Abstract
Background
Diabetes mellitus (DM) has shown a trend of reaching pandemic levels in the world. Chronic inflammation is a key factor in the development of diabetic retinopathy (DR). Red blood cell distribution width‐to‐albumin ratio (RA) is used to assess immune status and the immune response. Our study was conducted to assess the association between DR and RA levels to determine the value of RA in predicting DR.
Methods
The data came from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2006, The RA was calculated as the Red Blood Cell Distribution Width/Albumin Ratio. Multivariable logistic regression and propensity score‐matched analysis were used to examine the association between RA and DR levels.
Results
The clinical and demographic features of the 1,751 patients with DM. The eligible participants included 874 females and 870 males with mean age 62.2 ± 14.0 years, and mean RA 3.2 ± 0.5. RA ≥ 2.9659 was a risk factor for DR (OR = 1.66 95% CI: 1.31–2.11, p < 0.0001). After adjusting for age, sex, race, education, marital status, ratio of family income to poverty, body mass index, fasting glucose, hypertension, and coronary heart disease, RA ≥ 2.9659 was an independent risk factor for DR (OR = 1.64, 95% CI: 1.23–2.19, p = 0.0008). The propensity score‐matched analysis also showed that high RA was an independent risk factor for DR.
Conclusions
Our study shows that RA is a risk factor for patients with DR. The findings of this study should be validated the role of RA in DR in diabetic patients.
Keywords: diabetes mellitus, diabetic retinopathy, National Health and Nutrition Examination Survey, red blood cell distribution width‐to‐albumin ratio
Diabetes mellitus (DM) has shown a trend of reaching pandemic levels in the world. Chronic inflammation is a key factor in the development of diabetic retinopathy (DR). Red blood cell distribution width to albumin ratio (RA) is used to assess immune status and the immune response. Our study was conducted to assess the association between DR and RA levels to determine the value of RA in predicting DR. The data came from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2006, The RA was calculated as the red blood cell distribution width/albumin ratio. Multivariable logistic regression and propensity score matched analysis were used to examine the association between RA and DR levels. The clinical and demographic features of the 1751 patients with DM. The eligible participants included 874 female and 870 males with mean age 62.2 ± 14.0 years, and mean RA 3.2 ± 0.5. RA ≥ 2.9659 was a risk factor for DR (OR = 1.66 95% CI: 1.31–2.11, p < 0.0001). After adjusting for age, sex, race, education, marital status, ratio of family income to poverty, body mass index, fasting glucose, hypertension, and coronary heart disease, RA ≥ 2.9659 was an independent risk factor for DR (OR = 1.64, 95% CI: 1.23–2.19, p = 0.0008). The propensity score‐matched analysis also shown that high RA was an independent risk factor for DR. Our study shown that RA is a risk factor for patients with DR. The findings of this study should be validated the role of RA in DR in diabetic patients.
1. INTRODUCTION
Diabetes mellitus (DM) has shown a trend of reaching pandemic levels in the world. 1 , 2 , 3 It has rapidly become a global health problem. Diabetic retinopathy (DR) is the most common microvascular complication of type 2 diabetes mellitus (T2D) and the most frequent. 4 DR is one of the major eye diseases causing blindness in people aged 50 years and above. 5 Its basic pathological changes are destruction of blood retinal barrier (BRB) and the formation of retinal neovascularization. 6 Studies 7 on the prevalence of DR in China show that between 9.4% and 43.1% of patients with diabetes are diagnosed with DR. In addition, DR is always asymptomatic before entering the later stage because DR seriously harms human health and the economic sustainability of the national health system, both for individual vision and society. 6 , 8 , 9 Early detection of predictors of the occurrence of DR allows doctors to identify high‐risk patients earlier for early prevention and quality treatment.
Chronic inflammation is the non‐specific response of body to injury or stress. 10 , 11 There is growing evidence that chronic inflammation is a key factor in the development of DR. 12 , 13 Pathological manifestations of chronic retinal inflammation, such as increased retinal blood flow, abnormal stasis of white blood cells, infiltration of neutrophils and macrophages, activation of complement and microglia, up‐regulation of cytokines, and increased vascular permeability and tissue edema, have been confirmed in animal models as well as in patients with DR during the development of chronic retinal inflammation. 14 Red blood cell distribution width (RDW) is a parameter that reflects the heterogeneity of red blood cells (RBC) measured using hematology analyzer. 15 The normal range of RDW in human is between 11 and 15%. The larger the RDW value, the greater the difference of red blood cell shape and size. This suggests that there is poor blood and various hematopoietic abnormalities. It was evident that RDW was generally elevated in patients with diabetes and its complications. 16 , 17 Moreover, elevated RDW is associated with severe imbalance of RBC homeostasis, abnormal RBC production and survival. This may be associated with oxidative stress, vascular inflammation, and malnutrition such as iron, vitamin B12, and folic acid deficiencies. In addition, excessive RDW may increase mortality from diabetes mellitus and macrovascular as well as microvascular diseases. Recent studies 18 , 19 have shown that albumin is involved in inflammation. The RA is an integrated and novel inflammatory biomarker based on RDW and albumin. Its index was originally used to assess the outcomes in patients with solid stroke 19 and ARDS 20 and is believed to accurately reflect inflammation. However, the role of RA in patients with diabetic retinopathy is still unclear. In the present study, it was hypothesized that patients with DM and higher levels of inflammation were at higher risk of developing retinopathy as measured by RA. Therefore, a cross‐sectional study was conducted to assess the association between diabetic retinopathy and RA levels to determine the value of RA in predicting DR.
2. METHODS
2.1. Data and sample sources
The data came from the National Health and Nutrition Examination Survey (NHANES). NHANES was a 2‐year cross‐sectoral, hierarchical and multi‐stage probability cluster survey. It aimed to gather information on a variety of potential risk factors and nutrition in the non‐institutionalized, democratized and U.S. population. The NHANES was approved by the Institutional Review Board of the National Centre for Health Statistics with the informed consent of all participants. The National Center for the Health Statistics Ethics Review Board also approved the NHANES, and all participants gave written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was followed in this study. The Medical Ethics Committee of the Second Affiliated Hospital and Yuying Children's Hospital approved our study (ethical review batch number: 20211090).
2.2. Study variables
Definition of DM: the selection criteria for diabetes included self‐reported diabetes, on anti‐diabetes drugs, taking insulin, fasting glucose ≥110 mg/dl, or glycohemoglobin (HbA1c) ≥6.5%. Definition of DR: using the Canon CR6‐45NM ophthalmic digital imaging system and Canon EOS 10D digital camera (Canon), or has a doctor ever told that diabetes has affected eyes or that had retinopathy. Demographic characteristics included age, sex, race, marital status, education level, body mass index (BMI), C‐reactive protein (CRP), RDW, Albumin, Insulin use, fasting glucose, ratio of family income to poverty (PIR), hypertension, and coronary heart disease.
2.3. Statistical analyses
Continuous data were expressed as mean ± standard deviation, and categorical variables were expressed as frequency. Differences in baseline characteristics were compared via an independent sample t test in continuous variables and χ2 tests in categorical variables. For the current study, receiver operating characteristic curve analysis was used to determine the optimal cutoff value for the RA. A multivariable logistic regression analysis was performed to examine the association between RA and diabetic DR thus calculating 95% confidence intervals (CI) and odds ratios (OR). In model 1, there was no adjustment any confounding factors. Age, sex, race, education, marital status, PIR, BMI, fasting glucose chronic conditions including hypertension and CHD was adjusted in model 2. To avoid potential bias, propensity score matching (PSM) was also determined in this study. This study ensured that all reported selection factors for DR were included in the model covariation to further reduce potential confusion. The PSM was performed at a ratio of 1:1, using the 0.05 caliper width of the SD by the propensity score log. After PSM, model 3 was analyzed. All analyses were conducted using R software (version 4.01, http://www.r‐project.org). The double‐sided significant difference was set at p < 0.05.
3. RESULTS
3.1. Subject characteristics
The clinical and demographic features of the 1,751 patients with DM were as presented in Table 1. However, it was found that the data of some patients were partly incomplete. The eligible participants included 874 female and 870 males with mean age 62.2 ± 14.0 years, and mean RA 3.2 ± 0.5. The outcomes of this study showed that there was no statistical difference in the age, sex, race, marital status, BMI, and CRP. Patients with DR had high levels of RA, RDW, albumin, fasting glucose insulin using hypertension and CHD (p < 0.05).
TABLE 1.
Basic characteristics of study population
| All | No DR | DR | p | |
|---|---|---|---|---|
| Sample size, N | 1751 | 1309 | 442 | |
| Age (years) | 62.2 ± 14.0 | 62.1 ± 14.5 | 62.6 ± 12.4 | 0.957 |
| Female (%) | 874 (49.9) | 656 (50.1) | 218 (49.3) | 0.773 |
| Race (%) | ||||
| Non‐Hispanic White | 674 (38.5) | 516 (39.4) | 158 (35.7) | 0.450 |
| Black | 448 (25.6) | 326 (24.9) | 122 (27.6) | |
| Mexican American | 485 (27.7) | 356 (27.2) | 12 (2.6) | |
| Other Hispanic | 75 (4.3) | 60 (4.6) | 12 (2.6) | |
| Other | 69 (3.9) | 60(3.9) | 9 (2.0) | |
| Education level (%) | ||||
| Primary school | 812 (46.4) | 586 (44.8) | 226 (51.1) | 0.041 |
| High school | 378 (21.6) | 284 (21.7) | 94 (21.3) | |
| Above | 559 (32.0) | 437 (33.4) | 122 (27.6) | |
| PIR | 2.2 ± 1.5 | 2.3 ± 1.5 | 1.9 ± 1.4 | <0.001 |
| Marital status (%) | ||||
| Married/living with partner | 1036 (60.8) | 779 (60.9) | 257 (60.3) | 0.202 |
| Widowed/divorced | 552 (32.4) | 405 (31.7) | 147 (34.5) | |
| Never married | 117 (6.9) | 95 (7.4) | 22 (5.2) | |
| BMI (kg/m2) | 31.3 ± 7.0 | 31.2 ± 6.8 | 31.7 ± 7.6 | 0.373 |
| Waist circumference (cm) | 106.6 ± 15.2 | 106.2 ± 14.8 | 107.7 ± 16.1 | 0.187 |
| RDW (%) | 13.2 ± 1.3 | 13.1 ± 1.3 | 13.3 ± 1.4 | 0.016 |
| Albumin (g/dl) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.4 | <0.001 |
| RA | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.3 ± 0.5 | <0.001 |
| Fasting glucose (mg/dl) | 164.9 ± 73.2 | 161.5 ± 71.3 | 176.1 ± 77.9 | 0.012 |
| HDL (mg/dl) | 47.9 ± 14.0 | 47.7 ± 14.0 | 48.3 ± 14.0 | 0.429 |
| LDL (mg/dl) | 110.2 ± 36.0 | 111.1 ± 36.6 | 107.4 ± 33.8 | 0.608 |
| Insulin use (%) | 163 (9.3) | 93 (7.1) | 70 (15.8) | <0.001 |
| CRP (mg/dl) | 0.7 ± 1.4 | 0.7 ± 1.4 | 0.7 ± 1.4 | 0.440 |
| Chronic conditions | ||||
| Coronary heart disease (%) | ||||
| Yes | 1307 (74.9) | 1007 (77.3) | 300 (67.9) | <0.001 |
| No | 438 (25.1) | 296 (22.7) | 142 (32.1) | |
| Hypertension (%) | ||||
| Yes | 621 (35.5) | 483 (37.0) | 138 (31.3) | 0.031 |
| No | 1126 (64.5) | 823 (63.0) | 303 (68.7) | |
Data are weighted estimates, and values are presented as means ± standard deviation or means (percentage).
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; PIR, ratio of family income to poverty; RA, red blood cell distribution width‐to‐albumin ratio; RDW, red blood cell distribution width.
3.2. RA was an independent risk factor for DR
Smooth curve fitting was used to assess the effects of RA and DR (Figure 1). We constructed various models to assess the independent effects of RA and DR, after adjusting for other potential confounders. Results of logistic regression analysis found that shows effect sizes (OR) and 95% CI (Table 2). In univariate analysis, RA ≥ 2.9659 was a risk factor for diabetic DR (OR = 1.66 95% CI: 1.31–2.11, p < 0.0001). After adjusting for age, sex, race, education, marital status, PIR, BMI, fasting glucose chronic conditions including hypertension (yes/no) and CHD (yes/no), RA ≥ 2.9659 was an independent risk factor for DR (OR = 1.64, 95% CI: 1.23–2.19, p = 0.0008).
FIGURE 1.
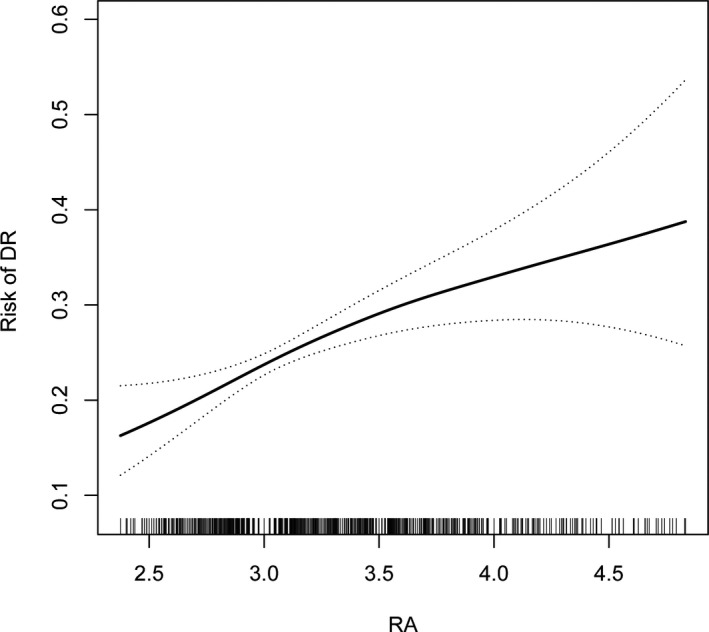
Association between RA and risk of DR. Abbreviation: DR, diabetic retinopathy; RA, red blood cell distribution width‐to‐albumin ratio
TABLE 2.
Association between RA and diabetic retinopathy
| RA | OR | 95% CI | p |
|---|---|---|---|
| Before propensity score | |||
| <2.9659 | Reference | 1.31–2.11 | <0.00001 |
| ≥2.9659 | 1.66 | ||
| Before propensity score a | |||
| <2.9659 | Reference | 1.23–2.19 | 0.0008 |
| ≥2.9659 | 1.64 | ||
| After propensity score | |||
| <2.9659 | Reference | 1.26–2.40 | 0.0008 |
| ≥2.9659 | 1.74 | ||
Abbreviations: CI, confidence interval; OR odds ratio; RA, red blood cell distribution width‐to‐albumin ratio.
Adjusted for age, sex, race, education, marital status, ratio of family income to poverty, body mass index, fasting glucose, hypertension, and coronary heart disease.
3.3. Propensity score‐matched analysis
The propensity score‐matched analysis was also performed to further verify the relationship between RA and DR. After propensity score‐matched analysis, the baseline characteristics of patients in different RA groups were not significantly different (Table 3). Results of logistic regression analysis showed that RA ≥ 2.9659 was an independent risk factor for DR (OR = 1.74, 95% CI: 1.26–2.40, p = 0.0008).
TABLE 3.
Characteristics of patients before and after PSM
| Before propensity score | After propensity score | |||
|---|---|---|---|---|
| <2.9659 | ≥2.9659 | <2.9659 | ≥2.9659 | |
| Sample size, N | 553 | 1198 | 432 | 432 |
| Age, years | 60.2 ± 14.0 | 63.1 ± 13.9* | 61.7 ± 13.2 | 61.1 ± 13.4 |
| Sex, n (%) | * | |||
| Male | 339 (61.3) | 538 (44.9) | 250 (57.9) | 244 (56.5) |
| Female | 214 (38.7) | 660 (55.1) | 182 (42.1) | 188 (43.5) |
| Ethnicity, n (%) | * | |||
| Non‐Hispanic White | 236 (42.7) | 438 (36.6) | 186 (43.1) | 142 (32.9) |
| Black | 65 (11.8) | 383 (32.0) | 48 (11.1) | 132 (30.6) |
| Mexican American | 192 (34.7) | 293 (24.5) | 156 (36.1) | 119 (27.5) |
| Other Hispanic | 29 (5.2) | 46 (3.8) | 19 (4.4) | 24 (5.6) |
| Other | 31 (5.6) | 38 (3.2) | 23 (5.3) | 15 (3.5) |
| Education level (%) | ||||
| Primary school | 250 (45.2) | 562 (47.0) | 191 (44.2) | 192 (44.4) |
| High school | 107 (19.3) | 271 (22.7) | 84 (19.4) | 101 (23.4) |
| Above | 196 (35.4) | 363 (30.4) | 157 (36.3) | 139 (32.2) |
| PIR | 2.3 ± 1.5 | 2.2 ± 1.4 | 2.4± 1.5 | 2.4± 1.5 |
| Marital status (%) | * | |||
| Married/living with partner | 365 (69.1) | 671 (57.0) | 292 (67.6) | 293 (67.8) |
| Widowed/divorced | 132 (25.0) | 420 (35.7) | 120 (27.8) | 119 (27.5) |
| Never married | 31 (5.9) | 86 (7.3) | 20 (4.6) | 20 (4.6) |
| BMI (kg/m2) | 29.0 ± 5.2 | 32.4 ± 7.5* | 29.4 ± 5.0 | 29.8± 6.0 |
| Waist circumference (cm) | 102.4 ± 12.8 | 108.6 ± 15.8* | 103.7± 12.4 | 104.3 ± 15.1 |
| RA | 2.8 ± 0.1 | 3.4 ± 0.5* | 2.8± 0.1 | 3.3 ± 0.4* |
| RDW (%) | 12.3 ± 0.5 | 13.6 ± 1.4* | 12.3 ± 0.5 | 13.4± 1.3* |
| Albumin (g/dl) | 4.5 ± 0.2 | 4.0 ± 0.3* | 4.5± 0.2 | 4.1± 0.3* |
| Fasting glucose (mg/dl) | 164.0 ± 71.6 | 165.4 ± 73.9 | 162.6 ± 67.1 | 160.9 ± 71.3 |
| CRP (mg/dl) | 0.3 ± 0.4 | 0.9 ± 1.6* | 0.4± 0.4 | 0.4± 0.4 |
| HDL (mg/dl) | 46.9 ± 13.2 | 48.4 ± 14.4 | 46.6 ± 13.2 | 47.02± 14.3 |
| LDL (mg/dl) | 117.3 ± 33.4 | 107.0 ± 36.8 | 117.8 ± 34.0 | 110.0 ± 36.0 |
| Insulin use (%) | 32 (5.8) | 131 (10.9) * | 24 (5.6) | 28 (6.5) |
| CHD (%) | * | |||
| Yes | 84 (15.3) | 354 (29.6) | 75 (17.4) | 72 (16.7) |
| No | 466 (84.7) | 841 (70.4) | 357 (82.6) | 360 (83.3) |
| Hypertension (%) | * | |||
| Yes | 293 (53.2) | 833 (69.6) | 246 (56.9) | 240 (55.6) |
| No | 258 (46.8) | 363 (30.4) | 186 (43.1) | 192 (44.4) |
| DR | * | * | ||
| Yes | 105 (19.0) | 337 (28.1) | 79 (18.3) | 121 (28) |
| No | 448 (81.0) | 861 (71.9) | 353 (81.7) | 311 (72) |
*p < 0.05.
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; DR, diabetic retinopathy; PIR, ratio of family income to poverty; RA, red blood cell distribution width‐to‐albumin ratio; RDW, red blood cell distribution width.
4. DISCUSSION
This is the first study which has shown that RA is an independent risk factor for DR. Further results revealed that patients with DR had higher levels of RA than those without DR.
Diabetes is a chronic, low‐grade and persistent inflammatory disease. 21 , 22 Normal tissue immune cells, such as regulatory T cells, macrophages, and eosinophils, exert anti‐inflammatory effects by synthesis of cytokines such as IL‐3, IL‐10, and IL‐4 thereby maintaining microenvironmental homeostasis. 23 , 24 When diabetes and its complications occur, the AGEs, lipid metabolism disorders and OS induce monocytes, macrophages as well as natural killer cells to infiltrate into the non‐inflammatory tissues such as fat and muscle, resulting in changes in the number and types of immune cells and the release of inflammatory factors which causes chronic inflammation. 25 The serum and vitreous and aqueous humor tissue of patients with diabetes contain a variety of inflammatory cytokines and chemokines. A variety of eye tissue of patients with NPDR also contains inflammatory cytokines IL‐1 beta, IL‐6, IL‐8, TNF alpha, and mononuclear cells chemokine (monocyte chemotactic protein, MCP‐1) such as add. 26 Recent studies have reported that Il‐8 and TNF ‐ α levels were even higher in patients with active PDR. 27 In ischemia mice models, some cytokines such as macrophage inflammatory protein 1 (MIP), IL–1, and IL‐3 of these inflammatory mediators. First, the formation of new blood vessels is considered to be the early diabetic retinal nerve cells, the cause of death, activated microglia and endothelial cells, glial cells even later these cytokines increase of neuron, illustrates this some inflammatory cytokines lead to inflammation in the development of early DR and the DR has always been.
Inflammatory response may promote the heterogeneity of RBC volume and lead to the increase of RDW 28 , 29 by affecting RBC genesis, RBC circulating half‐life and RBC deformability. Red cells with 1/4 higher RDW have higher IL‐6 levels. Inflammatory factors in DR patients have serious damage to endothelial cells and glomerular permeability. The TNF‐α and IL‐6 are related to proteinuria and reflect the level of inflammation. Further, RDW is positively correlated with proteinuria. In addition, the nuclear factor κB (NF‐κB) signaling pathway plays an important role in the early pathogenesis of diabetes mellitus and its complications. In inflammatory response, this pathway is mainly involved in the expression of inflammatory cytokines and is a key nuclear factor in the initiation and regulation of inflammatory response in diabetes mellitus. In addition, NF‐κB‐mediated inflammation may lead to cell damage through increased production of active oxygen clusters (ROS). Albumin is negative acute phase protein with antioxidant properties in contrast to CRP. 30 Inflammatory activities are inversely associated with albumin synthesis rate which raises blood viscosity, aggregation, and platelet activation. 31 Earlier studies have shown that decreased albumin increases the general population risk for cardiovascular events. In our research, the levels of albumin in patients with higher RDW were considerably lower.
HbA1c is produced in red blood cells through a slow and irreversible reaction between hemoglobin and glucose. 32 It reflects the average blood glucose level of body in recent 3 months, with no obvious short‐term fluctuation, which is suitable for long‐term 33 monitoring. HbA1c is mainly determined by blood glucose concentration and the lifespan of RBC. In addition to promoting the formation of HbA1c, hyperglycemia also changes the mechanical properties of red blood cells, such as increased osmotic brittleness of red blood cells, enhanced adhesion, and increased density of red blood cells, erythrocyte membrane rearrangement and deficiency of oxygen‐binding activity of hemoglobin. In addition, hyperglycemia also has an impact on RBC lifespan, resulting in highly variable RBC volume, which may affect RDW machine.
Some studies have found that RDW is correlated with the increase of HbA1c concentration, but not with blood glucose concentration. This indicates that RDW may be related to HbA1c through non‐glucose pathway. The RBC survival time increases with decrease in rate of RBC renewal and the small RBC in the circulation is delayed, resulting in uneven RBC size. Further, it was found the increase of RBC survival time increased HbA1c concentration, suggesting that the correlation between RDW and HbA1c was caused by an increase in RBC survival time and high RDW. It was evident that the level of RDW was positively correlated with HbA1c and negatively correlated with blood glucose. 33 , 34
Serum albumin is only synthesized in the liver and is a biochemical marker of nutritional status and a major component of colloidal osmotic pressure. Albumin is a negative acute phase protein whose synthesis rate is negatively correlated with inflammatory activity.
Currently, there are no effective biomarkers to monitor the DR or to effectively classify patients in order to best assess the efficacy of treatment, and our study has several advantages. The sample size of this study was large enough to determine a significant association between RA and patients with DR. In addition, analysis of detailed covariant data allowed the adjustment for potential confounding factors that might influence the association between RA and DR. However, there are some limitations to the current study. First, cross‐sectional study design means causality could not be determined. Prospective studies are thus needed to determine the cause and effect. Second, the data used in this study were extracted from a single blood test. Furthermore, a continuous testing may be more informative than admission testing because of the short life span of the blood cells. Third, RA is easily measured in clinical practice, but the loss of RDW and albumin is common.
CONFLICT OF INTEREST
None.
Zhao F, Liu M, Kong L. Association between red blood cell distribution width‐to‐albumin ratio and diabetic retinopathy. J Clin Lab Anal. 2022;36:e24351. doi: 10.1002/jcla.24351
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author (Lingzhen Kong).
REFERENCES
- 1. Dieren SV, Beulens J, Schouw Y, Grobbee DE, Neal BJ. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(1_Suppl):s3‐s8. [DOI] [PubMed] [Google Scholar]
- 2. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534‐548. doi: 10.1038/s41574-021-00512-2 [DOI] [PubMed] [Google Scholar]
- 3. Katulanda P, Sheriff M, Matthews DR. The diabetes epidemic in Sri Lanka – a growing problem. Ceylon Med J. 2006;51(1):26‐28. [DOI] [PubMed] [Google Scholar]
- 4. Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18(S3):S21‐S26. [DOI] [PubMed] [Google Scholar]
- 5. Flugelman M. Compositions and methods for treating ophthalmic disorders; 2008.
- 6. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124‐136. [DOI] [PubMed] [Google Scholar]
- 7. Cui J, Ren JP, Chen DN, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross‐sectional study. BMJ Open. 2017;7(8):e015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin T, Gubitosi‐Klug RA, Channa R, Wolf RM. Pediatric Diabetic Retinopathy: Updates in Prevalence, Risk Factors, Screening, and Management. Curr Diab Rep. 2021;21(12):56. doi: 10.1007/s11892-021-01436-x [DOI] [PubMed] [Google Scholar]
- 9. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480‐1487. [DOI] [PubMed] [Google Scholar]
- 10. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Investig. 2003;112(12):1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Festa A, D'Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation. 2000;102(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Wang JJ, Qiang Y, Min W, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450‐1452. [DOI] [PubMed] [Google Scholar]
- 14. Grigsby JG, Cardona SM, Pouw CE, et al. The role of microglia in diabetic retinopathy. J Ophthalmol. 2014;2014:705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaya A, Hernández J, Zorio E, Bautista D. Association between red blood cell distribution width and the risk of future cardiovascular events. Clin Hemorheol Microcirc. 2012;50(3):221‐225. [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Li S, Zhang A, et al. Association between red blood cell distribution width and diabetic retinopathy: a 5‐year retrospective case‐control study. J Ophthalmol. 2021;2021:1‐9. doi: 10.1155/2021/6653969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magri CJ, Fava S. Red blood cell distribution width and diabetes‐associated complications. Diabetes Metab Syndr Clin Res Rev. 2014;8(1):13‐17. [DOI] [PubMed] [Google Scholar]
- 18. Long J, Xie X, Xu D, et al. Association between red blood cell distribution width‐to‐albumin ratio and prognosis of patients with aortic aneurysms. Int J Gen Med. 2021;14:6287‐6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao N, Hu W, Wu Z, et al. The red blood cell distribution width–albumin ratio: a promising predictor of mortality in stroke patients. Int J Gen Med. 2021;14:3737‐3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoo J, Ju S, Lee S, Cho Y, Lee J, Kim HC. Red cell distribution width/albumin ratio is associated with 60‐day mortality in patients with acute respiratory distress syndrome. Infect Dis. 2020;52(4):266‐270. [DOI] [PubMed] [Google Scholar]
- 21. Bendek M, Canedo‐Marroquín G, Realini O, et al. Periodontitis and gestational diabetes mellitus: a potential inflammatory vicious cycle. Int J Mol Sci. 2021;22:(21):11831. doi: 10.3390/ijms222111831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frühbeck G, Gómez‐Ambrosi J, Ramírez B, et al. Increased levels of interleukin‐36 in obesity and type 2 diabetes fuel adipose tissue inflammation by inducing its own expression and release by adipocytes and macrophages. Front Immunol. 2022;13:832185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zychowska M, Rojewska E, Kreiner G, Nalepa I, Przewlocka B, Mika J. Minocycline influences the anti‐inflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J Neuroimmunol. 2013;262(1‐2):35‐45. doi: 10.1016/j.jneuroim.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Jin F, Li Q, Gao Y, Liu X, Hou R. IL‐34 and coronary heart disease complicated with diabetes mellitus. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46(12):1409‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cabrera S, Coren A, Pant T, et al. Probiotic normalization of systemic inflammation in siblings of type 1 diabetes patients: an open‐label pilot study. Sci Rep. 2022;12(1):3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khairul‐Anwar I, Wan‐Nazatul‐Shima S, Siti‐Lailatul‐Akmar Z, Siti‐Azrin A, Zunaina E. Evaluation of TNF‐α and IL‐6 in saliva among diabetic retinopathy patients in East Coast Malaysia. Trop Med Int Health. 2022;27(3):310‐316. doi: 10.1111/tmi.13724 [DOI] [PubMed] [Google Scholar]
- 27. Lu L, Zou G, Chen L, Lu Q, Wu M, Li C. Elevated NLRP3 inflammasome levels correlate with vitamin D in the vitreous of proliferative diabetic retinopathy. Front Med. 2021;8:736316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucijanić M, Jordan A, Jurin I, et al. Red cell distribution width is a potent prognostic parameter for in‐hospital and post‐discharge mortality in hospitalized coronavirus disease 2019 patients: a registry‐based cohort study on 3941 patients. Croat Med J. 2022;63(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katamreddy A, Kokkinidis D, Miles J, Siasos G, Giannakoulas G. Faillace RT. Elevated red cell distribution width and cardiovascular mortality in ASCVD risk cohorts: National Health and Nutrition Examination Survey (NHANES III). Rev Cardiovasc Med. 2022;23(2):51. [DOI] [PubMed] [Google Scholar]
- 30. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432‐437. [DOI] [PubMed] [Google Scholar]
- 31. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(4):281‐290. [DOI] [PubMed] [Google Scholar]
- 32. Delanghe J, Lambrecht S, Fiers T. Speeckaert MM. Labile glycated hemoglobin: an underestimated laboratory marker of short term glycemia. Clin Chem Lab Med. 2022;60(3):451‐455. [DOI] [PubMed] [Google Scholar]
- 33. Gu L. Xue S. The association between red blood cell distribution width and the severity of diabetic chronic kidney disease. Int J Gen Med. 2021;14:8355‐8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsilingiris D, Makrilakis K, Barmpagianni A, et al. The glycemic status determines the direction of the relationship between red cell distribution width and HbA1c. J Diabetes Complications. 2021;35(10):108012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (Lingzhen Kong).


