Fluid retention is a leading pathophysiological and clinical cause of acute heart failure (AHF). Therefore, diuretic agents facilitating sodium and water excretion are the first-line drugs that are administered. Inadequate response to diuretic agents is a major clinical challenge that, when it occurs, requires combining different classes of diuretic agents or ultrafiltration. However, the benefits of these strategies are unknown and might result in worsening renal function. Thus, an intervention that effectively targets volume overload and diuretic resistance (while preserving the renal function) is required, which could potentially modify the prognosis of patients with AHF.1,2
Revamp Medical has developed the Doraya catheter, for use as a temporary intravenous flow regulator that is percutaneously deployed in the inferior vena cava (IVC) below the level of the renal veins (Figure 1A). Furthermore, the distal frame opening is adjusted to create a partial obstruction of the venous flow, resulting in reduced cardiac preload and venous congestion. This triggers a cascade of hemodynamic effects, beginning with the unloading of the renal venous system. Venous congestion is considered a strong determinant of worsening renal function caused by exertion of increased venous pressure (VP) on renal veins and kidneys, thereby causing hypoxia of the renal parenchyma and lowering of the renal perfusion pressure. The second effect is the unloading of the right ventricle, which causes a reduction cardiac preload.
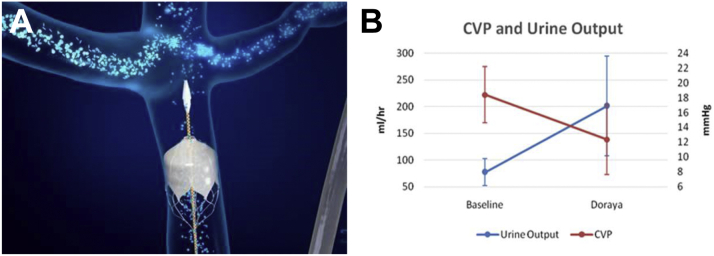
Figure 1.
The Location of the Catheter in the Inferior Vena Cava and its Clinical Effects
(A) The location of the catheter in the inferior vena cava and (B) mean central venous pressure (CVP) and urine output values improvement following catheter placement.
The Doraya catheter has been recently investigated to ascertain the decrease in renal VP in patients with AHF (First in Human Study of the Doraya Catheter for the Treatment of AHF Patients; NCT03234647). The study inclusion criteria comprised patients with fluid overload symptoms, insufficient diuretic response (defined as >80 mg furosemide/day resulting in urine output <0.75 mL/kg/h per 40 mg furosemide after 18-24 hours of diuretic agents initiation), N-terminal pro–B-type natriuretic peptide ≥1,600 pg/ mL, and central venous pressure (CVP) ≥12 mm Hg. The catheter device was deployed for up to 12 hours.3
The study followed the current guidelines of the European Society of Cardiology. Each patient served as their own control and underwent at least 24 hours of standard care and assessment prior to catheter deployment. Patients were monitored for adverse events (AEs), particularly for catheter thrombosis, misplacement or migration, edema of lower extremities, and other potential complications. The monitoring of the hemodynamic parameters during the procedure was performed by Swan-Ganz catheter. All patients were followed up for 60 days.
The primary safety outcome was the rate and nature of device- and procedure-related AEs until 1 month following the procedure. The primary feasibility end point was technical success, defined as the ability to position the catheter below the renal veins, to regulate the flow in the IVC using the catheter by creating a gradient pressure of at least 2 mm Hg, and to withdraw the catheter safely. Additional observational measures included the rate of urine output, changes of serum creatinine, and natriuresis.
A total of 9 patients were treated with the catheter (7 male and 2 female patients, mean age 69 ± 9 years, mean left ventricle ejection fraction 24 ± 12%). The technical aspects of catheter use such as its placement, deployment, and removal were successfully achieved, and there were no incidents of device malfunctions. The mean intervention duration was 8.5 hours (range 7–11.5 hours). No device-related AE or embolic event was observed during the procedure nor within 30 days of follow-up; however, 1 procedure-related severe AE was reported (ie, bleeding hematoma from the Swan-Ganz catheter insertion site), which was resolved without sequelae.
The catheter created a transient and fully controllable gradient in the IVC. The mean IVC pressures obtained above and below the catheter were 18.4 ± 3.8 mm Hg and 17.8 ± 4.0 mm Hg, respectively, at baseline. The catheter deployment resulted in significant pressure reduction above the device: 12.4 ± 4.7 mm Hg, when compared to unchanged pressure below the catheter: 18.5 ± 6.2 mm Hg at the end of procedure (P < 0.01; by Student’s t-test for paired samples). Additionally, the systolic blood pressures were stable during the study at baseline, minimum during procedure, and at end of procedure and were recorded as 110 ± 13, 100 ± 13, and 109 ± 17 mm Hg, respectively. Heart rates were not recorded during the procedures.
He rate of diuresis was measured as 77.1 ± 25 mL/h at baseline, and 200.8 ± 93 mL/h during device deployment, while maintaining the same diuretic dose. Furthermore, the average peak urine output rate during deployment was 294 ± 139 mL/h. Figure 1B presents the correlation between increased urine output and CVP reduction during the intervention.
A positive natriuretic signal also existed during the procedure; however, the spot urine sodium was measured only in a limited number of patients (n = 3) and the mean increased from 35 to 101 mmol/L. The clinical signs of congestion, namely edema and dyspnea, respectively, improved from baseline (1.8 ± 0.8 and −1.4 ± 1.1)to postprocedural period (0.7 ± 0.9 and 1.1 ± 0.9). Dyspnea was measured by 7-point Likert scale and edema by pitting edema score.
To conclude, the first-in-human study shows the feasibility and safety of the catheter for the modification of the IVC pressure in patients with AHF, in conjunction with diuretic therapy. The catheter led to a controllable drop of IVC pressure with a mean reduction of 6 mm Hg, translated into a pressure decrease in renal veins and resulted in a better diuretic response. Although the study was not designed to investigate the clinical efficacy of the catheter, early positive signs suggest a potential for future development, and it may be speculated that new interventions directed toward CVP may be beneficial in patients suffering from inadequate diuretic response.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2022-shark-tank
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Mullens W., Abrahams Z., Francis G.S., et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felker G.Michael, Ellison D.H., Mullens W., Cox Z.L., Testani J.M. Diuretic therapy for patients with heart failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(10):1178–1195. doi: 10.1016/j.jacc.2019.12.059. [DOI] [PubMed] [Google Scholar]
- 3.Dierckx R., Vanderheyden M., Heggermont M., Goethals S., Verstreken J., Bartunek J. Treatment of diuretic resistance with a novel percutaneous blood flow regulator: concept and initial experience. J Card Fail. 2019;25(11):932–934. doi: 10.1016/j.cardfail.2019.08.017. [DOI] [PubMed] [Google Scholar]