Heart failure (HF) affects 6.2 million Americans and contributes to approximately 300,000 deaths per year.1 There are more than 1 million hospital discharges for HF annually and, although many efforts have been made to reduce these hospitalizations, the number has remained relatively constant during the past decade.1
The standard of care for managing HF is still largely based on clinician assessment of symptoms and physical examination. However, these criteria are focused on identifying manifestations of clinical congestion and are not sensitive or specific for identifying asymptomatic hemodynamic congestion, which is characterized by elevation of intracardiac filling pressures (eg, pulmonary capillary wedge pressure [PCWP]). To date, there is only 1 device approved by the US Food and Drug Administration for outpatient hemodynamic monitoring in patients with HF. This implantable pulmonary artery pressure sensor is implanted in the pulmonary artery via a transcatheter procedure, and it has successfully demonstrated a reduction in hospitalizations for HF. However, owing to reimbursement challenges associated with the nearly $20,000 price tag and patient reluctance to undergo invasive implantation of a permanent device, it is estimated that <2% of patients hospitalized with HF have received the device since approval in 2014.2 This leaves the overwhelming majority of patients hospitalized with HF in need of an alternative solution.
Cardiosense, Inc is developing such a solution: a noninvasive cardiac monitoring platform that analyzes seismocardiogram, electrocardiogram, and photoplethysmogram signals, captured by the CardioTag, a wearable device, to noninvasively estimate hemodynamic parameters, with a primary focus on estimating changes in PCWP. Specifically, Cardiosense applies advanced signal processing and machine learning techniques to features extracted from these waveforms to develop algorithms to noninvasively estimate valuable hemodynamic parameters including changes in PCWP, cardiac output, contractility, and blood pressure. Furthermore, the Cardiosense Monitoring Platform was granted a U.S. Food and Drug Administration Breakthrough Device Designation to noninvasively measure PCWP for patients diagnosed with New York Heart Association functional class III or IV HF with reduced ejection fraction (≤40%) in professional health care facilities or at home.
Relevant studies involving our platform’s noninvasive hemodynamic capabilities and correlations to gold standard parameters are summarized in Table 1. All studies referenced in Table 1 were conducted under protocols reviewed and approved by the Institutional Review Boards of University of California-San Francisco; Georgia Institute of Technology; Northwestern University; and/or Stanford University. All study subjects provided written consent prior to participation.
Table 1.
Summary of Relevant Clinical Studies
| Parameter | First Author, Journal (Year) | Reference | Sample Size | Correlation |
|---|---|---|---|---|
| ΔPCWP | Shandhi et al,3IEEE Trans Biomed Eng (2022) | Right heart catheterization | 20 Patients with HF who were undergoing vasodilator challenge | R2 = 95% |
| Oxygen uptake | Shandhi et al,4J Card Fail (2020) | Cardiopulmonary exercise test | 59 Patients with HF | R2 = 76% |
| Mean arterial pressure | Carek et al, under review (2020) | Radial arterial line | 15 Intensive care unit patients | R2 = 88% |
| Stroke volume | Semiz et al,5IEEE J Biomed Health Inform (2021) | Transesophageal Doppler | 12 Perioperative patients | R = 81% |
| ΔCardiac output | Inan et al,6Physiol Meas (2009) | Doppler echocardiography | 9 Volunteers | R2 = 85% |
| ΔContractility | Ashouri et al,7Sensors (2016) | Impedance cardiography | 17 Volunteers | R2 = 92% |
HF = heart failure; PCWP = pulmonary capillary wedge pressure.
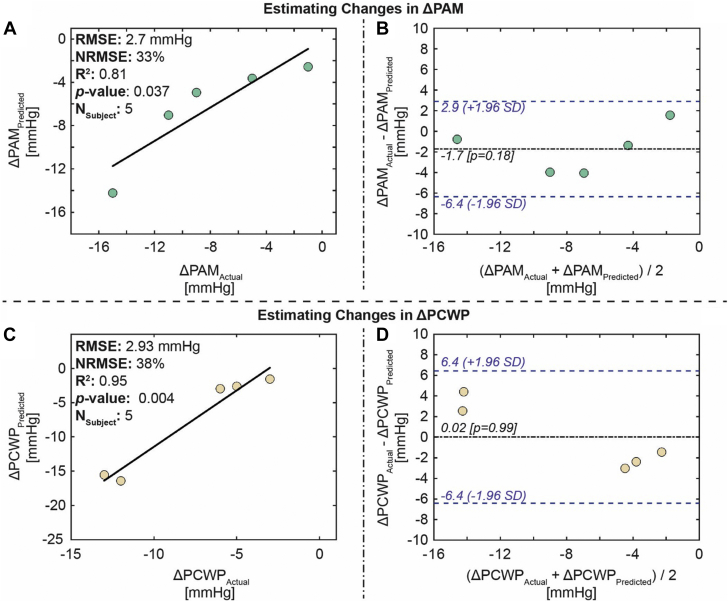
The first study listed in Table 1 provides proof-of-concept evidence that Cardiosense can use the CardioTag’s capabilities combined with our expertise in signal processing and machine learning to develop accurate algorithms to estimate changes in PCWP. For the proof-of-concept study, data were collected from 20 patients with HF (20% women; median age 55 years) at the University of California-San Francisco during their right heart catheterization procedures.3 Each patient underwent a right heart catheterization and vasodilator challenge while wearing an earlier iteration of the CardioTag. The signals obtained by the CardioTag simultaneously during the right heart catheterization and vasodilator challenge were used to estimate the changes in PCWP. A population regression model was developed to estimate changes in PCWP using the changes in the CardioTag’s signals. The model estimated changes in PCWP with reasonable accuracy for the validation set (root-mean-square error = 2.9 mm Hg; R2 = 0.95), indicating that changes in the CardioTag’s signals can be used to track changes in PCWP in patients with HF (Figure 1C).3
Figure 1.
Estimation Results for the Validation Set
(A) Correlation analysis for ΔPAM predicted versus ΔPAM actual, (B) Bland-Altman analysis for ΔPAM predicted and ΔPAM actual, (C) correlation analysis for ΔPCWP predicted versus ΔPCWP actual, and (D) Bland-Altman analysis for ΔPCWP predicted and ΔPCWP actual. In the Bland-Altman plots, the black line indicates the mean, and the blue dashed lines indicate mean ± 1.96. © 2022 IEEE. Reprinted, with permission, from Shandhi et al.3 NRMSE = normalized root-mean-square error; PAM = pulmonary artery mean pressure; PCWP = pulmonary capillary wedge pressure; RMSE = root-mean-square error.
The Cardiosense Monitoring Platform, comprising the CardioTag and machine learning techniques, has the potential to provide a noninvasive alternative to implantable sensors and extend the benefits of hemodynamic-guided therapy to both a broader HF population (eg, patients classified as New York Heart Association functional class II and IV, patients classified as New York Heart Association functional class III who were not hospitalized in the last 12 months) as well as increase use in patients who are already known to benefit from a hemodynamically guided HF management strategy.
Footnotes
Dr Neill has an equity stake in Cardiosense, Inc; and is an employee of Cardiosense. Dr Etemadi is a cofounder, board member, and advisor for Cardiosense, Inc; and has an equity stake in Cardiosense. Dr Inan is a scientific advisor for Physiowave, Inc; a cofounder, board member, and scientific advisor for Cardiosense, Inc; has an equity stake in Cardiosense; is a cofounder, board member, and scientific advisor for Arthroba, Inc; has an equity stake in Arthroba, Inc; has received research funding for his lab from Hillrom, Inc and Cisco, Inc; and has intellectual property licensed by Physiowave, TandemLaunch, and Cardiosense. Dr Klein has an equity stake in Cardiosense, Inc; and is a clinical advisor for Cardiosense.
Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2022-shark-tank.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Singh R., Varjabedian L., Kaspar G., Zughaib M. CardioMEMS in a busy cardiology practice: less than optimal implementation of a valuable tool to reduce heart failure readmissions. Cardiol Res Pract. 2018;2018:4918757. doi: 10.1155/2018/4918757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shandhi M.M.H., Fan J., Heller J., Etemadi M., Klein L., Inan O. Estimation of changes in intracardiac hemodynamics using wearable seismocardiography and machine learning in patients with heart failure: a feasibility study. IEEE Trans Biomed Eng. Published online January 31, 2022 doi: 10.1109/TBME.2022.3147066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shandhi M.M.H., Hersek S., Fan J., et al. Wearable patch-based estimation of oxygen uptake and assessment of clinical status during cardiopulmonary exercise testing in patients with heart failure. J Card Fail. 2020;26(11):948–958. doi: 10.1016/j.cardfail.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semiz B., Carek A.M., Johnson J.C., et al. Non-invasive wearable patch utilizing seismocardiography for peri-operative use in surgical patients. IEEE J Biomed Health Inform. 2021;25(5):1572–1582. doi: 10.1109/JBHI.2020.3032938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inan O.T., Etemadi M., Paloma A., Giovangrandi L., Kovacs G.T. Non-invasive cardiac output trending during exercise recovery on a bathroom-scale-based ballistocardiograph. Physiol Meas. 2009;30(3):261–274. doi: 10.1088/0967-3334/30/3/003. [DOI] [PubMed] [Google Scholar]
- 7.Ashouri H., Orlandic L., Inan O.T. Unobtrusive estimation of cardiac contractility and stroke volume changes using ballistocardiogram measurements on a high bandwidth force plate. Sensors (Basel) 2016;16(6):787. doi: 10.3390/s16060787. [DOI] [PMC free article] [PubMed] [Google Scholar]