Elevated left-side filling pressures at rest and particularly during exertion are well described prognostic indicators and targets for cardiovascular therapies with the intention to improve exertional capacity, quality of life, and cardiovascular morbidity associated with heart failure (HF). The shift of blood between the peripheral vascular compartments (most significantly the splanchnic vascular bed) and the central vascular compartment have been identified as a central contributor to these elevated pressures.
The splanchnic vascular compartment is highly compliant and contains around 30% of the body’s intravascular blood volume. Preclinical and clinical investigations support the pivotal role of the sympathetic nervous system to affect the capacitance and compliance of the splanchnic bed by modulation of the greater splanchnic nerve (GSN). Stimulation of the GSN induces a rapid increase in cardiac preload and increased central pressures, particularly in the setting of HF.1
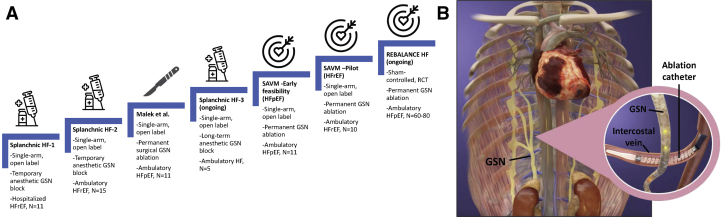
Recent investigations have explored the impact of temporary and permanent block of the GSN across the HF spectrum. Studies progressed from temporary anesthetic GSN blockade in hospitalized patients with HF with reduced ejection fraction (HFrEF) (bilateral, n = 11; Abdominal Nerve Blockade in Chronic Heart Failure, NCT03453151),2 to chronic HFrEF (bilateral and unilateral, n = 15; Splanchnic Nerve Anesthesia in Heart Failure, NCT02669407),3 to surgical ablation of the splanchnic nerves in HF with preserved ejection fraction (HFpEF) (n = 11; Surgical Resection of the Greater Splanchnic Nerve in Subjects Having Heart Failure With Preserved Ejection Fraction, NCT03715543) (Figure 1). These single-arm open-label studies demonstrated a common effect independent from HF type: GSN blockade reduces resting and exercise-induced pulmonary capillary wedge pressure (PCWP) with positive effects on cardiac output and exercise function. Permanent surgical ablation of the right-side GSN confirmed the long-term safety and efficacy of unilateral GSN ablation through at least 12 months in an open-label study.4 There was no change in arterial blood pressure, resting heart rate, and serum creatinine. Similarly to temporary GSN block, at 3 months of follow-up, permanent GSN ablation reduced the mean PCWP by −4.5 mm Hg (95% CI: −14 to −2; P = 0.006) at 20 W exercise compared with baseline, and the effect carried over to peak exercise with a −5 mm Hg (95% CI: −11 to 0; P = 0.016) reduction. Hemodynamic improvements persisted out to 12 months, with parallel improvement in functional capacity and health status. The duration of exercise on the cardiopulmonary exercise test improved by 73.5 seconds (95% CI: −13 to 191; P = 0.084) at 1 month (compared with baseline), to 173 seconds (95% CI: −11 to 228; P = 0.039) at 3 months, 123 seconds (95% CI: 2-368; P = 0.027) at 6 months, and 134 seconds (95% CI: 12-261; P = 0.008) at 12 months. The peak oxygen consumption at 6 and 12 months after surgery increased by +2.3 mL/kg/min (95% CI: −0.2 to 4.5; P = 0.039) and +1.6 mL/kg/min (95% CI: −0.3 to 5.7; P = 0.050), respectively. At least 70% of patients experienced a symptomatic improvement by at least 1 New York Heart Association functional class at 1 month out to 12 months.
Figure 1.
Evidence Pipeline for Splanchnic Nerve Modulation in Heart Failure and Evolution to a Percutaneous Device Strategy
(A) Evidence ladder for greater splanchnic nerve ablation in heart failure. (B) Venous and GSN anatomy. GSN = greater splanchnic nerve; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; SAVM = splanchnic ablation for volume management.
Previous studies have demonstrated the long-term safety of permanent GSN ablation for the treatment of intractable abdominal pain. In general, permanent GSN ablation is well tolerated, with infrequent incidence of transient gastrointestinal side-effects and transient hypotension. Although those studies did not include HF patients, the long-term safety in patients with intractable abdominal pain (described over the past 40 years), coupled with the safety profile seen in recent HF studies, is reassuring.
With this foundation in place, a novel, minimally invasive, endovascular, transvenous procedure to permanently denervate the right-side GSN was developed in a therapy coined splanchnic ablation for volume management (SAVM). SAVM was first investigated in an open-label early feasibility study using the Axon Ablation System (Axon Therapies) (Endovascular GSN Ablation in Subjects With HFpEF, NCT04287946). The trial is completed and is pending publication. The trial enrolled 11 patients with HFpEF with New York Heart Association functional class II or III symptoms, ejection fraction ≥50%, and elevated PCWP ≥15 mm Hg at rest or ≥25 mm Hg during exercise.5 The Axon Ablation System delivers radiofrequency energy via the intravascular catheter temporarily deployed in close proximity to the right-sided GSN (right femoral venous access to the superior vena cava and retrograde passage via the azygous vein to the right-side intercostal veins at the 10th and 11th thoracic vertebral level) (Figure 1). The primary safety end point was a composite incidence of major adverse cardiac, cerebrovascular embolic, or renal events (MACCRE) through 1 month of follow-up. The primary efficacy end point was a mean change of PCWP with exercise at 1 month compared with baseline. Patients underwent assessment at baseline as well as 1 month, 3 months, 6 months, and 12 months after the intervention. At all time points, the tests included physical examination, 6-minute walk test, health status assessment with the Kansas City Cardiomyopathy Questionnaire, and comprehensive echocardiography.
The cumulative evidence to date supports the safety and efficacy of GSN ablation. The early feasibility and pilot studies of the SAVM procedure in HFpEF and HFrEF will extend the safety and efficacy data, as well as provide additional data on the durability of percutaneous right-side GSN ablation in the management of HF. Finally, a randomized, sham-controlled, phase 2 clinical trial (Endovascular Ablation of the Right Greater Splanchnic Nerve in Subjects Having HFpEF; Rebalance-HF, NCT04592445) is ongoing to expand on the initial findings and definitively investigate these promising results.
Footnotes
Dr Fudim was supported by the National Heart, Lung, and Blood Institute (K23HL151744), an American Heart Association Mario Family Award (20IPA35310955), Duke Chair’s Award, Translating Duke Health Award, Bayer, Bodyport, and BTG Specialty Pharmaceuticals, and receives consulting fees from Abbott, Audicor, Axon Therapies, Bodyguide, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, NXT Biomedical, Pharmacosmos, PreHealth, Splendo, Vironix, Viscardia, and Zoll. Dr Engelman consults for and owns stock in Axon Therapies. Dr Reddy serves as a consultant to and holds stock options in Axon Therapies and has other disclosures not related to this manuscript: Abbott (consultant), Ablacon (consultant, equity), Acutus Medical (consultant, equity), Affera (consultant, equity), Apama Medical (consultant, equity), APN Health (consultant, equity), Aquaheart (consultant, equity), Atacor (consultant, equity), Autonomix (consultant, equity), Backbeat (consultant, equity), BioSig (consultant, equity), Biosense-Webster (consultant), BioTel Heart (consultant), Biotronik (consultant), Boston Scientific (consultant), Cardiac Implants (consultant, equity), CardiaCare (consultant, equity), Cardiofocus (consultant), Cardionomic (consultant), CardioNXT/AFTx (consultant, equity), Circa Scientific (consultant, equity), CoreMap (consultant), Corvia Medical (consultant, equity), Dinova-Hangzhou DiNovA EP Technology (consultant, equity), East End Medical (consultant, equity), EBR (consultant), EPD (consultant, equity), Epix Therapeutics (consultant, equity), EpiEP (consultant, equity), Eximo (consultant, equity), Farapulse (consultant, equity), Fire1 (consultant), Gore & Associates (consultant), HRT (consultant, equity), Impulse Dynamics (consultant), Intershunt (consultant, equity), Javelin (consultant, equity), Kardium (consultant, equity), Keystone Heart (consultant, equity), LuxMed (consultant, equity), Manual Surgical Sciences (Equity), Medlumics (consultant, equity), Medtronic (consultant), Middlepeak (consultant, equity), Newpace (Equity), Nuvera (consultant, equity), Philips (consultant), Pulse Biosciences (consultant), Restore Medical (consultant, equity), Sirona Medical (consultant, equity), Surecor (Equity), Valcare (consultant, equity), and Vizaramed (Equity). Dr Shah has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics.
Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2022-shark-tank
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Bapna A., Adin C., Engelman Z.J., Fudim M. Increasing blood pressure by greater splanchnic nerve stimulation: a feasibility study. J Cardiovasc Transl Res. 2020;13(4):509–518. doi: 10.1007/s12265-019-09929-7. [DOI] [PubMed] [Google Scholar]
- 2.Fudim M., Ganesh A., Green C., et al. Splanchnic nerve block for decompensated chronic heart failure: Splanchnic-HF. Eur Heart J. 2018;39(48):4255–4256. doi: 10.1093/eurheartj/ehy682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fudim M., Boortz-Marx R.L., Ganesh A., et al. Splanchnic nerve block for chronic heart failure. J Am Coll Cardiol HF. 2020;8(9):742–752. doi: 10.1016/j.jchf.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Malek F., Gajewski P., Zymlinski R., et al. Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: first-in-human clinical trial. Eur J Heart Fail. 2021;23(7):1134–1143. doi: 10.1002/ejhf.2209. [DOI] [PubMed] [Google Scholar]
- 5.Shah S., Zirakashvili T., Shaburishvili N., et al. Durability of right greater splanchnic nerve ablation for the treatment of heart failure with preserved ejection fraction in an open-label first-in-human clinical trial (abstract) Heart Rhythm. 2021;18(8) [Google Scholar]