Corresponding Author

Key Words: E3 ligase, mitophagy, TLR9, TRAF2
Mitochondria are the powerhouses of cardiomyocytes, but they are easily damaged by oxidative stress, and, once they are damaged, they become a major source of reactive oxygen species and promote programmed cell death, including apoptosis.
Mitophagy is the most prominent mechanism by which damaged or unnecessary mitochondria are removed in cardiomyocytes. Because of their continuous use of oxidative phosphorylation and the consequent production of reactive oxygen species as byproducts, cardiomyocytes are equipped with stringent mitochondrial quality control mechanisms, including mitophagy. A comparison of mitophagic activity among multiple organs in a mitophagy-indicator mouse showed that the heart and cardiomyocytes have high mitophagic activities even under baseline conditions. Previous studies have shown that cardiomyocytes activate mitophagy in response to pathological stimuli, including ischemia, pressure overload, and high fat diet consumption, through either conventional or unconventional mechanisms.1 However, the molecular mechanism through which mitophagy is mediated in the heart at baseline and its functional significance remain obscure. PINK1-Parkin−mediated mitophagy is the most well-characterized form of mitophagy in many organs and cell types. Stabilization of PINK1 in depolarized mitochondria induces mitochondrial translocation of Parkin, an E3 ligase. Damaged mitochondria are marked with K63-linked polyubiquitination for engulfment by autophagosomes and lysosomal degradation. Mice genetically deficient in PINK1 and Parkin exhibit a normal cardiac phenotype at young ages, and mitophagy appears to be maintained at baseline. Thus, how mitophagy at baseline is maintained in the heart has been unclear.
In this issue of JACC: Basic to Translational Science, using conditional cardiac-specific TRAF2 knock-out (KO) mice, Ma et al2 showed that TNF-receptor−associated factor 2 (TRAF2), a mitochondrially localized E3 ubiquitin ligase, mediates mitophagy, and therefore plays a protective role in the adult heart at baseline. Previous studies showed that TRAF2 mediates cytoprotective TNF receptor signaling in the heart during stress, and, that TRAF2, in concert with Parkin, mediates mitophagy in cardiomyocytes in response to stress.3 By extending these findings, Ma et al2 showed that the loss of TRAF2 function attenuated mitophagy at baseline in the adult heart, accompanied by accumulation of mitochondrial proteins and mitochondrial dysfunction. Furthermore, the loss of TRAF2 function led to stimulation of TLR9 and macrophage infiltration, apoptosis, and cardiac dysfunction, which suggested that endogenous TRAF2 protects the heart against TLR9-induced sterile infection by facilitating safe disposal of mitochondrial DNA through activation of mitophagy, even without stress.2
The study by Ma et al2 is highly significant for multiple reasons. To my knowledge, this is the first report that clearly shows the molecular mechanism that mediates mitophagy, namely, the involvement of TRAF2 in the adult heart at baseline. The study showed that mitophagy at baseline not only maintains mitochondrial function but also protects the heart against sterile infection caused by TLR9 activation, which is caused by undigested mitochondrial DNA. Because TRAF2 is involved in innate immunity by transducing TNF receptor signaling, TRAF2 also mediates an additional cell-protective mechanism by preventing TLR9 activation through activation of mitophagy.
Although the investigators showed convincing evidence to support the role of TRAF2 in mediating physiological mitophagy, several questions remain. First, TRAF2 mediates multiple cardioprotective cellular mechanisms. For example, TRAF2 inhibits necroptosis and apoptosis in the heart. Although the investigators showed that conditional down-regulation of TRAF2 in the adult heart did not induce necroptosis or systemic inflammation, it was unclear why these mechanisms were unaffected in the adult TRAF2 KO heart. Because TRAF2 can polyubiquitinate multiple targets at K63, it would have been useful to conduct the specific rescue of mitochondrial E3 ligase targets in TRAF2 KO mice.
Although the investigators showed that the rescue of mitophagy through Parkin overexpression improved the function of TRAF2-deficient murine embryonic fibroblast cells, ideally, it would have been good to test whether the rescue of mitophagy could normalize cardiac function in TRAF2 KO mice in vivo.
Second, the investigators previously showed that TRAF2 and Parkin, both of which are E3 ligases, have well-coordinated roles in mediating mitophagy during stress. However, Parkin does not compensate for the loss of TRAF2 function in the adult heart at baseline. Because Parkin KO mice show no obvious cardiac phenotype at baseline in the adult heart, it is possible that TRAF2 may compensate for the loss of Parkin. Parkin promotes proteasomal degradation of TRAF2, and down-regulation of Parkin extends the half-life of TRAF2 in 293 and SH-SY5Y cells.4 Ma et al2 showed that the level of Parkin was not altered in TRAF2 KO mice. Although overexpression of Parkin can rescue the loss of mitophagy in TRAF2-deficient murine embryonic fibroblast cells, the insufficient upregulation of Parkin in the presence of TRAF2 down-regulation appears to explain the distinct cardiac phenotype in TRAF2 and Parkin KO mice. Further investigation is needed to test this hypothesis.
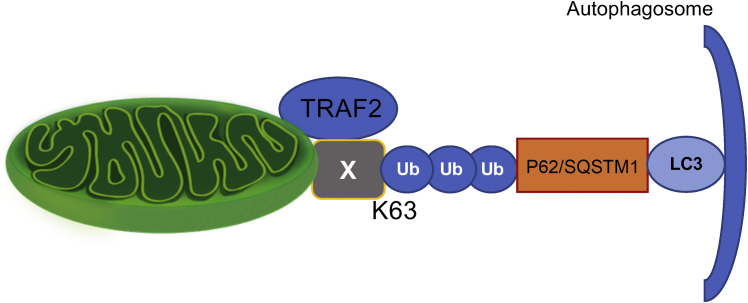
Finally, the molecular mechanism through which TRAF2 mediates mitophagy at baseline requires further investigation. The investigators suggested that TRAF2-induced mitophagy at baseline was PINK1-independent. This raises a question as to how damaged mitochondria are selectively eliminated by mitophagy. Although mitophagy is often triggered by mitochondrial depolarization, it can be activated even in polarized mitochondria. For example, activation of the mitochondrial unfolded protein response can induce PINK1 accumulation.5 Because TRAF2-induced mitophagy at baseline appears to be PINK1-independent, further investigation is required to determine how physiological mitophagy is executed. A previous study showed that TRAF2 K63 polyubiquitinates Nur77, thus promoting its interaction with p62, an LC3 adapter protein, and mitophagy (Figure 1). TRAF2 also interacts with RIPK1, thereby mediating NF-κB activation in 293 cells. We have shown recently that RIPK1 plays an essential role in mediating alternative mitophagy, which does not rely on the conventional mechanism of mitophagy in cardiomyocytes.1 It would be interesting to investigate whether K63 polyubiquitination of RIPK1 mediates TRAF2-induced mitophagy.
Figure 1.
Baseline (Physiologic) Mitophagy
TNF-receptor−associated factor 2 (TRAF2), an E3 ligase localized at mitochondria, K63-polyubiquitinates proteins (X), including Nur77, on the outer mitochondrial membrane. How TRAF2 is activated and which proteins are polyubiquitinated in cardiomyocytes are unknown. This process appears to be independent of PINK1, a mitochondrial kinase stabilized by mitochondrial depolarization and involved in Parkin-mediated mitophagy. How mitochondria are selected for degradation is unknown. Once mitochondrial outer membrane proteins are K63 polyubiquitinated, tagged mitochondria are recognized by LC3 adapters, including p62/SQSTM1, sequestrated by autophagosomes, and degraded through lysosomes. Ma et al2 showed that physiologic mitophagy is important in preventing TLR9 activation and consequent sterile infection and for maintaining mitochondrial quality.
In summary, the study by Ma et al2 clearly shows the importance of TRAF2 in mediating physiological mitophagy in the heart and the cardiomyocytes therein. Increasing lines of evidence suggest that preserving mitochondrial quality is the key to maintaining cardiac function. For example, mitophagy is downregulated during aging, and aging is an important risk factor for incident heart failure.1 Thus, a better understanding of the molecular mechanisms involved in maintaining mitochondrial quality in the heart at baseline is essential, and pharmacological modulation of TRAF2 is certainly an attractive approach to addressing this issue. However, caution should be exercised because the function of TRAF2 is dose-dependent and cell type-specific.
Funding Support and Author Disclosure
This work was supported in part by U.S. Public Health Service Grants (HL67724, HL91469, HL102738, HL112330, HL138720, HL144626, HL150881, and AG23039), the American Heart Association Merit Award (20 Merit 35120374), and the Fondation Leducq Transatlantic Network of Excellence (15CVD04). Dr Sadoshima has reported that he has no relationships relevant to the content of this paper to disclose.
Acknowledgment
The author thanks Daniela Zablocki for critical reading of the manuscript.
Footnotes
Michael Bristow, MD, PhD, served as Guest Editor in Chief for this paper.
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Saito T., Hamano K., Sadoshima J. Molecular mechanisms and clinical implications of multiple forms of mitophagy in the heart. Cardiovasc Res. 2020;17:cvaa340. doi: 10.1093/cvr/cvaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma X., Rawnsley D.R., Kovacs A., et al. TRAF2, an innate immune sensor, reciprocally regulates mitophagy and inflammation to maintain cardiac myocyte homeostasis. J Am Coll Cardiol Basic Trans Science. 2022;7:223–243. doi: 10.1016/j.jacbts.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang K.C., Ma X., Liu H., et al. Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ Heart Fail. 2015;8:175–187. doi: 10.1161/CIRCHEARTFAILURE.114.001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung J.Y., Park H.R., Lee S.J., et al. Elevated TRAF2/6 expression in Parkinson's disease is caused by the loss of Parkin E3 ligase activity. Lab Invest. 2013;93:663–676. doi: 10.1038/labinvest.2013.60. [DOI] [PubMed] [Google Scholar]
- 5.Jin S.M., Youle R.J. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy. 2013;9:1750–1757. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]