The outbreak of new SARS-CoV-2 variants represents a second pandemic emergency among paediatric populations. Since the second semester of 2021, epidemiological reports have confirmed that the diffusion of the Omicron SARS-CoV-2 variant in the general population is not limited to adults, highlighting the need for new studies on the incidence in young immunocompromised subjects, potentially exposed to severe complications [1].
In 2020, at the beginning of the pandemic, children seemed to have a lower incidence and milder clinical course of coronavirus disease 2019 (COVID-19), including those receiving chronic treatment with rituximab for nephrotic syndrome [2]. In April 2020, during the first pandemic wave, we prospectively followed up a cohort of 159 paediatric patients on chronic immunosuppression with the two B cell-depleting antibodies, rituximab vs. ofatumumab, due to steroid-dependent nephrotic syndrome (NCT02394119) [3] and we observed no cases of COVID-19 [2]. Such findings supported the therapeutic strategies to maintain administration of rituximab in these children during the pandemic. However, after almost 2 years, with the diffusion of the new variants characterized by faster spread also among the paediatric population, a re-analysis of COVID-19 incidence in children with nephrotic syndrome on immunosuppression was considered mandatory so as to exclude underestimating risks.
We report here an update on the incidence and clinical outcome of SARS-CoV-2 infection in paediatric and young adults with nephrotic syndrome on chronic immunosuppression. This large cohort includes 228 patients, 176 children [≤ 18 years] and 52 young adults [19–24 years]), enrolled in two randomized controlled trials comparing the safety/efficacy profile of the B cell-depleting antibodies rituximab vs. ofatumumab (NCT02394119) [3] and rituximab vs. mycophenolate mofetil (MMF) (NCT04585152) [4]. Swab tests for Sars-Cov2 were performed based on clinical signs or according to the Italian surveillance protocols, in case of close contact, or in order to obtain a Green Certificate (vaccination/exemption certificate) without vaccination for > 12 year-old.
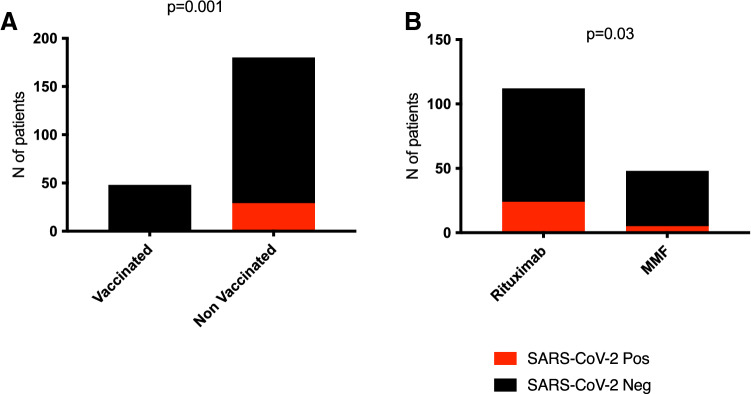
Of the 228 subjects considered, 29 (13%) reported SARS-CoV-2 infection. Among the entire cohort, only 48 patients received regular mRNA vaccination for SARS-CoV-2 (Pfizer/BioNtech BNT162b2 or Moderna m-1273) and none reported SARS-CoV-2 infection vs. 29/180 (21%) non vaccinated individuals (p = 0.0011) (Fig. 1A). Median age of unvaccinated subjects was 9 ± 5 vs. 14 ± 6 years of vaccinated patients. Of note, one subject reported an increase to 2 g/die of proteinuria two weeks after the second dose of vaccine, but recovered in one week without treatment.
Fig. 1.
Positivity for SARS-CoV-2. A The 29 positive subjects were not vaccinated for SARS-CoV-2 at the time of infection. None of the vaccinated patients developed SARS-CoV-2 infection (p = 0.0011). B Prevalence of infection in patients receiving rituximab is statistically significant when compared to subjects receiving MMF (p = 0.03)
No cases of relapse of the nephrotic syndrome were observed after the infection and 7/29 patients (24%) reported low or moderate positive dipsticks for 18 ± 6 days after positivity, that spontaneously recovered.
Therefore, contrary to what was reported in the first phase of the COVID-19 pandemic, the incidence of infection in a large cohort of paediatric and young patients with nephrotic syndrome receiving chronic immunosuppression has rapidly increased, in particular in the last months, as occurred in the general population. Of relevance, 9/27 (33%) positive subjects developed infection within only 6 weeks of the Omicron variant spread, from December 2021 to January 2022.
Between April 2020 and January 2022, 24 of the 112 patients on rituximab (21%) and 5 of the 48 on MMF (8%) reported COVID-19 infection (p = 0.03) (Fig. 1B). Comparing positive and negative subjects in the rituximab group, no significant differences were observed in median time since last infusion (15 months [0–56] in positive vs. 14 months [0–64] in negative subjects). Two patients reported SARS-CoV-2 infection a few days after their last infusion of rituximab. The clinical outcome was good in all cases and no patient required hospitalization: 5/24 on rituximab and 1/5 on MMF had mild symptoms, including fever (> 37 °C), cough, fatigue or gastrointestinal disorders. Median time to achieve negative swab test was 24 (7–38) and 23 (14–32) days in the rituximab and MMF groups, respectively.
The use of B cell–depleting therapies like rituximab may represent a significant risk factor compared to MMF. According to our observation, vaccination appears to be the greatest protective factor towards COVID-19. Unlike previous reports in the context of rheumatic diseases [5], no major or fatal COVID-19-related complications were reported in our patients receiving rituximab: our therapeutic scheme is limited to a single infusion of 375 mg/m2 and this strategy may protect against severe complications.
The main conclusion of the present update on COVID-19 incidence in a large cohort of paediatric and young subjects receiving immune-treatments is that therapeutic strategies need not be altered, mostly considering the benign clinical course in our cases of SARS-CoV-2 infection.
We also strongly recommend vaccination for SARS-CoV-2 at any age. In our cohort, only a minority of subjects were vaccinated (21%), mostly due to the age limit (14%) or because still on a waiting list to receive it (46%). To avoid a long wait after B-cell depletion, we suggest vaccination before rituximab infusion.
Author contributions
GMG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: GMG and AA. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: GMG and AA. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: MB. Obtained funding: GMG and AA. Administrative, technical, or material support: GMG and FL. Supervision: GMG, AA and EV.
Funding
Funding was provided by Ricerca Corrente 2021 (grant no. 2021).
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeletti A, Drovandi S, Sanguineri F, et al. COVID-19 in children with nephrotic syndrome on anti-CD20 chronic immunosuppression. Clin J Am Soc Nephrol. 2020;15(10):1494–1495. doi: 10.2215/CJN.06400420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravani P, Colucci M, Bruschi M, et al. Human or chimeric monoclonal anti-CD20 antibodies for children with nephrotic syndrome: a superiority randomized trial. J Am Soc Nephrol. 2021;32(10):2652–2663. doi: 10.1681/ASN.2021040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugani F, Angeletti A, Ravani P, et al. Randomised controlled trial comparing rituximab to mycophenolate mofetil in children and young adults with steroid-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open. 2021;11(11):e052450. doi: 10.1136/bmjopen-2021-052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvaro Gracia JM, Sanchez-Piedra C, Manero J, et al. Role of targeted therapies in rheumatic patients on COVID-19 outcomes: results from the COVIDSER study. RMD Open. 2021;7(3):e001925. doi: 10.1136/rmdopen-2021-001925. [DOI] [PMC free article] [PubMed] [Google Scholar]