Abstract
The purpose of this study was to assess the effectiveness of echinacea for the prevention of experimental rhinovirus colds. Infection occurred in 44 and 57% and illness occurred in 36 and 43% of the echinacea- and placebo-treated subjects, respectively. This preparation of echinacea had no significant effect on either the occurrence of infection or the severity of illness.
Since the Dietary Supplement Health and Education Act was enacted by Congress in 1994 to modify the Food and Drug Administration regulation of dietary supplements, there has been a dramatic increase in the marketing and sale of these products. Echinacea is one of the most popular herbal remedies and is reported to have immunomodulatory activity that is beneficial for the treatment of common cold symptoms. Clinical studies on the effect of echinacea on the common cold have used a variety of different echinacea preparations and study designs (8, 13, 16). Different preparations of echinacea differ in chemical composition due variation in the plant species or the part of the plant that is used as the starting material or because of differences in the manufacturing process or dosage form (1, 3). The purpose of this study was to use the experimental cold model to evaluate the effectiveness of an echinacea preparation with a defined chemical profile for the prevention of rhinovirus colds.
Subjects ≥18 years of age were recruited for these studies from the university community of the Medical University of South Carolina. Volunteers with a serum titer of neutralizing antibody to the study virus of ≤1:4 were treated with echinacea (300 mg) or a placebo three times each day for 14 days prior to virus challenge. All volunteers were challenged with 100 to 300 50% tissue culture-infective doses of rhinovirus type 23, and then the treatment was continued for 5 days after the virus challenge. Virus infection was documented by virus cultures and antibody responses, and illness severity was assessed by a modification of a previously published method (12, 14). The echinacea preparation used in this study was analyzed by reversed-phase high-pressure liquid chromatography by Rudolf Bauer (Institut für Pharmazeutische Biologie, Düsseldorf, Germany) using published methods (2–6) and found to contain 0.16% cichoric acid with almost no echinacosides or alkamides. Written informed consent was obtained prior to study participation, and subjects were compensated for participation.
One hundred seventeen subjects were enrolled in the trial and treated with either echinacea (63 subjects) or a placebo (54 subjects) for 2 weeks. Sixteen subjects (eight active, eight placebo) had a respiratory illness on day 0 and were not challenged with rhinovirus, four subjects in each group withdrew from the study prior to challenge, and one subject treated with echinacea was removed from the study due to an adverse event. The remaining 92 subjects were challenged with rhinovirus type 23 and continued on study medication as outpatients for an additional 5 days. On day 0 prior to virus challenge, 30 (60%) of the 50 echinacea-treated subjects and 19 (45%) of the 42 placebo-treated subjects believed that they were receiving the active treatment (P = 0.21, Fisher exact test).
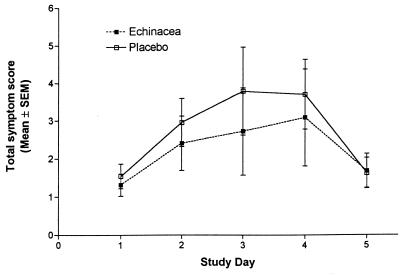
Rhinovirus infection occurred in 22 (44%) of the 50 echinacea-treated and in 24 (57%) of the 42 placebo-treated volunteers (P = 0.3, Fisher exact test). Clinical colds developed in 11 (50%) of 22 echinacea-treated and 14 (59%) of 24 placebo-treated subjects with documented rhinovirus infection (P = 0.77, Fisher exact test). The power of this study was approximately 75% to detect a reduction in the incidence of colds from 59 to 20%. The lack of effect of echinacea on the incidence of symptoms was associated with a lack of effect on the severity of symptoms. There was no significant effect of echinacea on the total daily symptom score in the virus-infected subjects (Fig. 1). The mean total symptom score over the course of the cold was 13.6 (95% confidence interval [CI], 7.5 to 19.7) in the placebo group and 11.4 (95% CI, 3.9 to 18.9) in the treated group. Similarly, the mean total rhinorrhea score over the course of the illness was 2.1 (95% CI, 1.0 to 3.1) in infected subjects who received the placebo, compared to 1.59 (95% CI, 0.4 to 2.8) in the echinacea-treated subjects. The mean nasal obstruction scores were 3.7 (95% CI, 2.3 to 5.0) and 2.8 (95% CI, 1.5 to 4.1) in the two groups, respectively. No significant side effects of echinacea were seen in this relatively small number of volunteers.
FIG. 1.
Comparison of total symptom score by day after rhinovirus challenge in volunteers treated with either echinacea (900 mg/day) or a placebo.
The genus Echinacea consists of nine species, three of which, Echinacea angustifolia, E. pallida, and E. purpurea, are used medicinally. Several substances are found in Echinacea species that could potentially produce a beneficial effect on common cold symptoms either by direct inhibition of virus replication or by modulation (i.e., enhancement or suppression) of the host immune response. (7, 9, 10, 15, 18–21). Variations in the Echinacea species or plant parts used and manufacturing processes may affect the final chemical composition of the product composition and may thus affect biologic activity. In spite of the considerable variation in echinacea preparations, they have generally been used interchangeably in clinical studies.
The efficacy of echinacea for the prevention of viral respiratory disease has been evaluated in several clinical trials (11, 16, 17). Melchart et al. (17) treated 302 volunteers with either a fluid extract of E. purpurea or a placebo for 12 weeks. In a similar study, Grimm and Muller (11) treated 109 volunteers with an ethanolic extract of E. purpurea or E. augustafolia root or a placebo for 8 weeks. Neither of these studies found a significant effect of prophylaxis on either the occurrence or the severity of common colds. The detection of common cold illnesses in these studies may have been affected by reliance on passive reporting of illnesses by the volunteers and by the 4-week interval for scheduled contacts between the investigators and the volunteers. Most importantly, the phytochemical profile of the echinacea preparations used in these studies was not determined.
Recent changes in the regulation of dietary supplements have dramatically increased both the availability and the marketing of these products. Evaluation of efficacy is hampered by the fact that many of these products lack appropriate quality control and cannot be standardized because the active component(s) is not known. Future clinical studies of echinacea and similar products should focus on the evaluation of well-characterized preparations in well-controlled studies with clearly defined endpoints.
Acknowledgments
Genny Connelly was the study coordinator.
This work was supported by a grant from the Procter & Gamble Company, Cincinnati, Ohio.
REFERENCES
- 1.Bauer R. Chemistry, analysis and immunological investigations of Echinacea phytopharmaceuticals. In: Wagner H, editor. Immunomodulatory agents from plants. Basel, Switzerland: Birkhauser Verlag; 1999. pp. 41–88. [Google Scholar]
- 2.Bauer R. Standardisierung von Echinacea purpurea-Preßsaft auf Cichoriensäure und Alkamide. Z Phytother. 1997;18:270–276. [Google Scholar]
- 3.Bauer R, Khan I A, Wagner H. TLC and HPLC analysis of Echinacea pallida and Echinacea augustifolia roots. Planta Med. 1988;54:426–430. doi: 10.1055/s-2006-962489. [DOI] [PubMed] [Google Scholar]
- 4.Bauer R, Remiger P. Der Einsatz der HPLC bei der Standardisierung von Echinacea-Drogen. Arch Pharm (Weinheim) 1989;322:324. [Google Scholar]
- 5.Bauer R, Remiger P. TLC and HPLC analysis of alkymides in Echinacea drugs. Planta Med. 1989;55:367–371. doi: 10.1055/s-2006-962030. [DOI] [PubMed] [Google Scholar]
- 6.Bauer R, Remiger P, Wagner H. Vergleichende DC- und HPLC-Analyse der Herba-Drogen von Echinacea purpurea, E. pallida und E. angustifolia. Dtsch Apoth Ztg. 1988;128:174–180. [Google Scholar]
- 7.Bodinet C, Buescher N. Antiviral and immunological activity of glycoproteins from Echinaea purpurea radix. Planta Med. 1991;57(Suppl. 2):A33–A34. [Google Scholar]
- 8.Brinkeborn R M, Shah D V, Degenring F H. Echinaforce and other Echinacea fresh plant preparations in the treatment of the common cold. A randomized, placebo controlled, double-blind clinical trial. Phytomedicine. 1999;6:1–5. doi: 10.1016/S0944-7113(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 9.Burger R A, Torres A R, Warren R P, Caldwell V D, Hughes B G. Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol. 1997;19:371–379. doi: 10.1016/s0192-0561(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 10.Cheminat A, Zawatzky R, Becker H, Brouillard R. Caffeoyl conjugates from Echinacea species: structure and biological activity. Phytochemistry. 1988;27:2787–2794. [Google Scholar]
- 11.Grimm W, Muller H H. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138–143. doi: 10.1016/s0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 12.Hendley J O, Edmonson W P, Jr, Gwaltney J M., Jr Relation between naturally acquired immunity and infectivity of two rhinoviruses in volunteers. J Infect Dis. 1972;125:243–248. doi: 10.1093/infdis/125.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schafer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res. 1997;9:261–268. [Google Scholar]
- 14.Jackson G G, Dowling H F, Spiesman I G, Boand A V. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. Arch Intern Med. 1958;101:267–278. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- 15.McDougall B, King P J, Wu B W, Hostomsky Z, Reinecke M G, Robinson W E., Jr Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob Agents Chemother. 1998;42:140–146. doi: 10.1128/aac.42.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea—a systematic review of controlled clinical trials. Phytomedicine. 1994;1:245–254. doi: 10.1016/S0944-7113(11)80072-3. [DOI] [PubMed] [Google Scholar]
- 17.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 18.Neamati N, Hong H, Sunder S, Milne G W, Pommier Y. Potent inhibitors of human immunodeficiency virus type 1 integrase: identification of a novel four-point pharmacophore and tetracyclines as novel inhibitors. Mol Pharmacol. 1997;52:1041–1055. doi: 10.1124/mol.52.6.1041. [DOI] [PubMed] [Google Scholar]
- 19.Robinson W E, Jr, Reinecke M G, Abdel-Malek S, Jia Q, Chow S A. Inhibitors of HIV-1 replication that inhibit HIV integrase. Proc Natl Acad Sci USA. 1996;93:6326–6331. doi: 10.1073/pnas.93.13.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See D M, Broumand N, Sahl L, Tilles J G. In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology. 1997;35:229–235. doi: 10.1016/s0162-3109(96)00125-7. [DOI] [PubMed] [Google Scholar]
- 21.Stimpel M, Proksch A, Wagner H, Lohmann-Matthes M-L. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun. 1984;46:845–849. doi: 10.1128/iai.46.3.845-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]