The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOC) widespread and breakthrough infections prompted additional preventive measures in fully vaccinated immunocompromised recipients, including actively treated cancer patients.1 Regulatory agencies recommended a third homologous (booster) dose of a messenger RNA-based vaccine for this condition based on evidence from immunosuppressed organ transplant recipients.2 This study aimed to evaluate the safety, immunogenicity, and clinical outcome of two or three doses of BNT162b2 vaccine (tozinameran) in patients with solid malignancies receiving systemic therapies.
The Vax-On-Third is a prospective, observational study that included adult cancer patients on active treatment within the previous 6 months and who had completed a two-dose schedule of tozinameran 26-22 weeks before enrollment. Coronavirus disease 2019 (COVID-19) infection at any time was an exclusion criterion. Patients who received booster dosing (Boost cohort) were compared with those who did not (Unboost cohort) for different reasons (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2022.04.002). All enrolled patients were tested for immunoglobulin G (IgG) antibody titer against receptor-binding domain of SARS-CoV-2 Spike protein (RBD-S1) at baseline (timepoint-1) and 4 weeks after the third dose (timepoint-2). We used the SARS-CoV-2 IgG II Quant immunoassay on the ARCHITECT i2000sr automated platform (Abbott, Sligo, Ireland) with a cut-point ≥50 AU/ml indicating a positive seroconversion response. A threshold ≥4446 AU/ml was selected as a correlate of 50% vaccine efficacy (VE) against symptomatic COVID-19 infection.3 The incidence of SARS-CoV-2 infections was monitored in both study cohorts by periodic swab testing. Dedicated safety questionnaires were delivered at timepoint-1 and collected at timepoint-2. Propensity score matching (PSM) was carried out to reduce potential selection bias between the cohorts. Proper two-sided tests were applied with a significance level of P < 0.05 for each comparison within the matched population (Statistical Analysis in the Supplementary Material, available at https://doi.org/10.1016/j.annonc.2022.04.002). The study followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guidelines and was approved by the referring Ethics Committee (protocol number: 1407/CE Lazio1; clinical study identifier: EudraCT number 2021-002611-54).
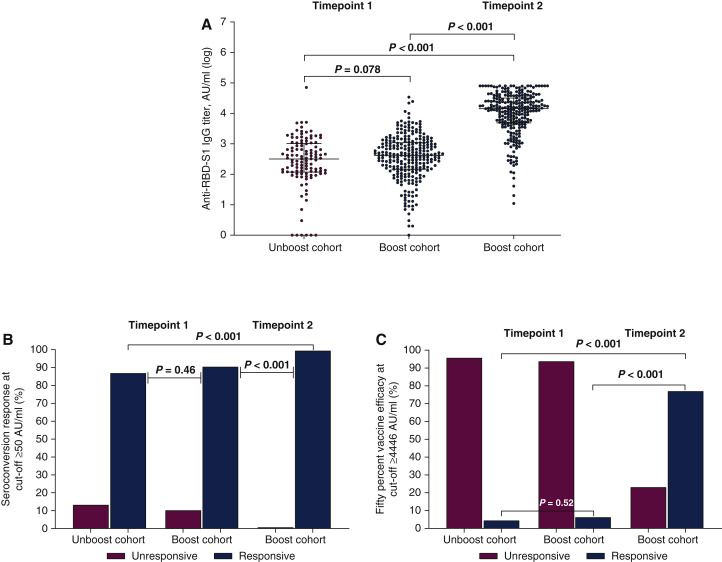
We enrolled 372 consecutive patients between 23 September and 7 October 2021 (Supplementary Figure S1 and Table S1, available at https://doi.org/10.1016/j.annonc.2022.04.002). All patients were assessable for safety, while 253 (98.1%) cases in the Boost cohort completed serologic testing at timepoint-2. Systemic adverse events were mostly mild to moderate and did not exceed 15% of cases, with only four patients (1.6%) reporting severe reactions (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2022.04.002). After PSM, 91 patients in the Boost cohort and 158 patients in the Unboost cohort were included in the comparative analysis, with no significant differences in confounding factors between the groups (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2022.04.002). Median anti-RBD-S1 IgG titer {Unboost cohort: 296 AU/ml [95% confidence interval (CI) 187-460 AU/ml] versus Boost cohort: 454 AU/ml (95% CI 359-584 AU/ml); P = 0.078}, seroconversion rate (Unboost cohort: 86.8% versus Boost cohort: 89.9%; P = 0.46), and 50% VE rate (Unboost cohort: 4.4% versus Boost cohort: 6.3%; P = 0.52) did not differ between cohorts at timepoint-1. The third dose of vaccine resulted in an exponential increase in median anti-RBD-S1 IgG titer [15 024 AU/ml (95% CI 11 598-19 447 AU/ml)], which was significantly higher than assessment at timepoint-1 in both Unboost (P < 0.001) and Boost cohorts (P < 0.001, Figure 1A). Accordingly, seroconversion rate (99.4%, P < 0.001) and 50% VE rate (76.9%, P < 0.001) improved significantly in the same comparison (Figure 1B and C). After a median follow-up of 145 days (interquartile range 140-153 days), 18 patients in the Unboost cohort (19.8%) and 10 in the Boost cohort (6.3%, P = 0.001) reported contracting SARS-CoV-2 infection, none of which was clinically severe. On multivariate analysis, only immunosuppressive corticosteroid therapy and Eastern Cooperative Oncology Group performance status 2 correlated significantly with an impaired antibody response at timepoint-2 (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2022.04.002).
Figure 1.
Antibody response after two or three doses of tozinameran vaccine within the propensity score matching (PSM) population.
(A) Comparison of scatter plot distributions and medians of immunoglobulin G (IgG) titers against the receptor-binding domain of severe acute respiratory syndrome coronavirus 2 Spike protein (anti-RBD-S1), logarithmic (log) values. Bars represent median values with interquartile range. (B) Comparison of seroconversion response rates at cut-off ≥50 AU/ml. (C) Comparison of 50% vaccine efficacy response rates at cut-off ≥4446 AU/ml.
This cohort study confirms a favorable safety profile of the third dose of tozinameran in a broad sample of cancer patients receiving active treatments. While residual confounding may still be present, comparative evaluation within the PSM population suggests improved immunogenicity of booster dosing, independent of types and timing of systemic therapies, and consistent with similar studies that employed the same serologic testing methodology.4 , 5 Although longer follow-up is required, the effects of booster vaccine dosing appear to translate into a reduced risk of infection during intense SARS-CoV-2 VOC outbreaks.
Acknowledgements
All authors express their gratitude to the Strategic Directorate of Viterbo Public Health Agency, whose unselfish commitment made the conduct of this research project possible.
Funding
None declared.
Disclorure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Schmidt A.L., Labaki C., Hsu C.Y., et al. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33:340–346. doi: 10.1016/j.annonc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamar N., Abravanel F., Marion O., et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng S., Phillips D.J., White T., et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenioux C., Teixeira L., Fourati S., et al. SARS-CoV-2 antibody response to 2 or 3 doses of the BNT162b2 vaccine in patients treated with anticancer agents. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2021.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligumsky H., Dor H., Etan T., et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2021 doi: 10.1016/S1470-2045(21)00715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.