Abstract
Background
Huntington’s disease (HD) patients often present with abnormal modulation of blood pressure and heart rate. We investigated whether cardiac autonomic innervation assessed by 123I-metaiodobenzylguanidine (MIBG) imaging is impaired in HD patients, in comparison with controls (Ctrl).
Methods
Fifteen patients (6 F and 9 M) were assessed by the motor section of the Unified HD Rating Scale, the Total Function Capacity, and the scale for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT) questionnaire. All patients and 10 Ctrl (5 F and 5 M) underwent 123I-MIBG imaging. From planar images, the early and late heart-to-mediastinum (H/M) ratios and myocardial washout rates (WR) were calculated.
Results
We did not find significant differences in early and late H/M ratios and WR between the two groups. At individual level, three patients showed reduced early and/or late H/M ratios. The most common autonomic complaints were gastrointestinal and genitourinary disorders. SCOPA-AUT questionnaire score results positively correlated with the disease duration and WR.
Conclusions
Our study indicates that myocardial postganglionic sympathetic innervation is essentially preserved or only minimally involved in HD. These findings suggest that the cardiovascular dysfunction might be mainly due to the impairment of brain areas associated with the regulation and modulation of the heart function.
Electronic supplementary material
The online version of this article (10.1007/s12350-020-02299-7) contains supplementary material, which is available to authorized users.
Keywords: Huntington’s disease, autonomic disorders, scintigraphy, MIBG
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by a CAG trinucleotide repeat expansion in the huntingtin gene on chromosome 4 which leads to the production of a protein with an abnormally long polyglutamine stretch.1 The prevalence is 10.6 to 13.7 × 10−5 in Western countries and the age at onset ranges between 30 and 50 years.1,2 The cardinal symptoms consist of movement disorders, usually chorea, cognitive impairment, and psychiatric disturbances.1,2
The clinical picture is also characterized by symptoms due to hypothalamic dysfunction as weight loss, sleep and endocrine disorders including increased cortisol levels, reduced testosterone levels and high prevalence of diabetes.3 The patients may complain of autonomic disorders that often precede the onset of motor manifestations. HD patients and pre-manifest CAG expansion carriers more significantly present with gastrointestinal, urinary, cardiovascular and, in men, sexual problems than the control subjects.4 In particular, potential cardiac manifestation of severe autonomic dysfunction, as light headedness on standing up, tachycardia, arrhythmias, and sudden cardiac death, seems to be more frequent in HD than in controls.4,5
Here, we aimed to investigate whether cardiac autonomic innervation assessed by 123I-metaiodobenzylguanidine (MIBG) imaging is impaired in HD patients, in comparison with control subjects (Ctrl).
Patients and Methods
We included fifteen HD patients (6 F and 9 M), with confirmation by genetic test, and ten Ctrl (5 F and 5 M), comparable for age. Written informed consent was obtained from all participants, according to the declaration of Helsinki and with the local Ethics Committee approval. Patients were assessed by the motor examination of the Unified HD Rating Scale (UHDRS, section III), and the Total Function Capacity (TFC). All patients underwent the Scale for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) questionnaire,6 to assess self-reported autonomic dysfunction. Subjects with MMSE score ≤ 23/30, cardiac disease, diabetes, untreated hypertension or receiving treatment with tricyclic and tetracyclic antidepressants, serotonin reuptake inhibitors (SSRI), sympathomimetics and sympatholytics, antipsychotics, calcium channel antagonists or ACE inhibitors were excluded, to avoid interference with MIBG uptake.7 Ten subjects served as the Ctrl group, undergoing 123I-MIBG scintigraphy to rule out disease of the adrenal medulla. None of these subjects had a history of neurological or cardiac diseases or diabetes and none of them was taking medication that might have been expected to interfere with MIBG myocardial uptake. All patients and Ctrl underwent 123I-MIBG cardiac imaging as previously described in detail.8 After an administration of 111 MBq of 123I-MIBG a 10-minute planar anterior chest image (256x256 matrix) was performed at 15 minutes (‘‘early’’ image) and again at 3 hours and 50 minutes (‘‘late’’ image). From planar images of the thorax, the early and late heart-to-mediastinum (H/M) ratios were computed by dividing the mean counts per pixel within the myocardium by the mean counts per pixel within the mediastinum. By comparing early and late activities, the 123I-MIBG washout rate (WR) from the myocardium was derived, providing a parameter that reflects retention of norepinephrine by sympathetic neurons. The MIBG WR was calculated using the formula:
Statistical Analysis
Differences in non-parametric data between HD patients and Ctrl were analyzed using the Mann–Whitney U test. The relationship between variables was examined using the Spearman’s correlation coefficient. A P value < 0.05 was considered statistically significant. The Statistical Package for the Social Sciences software for Windows (version 21.00, SPSS, Chicago, IL, USA) was used for the statistical analyses.
Results
Demographic and clinical characteristics and imaging findings are shown in Table 1. Ten patients were in stage 1 of disease (TFC 11 to 13), and 5 in stage 2 (TFC 7 to 10). At SCOPA-AUT questionnaire, dysphagia was reported by 9 subjects (64%; case 1, 4, 5, 6, 8, 11, 13, 14 and 15), drooling by 3 (20%; case 10, 13 and 14), gastrointestinal symptoms, as feeling that food gets stuck in throat or constipation, by 9 patients (60%; case 1, 6, 7, 8, 9, 10, 11, 13 and 14), genitourinary symptoms, as bladder and/or sexual dysfunction, by 10 (67%; case 1, 2, 4, 5, 7, 9, 10, 11, 13 and 14), symptoms related to orthostatic hypotension, as the feeling of either becoming lightheaded when suddenly standing up or after standing for a while, by 6 (40%; case 1, 7, 9, 10, 12 and 13), sweating dysfunction by 6 (40%; case 1, 4, 7, 10, 12 and 15), oversensitivity to bright light and trouble tolerating cold/heat both by 7 (47%; case 4, 7, 10, 11, 12, 13, 14; case 1, 4, 7, 10, 12, 13 and 15) (Table 2).
Table 1.
Demographic and clinical data and MIBG scintigraphy results
| Subjects | Gender | Age | DD | SCOPA-AUT | UHDRS-III | TFC | CAG | Early H/M | Late H/M | WR |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||||
| 1 | F | 50 | 10 | 15 | 20 | 13 | 43 | 2.18 | 2.04 | 29.27 |
| 2 | M | 51 | 1 | 5 | 27 | 11 | 44 | 1.48 | 1.62 | 7.31 |
| 3 | M | 65 | 3 | 10 | 25 | 11 | 40 | 1.7 | 1.5 | 39.0 |
| 4 | F | 68 | 5 | 10 | 33 | 9 | 41 | 2.5 | 2.7 | 11.5 |
| 5 | M | 47 | 5 | 13 | 34 | 10 | 46 | 1.96 | 1.9 | 25.66 |
| 6 | F | 64 | 8 | 7 | 35 | 11 | 43 | 2.06 | 2.17 | 16.7 |
| 7 | F | 41 | 4 | 26 | 15 | 10 | 44 | 2.28 | 1.82 | 25.6 |
| 8 | F | 39 | 3 | 4 | 11 | 13 | 48 | 1.88 | 1.85 | 15.0 |
| 9 | M | 46 | 2 | 0 | 4 | 13 | 42 | 1.95 | 2.05 | 18.5 |
| 10 | M | 66 | 11 | 22 | 42 | 7 | 42 | 2.02 | 1.98 | 37.5 |
| 11 | F | 74 | 3 | 14 | 11 | 13 | 40 | 2.3 | 2.2 | 19.21 |
| 12 | M | 35 | 1 | 6 | 8 | 13 | 47 | 2.31 | 2.45 | -6.55 |
| 13 | M | 47 | 6 | 10 | 12 | 12 | 44 | 2.05 | 2.59 | 3.64 |
| 14 | M | 42 | 7 | 12 | 45 | 8 | 49 | 2.32 | 2.10 | 39.0 |
| 15 | M | 55 | 4 | 8 | 22 | 11 | 45 | 2 | 2.2 | 1.35 |
| Mean ± SD | 52.6 ± 12.0 | 4.8 ± 3.0 | 10.8 ± 9.0 | 24.6 ± 12.0 | 2.06 ± 0.26 | 2.09 ± 0.35 | 18.84 ± 14.01 | |||
| Median | 11 | 43 | ||||||||
| Ctrl | 55.0 ± 7.0 | |||||||||
| Mean ± SD | 2.18 ± 0.11 | 2.11 ± 0.18 | 20.65 ± 9.28 |
DD, disease duration; SCOPA-AUT, Scale for outcomes in Parkinson’s disease-autonomic; UHDRS, section III of Unified Huntington’s Disease Rating Scale; TFC, total functional capacity; CAG, pathological repeat
Values below or above 2SD the mean of Ctrl were considered abnormal (in bold)
Table 2.
Prevalence of autonomic complaints at SCOPA-AUT questionnaire in 15 patients with Huntington’s disease.
| Patients (%) | |
|---|---|
| Gastrointestinal domain | 9 (60) |
| Genitourinary domain | 10 (67) |
| Cardiovascular domain | 6 (40) |
| Thermoregulatory domain | 7 (47) |
| Pupillomotor domain | 7 (47) |
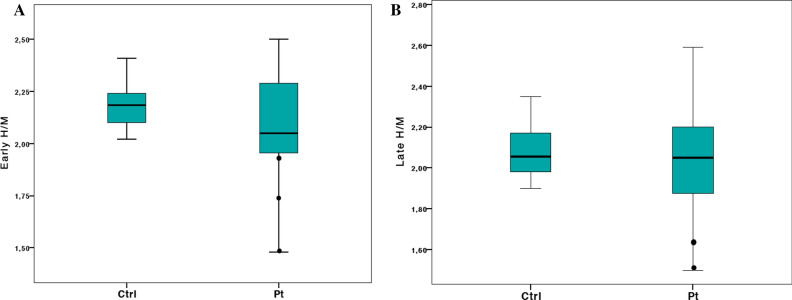
There were no significant differences in early H/M ratio (P = 0.18; cut-off ≥ 1.96), late H/M ratio (P = 0.85; cut-off ≥ 1.75) and WR (P = 0.78; cut-off ≤ 39.4%) between HD patients and Ctrl (Figure 1). At the individual level, compared to Ctrl, abnormal results (2SD below or above the mean of Ctrl) were found in only three patients: two subjects showed a significant reduction in both early and late H/M ratios (case 2 and 3) and one in early H/M ratio only (case 8) (Table 1).
Figure 1.
Early H/M (A) and late H/M ratio (B) results in patients (Pt) and controls (Ctrl). The central boxes represent the values from the lower to upper quartile (25° to 75° percentile). The middle lines represent the mean. The vertical lines extend from the minimum to the maximum value; pathological values are displayed as separate points
As expected, a correlation was observed between CAG expansion and age at onset (rho − 0.598; P < 0.02), and between disease duration and UHDRS-III score (rho 0.635; P = 0.011).
SCOPA-AUT questionnaire score results positively correlated with both disease duration (rho 0.598; P = 0.024) and WR (rho 0.631; P = 0.015) (Figure 2).
Figure 2.
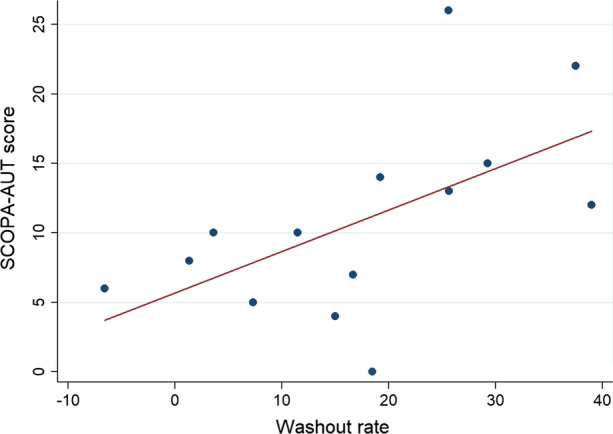
Relationship between SCOPA-AUT score and washout rate (Spearman’s correlation; rho 0.631; P = 0.015)
Discussion
In the last years, the evidence of an autonomic dysfunction in HD, even in presymptomatic and early stage of the disease, has strengthened. Abnormal modulation of blood pressure and heart rate, manifesting with syncope and cardiac arrhythmias, often occur in HD. Abnormal circadian rhythm of heart rate, decreased heart rate variability and baroreceptor reflex failure have been observed in preclinical and clinical research.9–11 Recent studies showed the presence of both sympathetic and parasympathetic autonomic nervous system dysfunction in HD.10–12
In a previous study in patients affected by spinocerebellar ataxia type 2, another degenerative disorder due to CAG triplet expansion and associated with abnormal aggregates of polyglutamine sequences, 123I-MIBG myocardial scintigraphy showed an impairment of cardiac sympathetic function.13
In view of the common pathogenic mechanism underlying the two disorders, we also assessed self-reported autonomic complaints and 123I-MIBG imaging in HD patients, to investigate if postganglionic sympathetic innervation is impaired in this neurodegenerative disorder, as so far this tool has never been applied in HD.
Autonomic dysfunction was present in our HD patients. Swallowing difficulties, erection and ejaculation problems, dysphagia, and drooling were the most common autonomic symptoms found in our patients in comparison with Ctrl using the SCOPA-AUT questionnaire, in agreement with previous report.4 We also found a high prevalence of gastrointestinal and genitourinary symptoms, but sweating dysfunction, symptoms related to orthostatic hypotension, oversensitivity to bright light and trouble tolerating cold/heat also resulted common. We observed a significant association between the total autonomic dysfunction score and the disease duration, suggesting that the autonomic nervous system involvement worsens over the time.
Interestingly, the SCOPA-AUT questionnaire score was not correlated with CAG expansion size, UHDRS-III score, and TFC, suggesting that the autonomic dysfunction could be an early non-motor manifestation of disease, in line with previous findings.4
The finding of oversensitivity to bright light might be due to retinal dysfunction/degeneration reported in mouse models and patients with HD. Attenuated pupillary light response accompanied by a progressive downregulation of retinal cone opsin and melanopsin expression has been observed in mouse models.14 The pupillary light reflex latency has been shown increased in the HD group in comparison with Ctrl.15
Thermoregulatory disorders as hypothermia, erratic thermogenesis, and an abnormal circadian temperature rhythm have already been reported in HD,16 so the trouble tolerating cold/heat complained by our patients may be explained by both metabolic factors, as low body weight, energy expenditure due to dyskinesias and adipose tissue composition, and hypothalamic pathology characterized by oxytocin and vasopressin neuron loss.16
We did not find a significant difference in early and late H/M ratios and WR between HD patients and Ctrl as a group (Table 1). At the individual level, two patients with HD showed, however, a significant reduction in both early and late H/M ratios, and one in early H/M only. This finding is not easy to interpret as the disease was at an early stage in all three patients. Moreover, those patients did not differ from the others in severity of the clinical and genetic features (age at onset, motor UHDRS score, CAG expansion, disease duration, age, SCOPA-AUT scores) (Table 1), and the presence of autonomic cardiovascular symptoms. The cerebral mechanisms for control of the autonomic nervous system are complex and not yet well understood.5 The wide and heterogeneous neuropathological findings reported in HD17,18 may explain the heterogeneity of both results in evaluation of cardiac sympathetic activity and of autonomic disorders detected at SCOPA-AUT questionnaire.
Interestingly, we observed a significant association between SCOPA-AUT questionnaire score and WR, that accounts for the retention of norepinephrine by sympathetic neurons and overall sympathetic tone, mainly representing noradrenaline uptake-1 (Figure 2).19 This finding is consistent with a relevant contribution of sympathetic impairment to the autonomic dysfunction in our patients.
Overall, the results of our study suggest that myocardial postganglionic sympathetic innervation as measure in vivo with 123I-MIBG is mostly preserved or only slightly reduced in HD, as suggested by the occurrence of significant early and/or late H/M ratios reduction in 20% of patients and the correlation found between increased WR and the severity of autonomic dysfunction.
The impairment of cortical and subcortical regions, as the prefrontal cortex, the bilateral insular cortex, the anterior cingulate gyrus, the amygdala and the hypothalamus, often reported in HD20 and implicated in the regulation and control of the cardiac function, might partly underly the cardiovascular dysfunction found in our HD patients.
Brain-derived neurotrophic factor (BDNF) is known to be involved in the neuro-mediated regulation of heart rate and blood pressure and to play a protective role against cardiac dysfunction.21 BDNF and its receptor TrkB are highly expressed in areas involved in cardiac control regions such as amygdala, frontal cortex, hypothalamus, and the brainstem. Interestingly, decreased levels of BDNF are reported in the striatum, cortex, and brainstem of HD patients.21 A deficient cortical transcription of the BDNF gene, its defective transport to the striatum and a reduced level of mRNA coding TrkB in the caudate nucleus have been also shown.21 Moreover, the accumulation of mutated huntingtin may be toxic to cardiomyocytes in humans, as showed in mouse models that developed cardiac dysfunction progressing to severe failure over a few weeks.22
So, cardiac dysfunction in HD is probably multifactorial and likely worsened by drugs, as neuroleptics and SSRI, that act on rhythm, heart rate and atrioventricular conduction.
Limitations
Caution is required in the interpretation of our results as the number of subjects included in this study is relatively small. However, HD is a rare disease and 123I-MIBG scintigraphy requires strict criteria of inclusion to avoid drugs interfering with the analysis. Further investigation in a larger number of patients is required to confirm these findings. Despite this limitation, our work provides for the first time the status of cardiac sympathetic function in vivo in a cohort of HD patients.
New Knowledge Gained
For the first time, 123I-MIBG has been performed in HD patients, showing that myocardial postganglionic sympathetic innervation is preserved in most cases. Therefore, the cardiovascular autonomic disorders may be due to central nervous system control impairment of the heart function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PPT 358 kb)
Electronic supplementary material 2 (M4A 2416 kb)
Acknowledgments
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Abbreviations
- HD
Huntington’s disease
- UHDRS
Unified HD Rating Scale
- TFC
Total functional capacity
- SCOPA-AUT
Scale for outcomes in Parkinson’s disease-autonomic
- MIBG
Metaiodobenzylguanidine
- H/M
Heart to mediastinum
- WR
Washout rate
- Ctrl
Control subjects
Disclosures
Anna De Rosa, Roberta Assante, Elena Salvatore, Carmela Nappi, Silvio Peluso, Giovanni De Simini, Luigi Di Maio, Gianluigi Rosario Palmieri, Alessandro Roca, Giuseppe De Michele, Alberto Cuocolo and Sabina Pappatà declare that they have no conflicts of interest.
Footnotes
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for reuse at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Jeroen J. Bax, MD.
Funding
Not applicable.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McColgan P, Tabrizi SJ. Huntington’s disease: A clinical review. Eur J Neurol. 2018;25(1):24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 2.Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;20(5):40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersén A, Björkqvist M. Hypothalamic–endocrine aspects in Huntington’s disease. Eur J Neurosci. 2006;24(4):961–967. doi: 10.1111/j.1460-9568.2006.04985.x. [DOI] [PubMed] [Google Scholar]
- 4.Aziz NA, Anguelova GV, Marinus J, van Dijk JG, Roos RA. Autonomic symptoms in patients and pre-manifest mutation carriers of Huntington’s disease. Eur J Neurol. 2010;17(8):1068–1074. doi: 10.1111/j.1468-1331.2010.02973.x. [DOI] [PubMed] [Google Scholar]
- 5.Abildtrup M, Shattock M. Cardiac dysautonomia in Huntington’s disease. J Huntingtons Dis. 2013;2(3):251–261. doi: 10.3233/JHD-130054. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19:1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 7.Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M. Proposal for standardization of 123Imetaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37:1802–1812. doi: 10.1007/s00259-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa A, Pellegrino T, Pappatà S, Pellecchia MT, Peluso S, Saccà F, et al. Myocardial 123I-metaiodobenzylguanidine scintigraphy in patients with homozygous and heterozygous parkin mutations. J Nucl Cardiol. 2017;24(1):103–107. doi: 10.1007/s12350-015-0332-z. [DOI] [PubMed] [Google Scholar]
- 9.Sharma KR, Romano JG, Ayyar DR, Rotta FT, Facca A, Sanchez-Ramos J. Sympathetic skin response and heart rate variability in patients with Huntington disease. Arch Neurol. 1999;56(10):1248–1252. doi: 10.1001/archneur.56.10.1248. [DOI] [PubMed] [Google Scholar]
- 10.Andrich J, Schmitz T, Saft C, Postert T, Kraus P, Epplen JT, et al. Autonomic nervous system function in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2002;72(6):726–731. doi: 10.1136/jnnp.72.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler TS, Park S, Loh DH, Jordan MC, Yokota T, Roos KP, et al. Neurocardiovascular deficits in the Q175 mouse model of Huntington’s disease. Physiol Rep. 2017 doi: 10.14814/phy2.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobal J, Meglic B, Mesec A, Peterlin B. Early sympathetic hyperactivity in Huntington’s disease. Eur J Neurol. 2004;11(12):842–848. doi: 10.1111/j.1468-1331.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa A, Pappatà S, Pellegrino T, De Leva MF, Maddaluno G, Fiumara G, et al. Reduced cardiac 123I-metaiodobenzylguanidine uptake in patients with spinocerebellar ataxia type 2: A comparative study with Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2013;40(12):1914–1921. doi: 10.1007/s00259-013-2524-6. [DOI] [PubMed] [Google Scholar]
- 14.Ouk K, Hughes S, Pothecary CA, Peirson SN, Morton AJ. Attenuated pupillary light responses and downregulation of opsin expression parallel decline in circadian disruption in two different mouse models of Huntington’s disease. Hum Mol Genet. 2016;25:5418–5432. doi: 10.1093/hmg/ddw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Den Heijer JC, Bollen WL, Reulen JP, van Dijk JG, Kramer CG, Roos RA, et al. Autonomic nervous function in Huntington’s disease. Arch Neurol. 1988;45(3):309–332. doi: 10.1001/archneur.1988.00520270087025. [DOI] [PubMed] [Google Scholar]
- 16.Weydt P, Dupuis L, Petersen Å. Thermoregulatory disorders in Huntington disease. Handb Clin Neurol. 2018;157:761–775. doi: 10.1016/B978-0-444-64074-1.00047-1. [DOI] [PubMed] [Google Scholar]
- 17.Nana AL, Kim EH, Thu DCV, Oorschot DE, Tippett LJ, Hogg VM, et al. Widespread heterogeneous neuronal loss across the cerebral cortex in Huntington’s disease. J Huntingtons Dis. 2014;3(1):45–64. doi: 10.3233/JHD-140092. [DOI] [PubMed] [Google Scholar]
- 18.Mehrabi NF. Symptom heterogeneity in Huntington’s disease correlates with neuronal degeneration in the cerebral cortex. Neurobiol Dis. 2016;96:67–74. doi: 10.1016/j.nbd.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi T, Nakata T, Hashimoto A, Yuda S, Tsuchihashi K, Travin MI, et al. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac events. J Nucl Med. 2001;42:1757–1767. [PubMed] [Google Scholar]
- 20.Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The neuropathology of Huntington’s Disease. Curr Top Behav Neurosci. 2015;22:33–80. doi: 10.1007/7854_2014_354. [DOI] [PubMed] [Google Scholar]
- 21.Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18(2):225–238. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihm MJ, Amann DM, Schanbacher BL, Altschuld RA, Bauer JA, Hoyt KR. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis. 2007;25(2):297–308. doi: 10.1016/j.nbd.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PPT 358 kb)
Electronic supplementary material 2 (M4A 2416 kb)