylococcus aureus infection. Biochem Soc Trans. 2015;43(4):715–719. [DOI] [PubMed] [Google Scholar]
- 69. Liuba P, Karnani P, Pesonen E, et al. Endothelial dysfunction after repeated Chlamydia pneumoniae infection in apolipoprotein E‐knockout mice. Circulation. 2000;102(9):1039–1044. [DOI] [PubMed] [Google Scholar]
- 70. Ayada K, Yokota K, Kobayashi K, Shoenfeld Y, Matsuura E, Oguma K. Chronic infections and atherosclerosis. Clin Rev Allergy Immunol. 2009;37(1):44–48. [DOI] [PubMed] [Google Scholar]
- 71. Koizumi Y, Kurita‐Ochiai T, Oguchi S, Yamamoto M. Nasal immunization with Porphyromonas gingivalis outer membrane protein decreases P. gingivalis‐induced atherosclerosis and inflammation in spontaneously hyperlipidemic mice. Infect Immun. 2008;76(7):2958–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. El‐Seweidy MM, Sarhan Amin R, Husseini Atteia H, El‐Zeiky RR, Al‐Gabri NA. Dyslipidemia induced inflammatory status, platelet activation and endothelial dysfunction in rabbits: Protective role of 10‐Dehydrogingerdione. Biomed Pharmacother. 2019;110:456–464. [DOI] [PubMed] [Google Scholar]
- 73. Cahill PA, Redmond EM. Vascular endothelium‐Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chukkapalli SS, Rivera MF, Velsko IM, et al. Invasion of oral and aortic tissues by oral spirochete Treponema denticola in ApoE(‐/‐) mice causally links periodontal disease and atherosclerosis. Infect Immun. 2014;82(5):1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fraser CM, Norris SJ, Weinstock GM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281(5375):375–388. [DOI] [PubMed] [Google Scholar]
- 76. Liu LL, Lin Y, Zhuang JC, et al. Analysis of serum metabolite profiles in syphilis patients by untargeted metabolomics. J Eur Acad Dermatol Venereol. 2019;33(7):1378–1385. [DOI] [PubMed] [Google Scholar]
- 77. Deka RK, Brautigam CA, Tomson FL, et al. Crystal structure of the Tp34 (TP0971) lipoprotein of Treponema pallidum: implications of its metal‐bound state and affinity for human lactoferrin. J Biol Chem. 2007;282(8):5944–5958. [DOI] [PubMed] [Google Scholar]
- 78. Giacani L, Denisenko O, Tompa M, Centurion‐Lara A. Identification of the Treponema pallidum subsp. pallidum TP0092 (RpoE) regulon and its implications for pathogen persistence in the host and syphilis pathogenesis. J Bacteriol. 2013;195(4):896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


