Abstract
Prevalence of anemia in India is almost 40% with no significant change since 1998–99, whereas globally this prevalence has been reduced to < 15%. This could be because our national nutritional programs (mainly National Nutritional Anemia Control Program-NNACP) focus on supplementation with iron and folate but not with vitamin B12. Some Indian studies, including our study (2012), indicated high prevalence of B12 deficiency in North Indian urban population. Hence, we conducted a retrospective analysis of 3 years’ data (2012–2014 including 48,317 subjects) and compared it with last year’s retrospective data (April 2019–March 2020 including 4775 subjects) to ascertain prevalence of deficiencies of these micronutrient with special reference to patients of anemia, and improvement therein over the subsequent 5-year period. Our results indicate that amongst our subjects with anemia, iron deficiency has reduced from 66.73% (2012–2014) to 56.86% (2019), but prevalence of vitamin B12 deficiency is still the same (36.54% in 2012–2014; 37.04% in 2019). Folate deficiency was similar in both sets of data (2.95% in 2012–2014 and 2.55% in 2019). Thus, NNACP has reduced prevalence of iron deficiency by ~ 10%points and folate deficiency marginally; B12 deficiency has not been addressed. It would, therefore, follow that we need to add to our current national programs to effectively deal with these deficiencies. Food fortification (with iron, folate and B12) seems the most likely means to add value to the existing programs. In addition, food diversification needs to be included in regular school curriculum to bring about community awareness and change in food habits.
Keywords: Micronutrient deficiencies, India, Food fortification, Food diversification, Iron and B12
Introduction
In addition to the increasing global burden of degenerative diseases, India also has a continued burden of infectious diseases and nutritional deficiencies. During the course of our studies on homocysteine as a risk factor for vascular (cardiovascular, cerebrovascular and peripheral vascular) disease we observed that, in our population, one of the major causes of homocysteinemia (high circulating homocysteine) was low vitamin B12 levels [1]. This was found to be prevalent in a very large number of our subjects- even those who came only for a health check-up, those with and without vascular disease, and those with and without homocysteinemia.
World Health Organisation (WHO) data on Global Burden of Disease showed that the prevalence of anaemia in children and women of India (and most of South-east Asia) in 1998–99 was > 40%, which was also the case in most of the countries in the African and South American continents. The remaining countries of Eurasia and Africa had a prevalence of 20–39.9%. It was further ascertained that this high prevalence of anaemia in India resulted in age-standardised disability adjusted life years (DALY) of 445 per 100,000. The Indian government introduced several supplementation plans including the National Nutritional Anaemia Control Program (NNACP) after this WHO report. Yet in 2004, the reported prevalence of anaemia in India was still 40%. Kotecha reported that the status of this prevalence has not changed from the 1970s to 2009—it remained as high as 79% in children below the age of 3 years and 40% in the population as a whole. The 2015–2016 National Family Health Survey reported a prevalence of deficiency anaemia of 59% in children below 3 years, 53% in women and 23% in men. In 2018, global data showed that prevalence of anaemia was still the highest in India at 39.86%, whereas it was < 15% in the rest of the world, indicating adequate corrective measures globally, except in India. Thus, despite successive nationwide programmes, the current burden of anaemia in our country still touches 40% and hence continues to be a severe public health problem. Therefore, anaemia in India has been classified as a persistent severe public health problem, which would require aggressive measures for amelioration [2–8].
The national programs implemented have not taken into account the prevalent deficiency of vitamin B12. Currently the national anaemia control programs that have been implemented, focus on supplementation of iron and folate, but do not include vitamin B12, even though deficiency of all three nutrients (iron, folate and B12) are implicated in causation of anaemia.
This prompted us to look into micronutrient status of North Indian urban population through a retrospective data analysis of subjects presenting in the out-patients’ department at a 675-bedded tertiary care hospital. Since, this hospital caters to all socioeconomic strata of the North Indian general population, it was felt that retrospective data extraction and analysis would provide a fairly representative prevalence.
Materials and Methods
The healthcare set-up in India allows individuals to present to the out-patients’ department of any hospital for a health check-up which includes a complete physical examination and a varied permutation-combination of imaging and laboratory investigations (fasting samples being drawn for the latter).
A 3-year (2012–2014) retrospective analysis of data on vitamin B12, folate and iron included 53,375 adult (adults > 18 years old) subjects who came for assessment of different types of routine health check-ups (which may or may not include vitamin estimations, but routinely do not include iron estimation unless an anaemia profile is included) and/or anaemia profile. These were sifted to exclude subjects with serum levels of vitamin B12 > 1500 pg/ml, folate > 24 ng/dl, or iron > 500 µg/dl (these are the upper limits of the analytical range of the assays used for serum estimations of B12, folate and iron). Since all the 53,375 subjects had serum levels of vitamin B12 estimated, this data was sifted first to delete those with B12 > 1500 pg/ml. 4841 had B12 > 1500 pg/ml, so the remaining 48,534 subjects were sifted to exclude subjects with serum folate > 24 ng/dl, which was observed in 217 subjects. No further segregation of data was required as serum iron > 500 µg/dl was not observed in any of the subjects. Hence, of the 53,375 subjects initially extracted, data of 48,317 subjects was finally included.
Of these, 36,167 came for a health check-up which included vitamin B12 estimation; 3597 of these had a package that included analysis of serum folate. The remaining 12,150 individuals came for an anaemia profile which includes estimation of iron, B12 and folate. The haemoglobin levels of those who came for anaemia profile were used to identify those with anaemia as per WHO definition (< 13.0 gm/dL for males and < 12.0 gm/dL for females) [9].
Thus, for statistical analyses, the data points included are as shown in Fig. 1.
Fig. 1.
This shows sifting of the extracted data with the final number of data points for each analyte
Vitamin B12 and Folate were estimated by chemiluminescent immunoassay. Ferrozine timed end-point immunoturbidimetric assay was used for quantifying serum iron. All estimations were done on fully automated platforms.
To assess for any change in prevalence of deficiency of these micronutrients in the North Indian urban population, one-year’s retrospective data (April 2019–March 2020) of subjects with anaemia was extracted and segregated as was the previous set of data with the same inclusion and exclusion criteria. The total number of data points for all three nutrients was 4775. It was then subject to statistical analysis to look for current prevalence of deficiencies of these nutritional markers of anaemia—iron, folate and vitamin B12 in patients with anaemia. The data was also analysed for correlation of haemoglobin with these micronutrients in subjects with anaemia.
Statistical Analysis
The data was analysed sex-wise (male and female), age-wise (< 18, 18–40, 41–60 and > 60 years) and analyte-wise by SPSS version 20 (Chicago, Ill). Parametric data are expressed as Mean ± standard error of mean (mean ± SEM). Confidence intervals are given with continuity correction. Classification of subjects includes identification of those with iron deficiency (serum iron < 40 µg/dl in females and < 50 µg/dl in males), folate deficiency (serum folate < 3 ng/ml), and vitamin B12 deficiency (serum B12 < 220 pg/ml).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the institutional ethics committee under the specific no. EC/05/19/1525.
Results
The number of subjects (Fig. 1) selected on basis of the inclusion/exclusion criteria was 48,317. Amongst the 12,150 subjects whose anaemia profile was estimated, 7562 were identified as anaemic as per WHO criteria on basis of haemoglobin (Hb < 12 gm/dl for females and < 13 gm/dl for males) [9]. The remaining 36,167 subjects came for a routine health check-up. Of these, 3,597 had both B12 and folate analysed while the remaining 32,570 had only B12 estimation done on their blood. The data of those who came for a general health check-up and anaemia profile was analysed separately from that of those who came for anaemia profile and were observed to be anaemic.
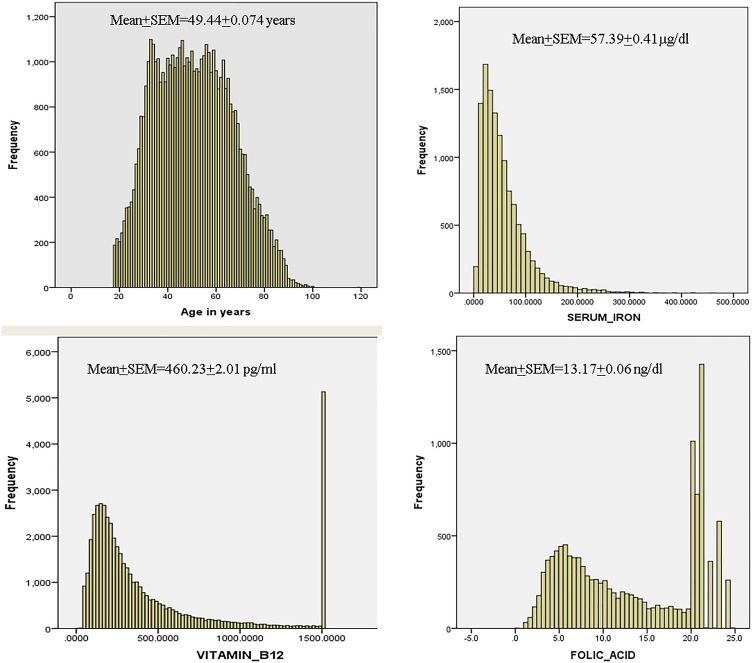
The composite data is represented in Fig. 2, which gives the histograms for all the subjects/analytes. The histogram for age shows a near normal distribution indicating that all ages are well represented. The preponderance of results for serum iron and folate are near the lower limit of the biological reference interval (BRI), and those for vitamin B12 are near the mean of the BRI. This is quantitatively represented in Table 1.
Fig. 2.
The histogram of age is a near normal curve, indicating that the data is representative of all ages (> 18 years). The histogram of iron indicates serum iron levels mostly in the very low/low normal range. Serum concentrations of B12 and folate are also in crowded in the low/low normal range with spikes in the high range (at 1500 pg/ml for B12 and in the range 20–24 ng/dl for folate), indicating a probable intake of vitamin B supplements
Table 1.
Data with sex-wise (mean ± SEM) of iron, vitamin B12, and folate (95% confidence interval includes continuity correction)
| Sex | Iron (µg/dL) | Vitamin B12 (pg/ml) | Folate (ng/dl) | |
|---|---|---|---|---|
| Total | N | 12,150 | 48,317 | 15,747 |
| Mean ± SEM | 57.40 ± 0.41 | 460.23 ± 2.01 | 13.17 ± 0.06 | |
| Range | 2–421 | 5–1500 | 0.2–24 | |
| Confidence interval (95%) | 56.586–58.204 | 456.289–464.173 | 13.047–13.293 | |
| Female | N | 6320 | 25,074 | 7838 |
| Mean ± SEM | 52.05 ± 0.53 | 446 ± 6.67 | 13.1 ± 0.21 | |
| Range | 4–323 | 5–1500 | 0.2–24 | |
| Male | N | 5830 | 23,243 | 7909 |
| Mean ± SEM | 63.22 ± 0.64 | 475 ± 6.88 | 13.2 ± 0.20 | |
| Range | 2–421 | 17–1500 | 1–24 | |
| p (female vs male) | ns | 0.002 | ns |
p < 0.05 significant
p < 0.005 highly significant
The mean serum vitamin B12 is significantly different in males and females but this difference is not significant in mean serum iron or folate levels
In the histograms for folate and vitamin B12 levels, there are several peaks in the 20–24 ng/ml range in the folate histogram and at the 1500 pg/ml level in the B12 histogram, indicating a possible high prevalence of folate and B12 supplementation.
Table 1 gives the sex-wise and Table 2 gives the age-wise serum concentrations (mean and SEM) of iron, folate, and vitamin B12. The mean vitamin B12 is significantly less in females as compared to males, whereas the iron and folate levels do not differ significantly in the two sexes.
Table 2.
Data with age-wise mean ± SEM of iron, vitamin B12, and folate
| Age group | Iron In µg/ml (Mean ± SEM) |
Vitamin B12 In pg/ml (Mean ± SEM) |
Folate In ng/ml (Mean ± SEM) |
|---|---|---|---|
| 18–40 years (a) |
60.438 ± 0.71 (n = 3253) |
329 ± 8.07 (n = 16,162) |
11.0 ± 0.25 (n = 5517) |
| 41–60 years (b) |
59.25 ± 0.70 (n = 4248) |
434 ± 7.43 (n = 19,152) |
13.2 ± 0.19 (n = 5682) |
| > 60 years (c) |
53.49 ± 0.66 (n = 4649) |
662 ± 8.99 (n = 13,003) |
14.9 ± 0.1 (n = 4548) |
| p value of a vs b | 0.386 | 0.26 | 0.012 |
| p value of b vs c | 0.098 | 1.0 | 0.001 |
| p value of a vs c | 0.001 | 1.0 | < 0.0001 |
p < 0.05 significant
p < 0.005 highly significant
Though the vitamin B12 showed an increasing trend with age, there was no significant difference between the age groups. Folate too showed an increasing trend and the inter-group variation was highly significant. However, serum folate levels were in the normal range in all three age groups. Serum iron showed a decreasing trend with age and the difference between the 18–40 years age group and the > 60 years age group was highly significant
Iron shows a decreasing trend with increase in age and the difference between the 18–40 years age group and the > 60 years age group was highly significant. Vitamin B12, despite showing an increasing mean concentration with age, is not significantly different in the three age groups, viz. 18–40, 41–60 and > 60 years. Folate is well within the BRI in all age groups, it shows an increasing trend with age, and the values are significantly different in all three age groups as compared to each other. Mean serum iron shows a decreasing trend with age but it is not statistical significant.
Table 3 shows the sex-wise prevalence of anaemia in the subjects whose anaemia profile was done. The overall prevalence of anaemia in these subjects was 62.24%, being 58.07% in males and 66.6% in females.
Table 3.
Sex-wise prevalence of anemia in subjects whose anemia profile was estimated
| No. of subjects | Total | Males | Females |
|---|---|---|---|
| Total | 12,150 | 6218 | 5932 |
| Subjects with anemia | 7562 | 3611 | 3951 |
| % age of subjects with anemia | 62.24 | 58.07 | 66.6 |
Table 4 describes the sex-wise prevalence of deficiency of iron, B12 and folate in those who had anaemia and those who came for a health check-up.
Table 4.
Prevalence of deficiency of iron, vitamin B12, and folate (95% confidence interval includes continuity correction)
| Deficiency of | Definition | Total no. of subjects | No. of subjects deficient | Prevalence of deficiency (%) | 95% CI |
|---|---|---|---|---|---|
| Iron only |
Serum iron ≤ 40 µg/dl in females ≤ 50 µg/dl in males |
7562 (An) F 3951 M 3611 |
5046 F 2534 M 2512 |
66.73 F 50.22 M 49.78 |
65.75–67.7% F 49.31–52.03% M 48.24–52.08% |
| B12 only |
Serum B12 ≤ 220 pg/ml |
7562 (An) F 3951 M 3611 |
2763 F 1523 M 1230 |
36.54 F 38.55 M 34.06 |
35.54–37.54% F 37.19–39.91% M 32.59–35.54% |
| Folate only |
Serum folate < 3 ng/ml |
7562 (An) F 3951 M 3611 |
223 F 94 M 129 |
2.95 F 2.38 M 3.57 |
2.59–3.29% F 1.99–2.86% 3.03–4.20% |
| Iron + B12 | As given above | 7562 (An) | 1428 | 18.88 | 18.08–19.71% |
| Iron + Folate | As given above | 7562 (An) | 134 | 1.77 | 1.51–2.07% |
|
Vitamin B12 deficiency |
Serum B12 ≤ 220 pg/ml |
36,167 (HCU) F 18,766 M 17,401 |
15,946 F 7695 M 8251 |
44.09 41.0 47.42 |
43.58–44.6% F 40.31–41.72% 46.68–48.17% |
| Vitamin B12 < population mean |
Serum B12 ≤ 460 pg/ml |
36,167 (HCU) F 18,766 M 17,401 |
27,169 F 14,049 M 13,120 |
75.12 74.86 75.4 |
74.67–75.56% F 74.23–75.48% M 74.75–76.04% |
| Folate |
Serum folate < 3 ng/ml |
3597 (HCU) F 1490 M 2107 |
80 F 25 M 55 |
2.2 F 1.67 M 2.61 |
1.29–3.11% F 1.05–2.29% M 1.92–3.3% |
An anemia, HCU health checkup
In Table 4, amongst anaemic subjects, prevalence of deficiency of serum iron was the highest (66.73%) followed by prevalence of deficiency of B12 (36.53%), combined deficiency of iron and B12 (18.88%), deficiency of folate (2.95%) and combined deficiency of iron and folate (1.77%). Amongst those who came for a routine health check-up, vitamin B12 deficiency was exhibited by 44.09% of the population and 75.12% showed B12 levels below the population mean.
Also, serum folate levels, though well within the normal range, showed a low mean—5.08 ng/ml (close to the lower limit of the BRI of 3–20 ng/ml).
Partial correlation of the data from the subjects of anaemia revealed that after correcting for age, there was a significant negative correlation of haemoglobin (Hb) with serum levels of iron (p = 0.013) and vitamin B12 (p = 0.019) but not with serum levels of folate (p = 0.084), indicating that serum concentrations of iron and vitamin B12 impacted Hb levels but serum folate did not in these subjects.
The data of April 2019 to March 2020 shows mean circulating iron, vitamin B12 and folate as similar to (though marginally lower than) the corresponding values 5 years ago (Table 5). The prevalence of iron, vitamin B12 and folate is 56.86, 37.04 and 2.55%, respectively (Table 6).
Table 5.
Mean circulating concentrations of iron B12 and folate in 4775 patients with anemia (Data from April 2019–March 2020)
| Descriptive | Iron | Vitamin B12 | Folate |
|---|---|---|---|
| Mean ± SEM | 53.682 ± 0.615 μg/dL | 440.455 ± 0.01 pg/ml | 12.653 ± 0.013 ng/dL |
| 95% confidence Interval | 53.67 to 53.70 μg/dL | 429.05 to 451.86 pg/ml | 12.48 to 12.83 |
Table 6.
Prevalence of deficiency of iron, vitamin B12, and folate in 4775 subjects with anemia (95% confidence interval includes continuity correction; Data from April 2019–March 2020)
| Deficiency of | Definition | No. deficient (N = 4775) | Percentage deficient in data of April 2019–March 2020 | Percentage deficient in previous data Jan 2011–Dec 2013 |
|---|---|---|---|---|
| Iron |
Serum iron ≤ 40 µg/dl in females ≤ 50 µg/dl in males |
2715 |
56.86% [CI 55.44–58.27%] |
66.73% |
| B12 |
Serum B12 ≤ 220 pg/ml |
1769 |
37.04% [CI 35.68–38.44%] |
36.54% |
| Folate |
Serum folate < 3 ng/ml |
123 |
2.55% [CI 2.16–3.08%] |
2.95% |
Discussion
The causes of anaemia can be classified into 4 groups: (a) due to nutritional deficiencies, including that due to inadequate and late dietary additions to breast milk, (b) due to parasitic infestations e.g. hookworm and ascaris, (c) chronic bacterial infections like tuberculosis, and (d) prevalence of rare conditions like thalassemia minor/major, sickle cell anaemia, irritable bowel syndrome, etc [4].
Of these, the parasitic infestations, chronic bacterial infections and rare conditions are best dealt with individually and do not lend themselves to generalised measures for reduction. Nutritional deficiencies, on the other hand, can be addressed collectively on a large scale, and, towards this, the Government of India has implemented the National Nutritional Anaemia Control Program (NNACP). This program aims to reduce the prevalence and incidence of anaemia in women of reproductive age through three main strategies—(a) enabling adequate intake of iron by provision of foods rich in the nutrient in school health programs (e.g. anganwadis), (b) providing supplements (tablets containing iron and folate) to the population at high risk, and (c) identifying and treating cases of severe anaemia [5]. In addition to NNACP, in the last two decades, the Indian Government collaborated with several international programmes like Micronutrient Initiative (MI), Global Alliance for Improved Nutrition (GAIN), Flour Fortification Initiative (FFI), but these have resulted in a few state-specific nutritional programmes rather than nationwide programmes [4].
Despite these successive programmes, the current burden of anaemia in our country is still touching 40% [6]. Moreover, the programs implemented have not taken into account the prevalent deficiency of vitamin B12 and supplements used in these programs do not include this micronutrient.
Our data suggests that the leading cause of anaemia amongst our 7562 subjects with anaemia still is iron deficiency which was found in 66.73%. However, in addition, deficiency of vitamin B12 was also present in 36.53% of these subjects. Folate deficiency was not so common, being present in only 2.92% of these subjects. The combined deficiency of iron and B12 was present in 18.88% and that of iron and folate was present in 1.77% of these subjects. We also observed that the mean serum iron was significantly (p = 0.031) less in females as compared to males, indicating a need to accelerate the NNACP.
Prevalence of anaemia in the Indian population has been reported by many scientists, but the number of subjects included in each study have been relatively small. Also, only few have ascertained the cause. Though iron, folate and vitamin B12 are well established causes of anaemia, to the best of our knowledge, there are scanty reports on their inclusion as the cause of anaemia in studies on Indian population amongst whom, usually, anaemia is attributed to iron deficiency.
Garcia et al. reported a prevalence of vitamin B12 deficiency in 20–30% of infants, children, adolescents and pregnant women in Venezuela in 2005 whereas Pathak et al. reported a prevalence of 74.1% of the same deficiency in pregnant women of Haryana State, India, in 2007. Ahmed et al. in 2008, reported a prevalence of this deficiency in adolescent anaemic schoolgirls in rural Bangladesh as just 7%. At the same time, Bhardwaj et al. reported very low mean vitamin B12 levels (34.7 ± 11.5 and 33.5 ± 11.0 pg/mL in adolescent males and females, respectively) but normal mean folate levels in the same category of subjects in Himachal Pradesh, observing that 100% of the 100 anaemic subjects tested had normal folate levels but very low vitamin B12 levels [10–13]. The prevalence thus reported have been varied and in a small subset of the population.
Analysis of the vitamin B12 and folate status in our data revealed a prevalence of B12 deficiency in 44.1% of the 36,167 healthy subjects, with a comparatively negligible prevalence of folate deficiency in the 7562 subjects with anaemia (2.39–5.97%) and the 3597subjects who came for a health check-up(1.29–3.11%) as shown in Table 4. The mean serum folate levels were similar in males and females whereas the mean serum levels of Vitamin B12 were significantly (p = 0.002) lower in females as compared to males.
This would indicate a higher prevalence of deficiency of iron and vitamin B12 in Indians as compared to other populations, without a concomitant deficiency in folate.
To explain the spikes in the histograms of folate and vitamin B12, we did a small prospective study to ascertain the prevalence of vitamin B supplement intake amongst those who came for health checkups. The percentage of subjects who were having supplements was 11.2% (112/1000), similar to the percentage of our subjects who had B12 ≥ 1500 pg/ml or folate ≥ 24 ng/ml which was 13.64% (7282/53,375). Hence the spikes in the histograms could be attributed to B vitamin supplement intake by the subjects.
Looking at the subsequent data, it seems that from 2014 to 2019 (5 years) there is only a marginal improvement in the prevalence of iron deficiency associated with anaemia, whereas prevalence of B12 and folate deficiency remain essentially the same as before.
The overall prevalence of vitamin B12 deficiency in India has been reported as ≈75% though our data reveals only 44.1% deficient adults [14]. As aptly pointed out by Antony in his editorial in American Journal of Clinical Nutrition (AJCN), dietary factors alone cannot account for this prevalence. He further states that no one single factor can be responsible as the Indian population is very heterogenous in social, religious, cultural, socioeconomic, and dietary considerations [15]. Moreover, the prevalence of malnutrition and tropical sprue are relatively common in India, with gastrointestinal infections and other nutrient deficiencies resulting in a malabsorptive state as well [16, 17].
Hence, some additional plans are required to address these deficiencies in the Indian population as a whole.
To be able to suggest a plan for mitigation of these deficiencies, it would be necessary to understand their implications and a few details of the absorption of these micronutrients.
Iron is essential for many varied functions and physiological reactions, e.g. cellular growth and differentiation, haemoglobin synthesis, intracellular as well as extracellular oxygen transport, enzymatic reactions, immune functions, cognitive functions, etc. its deficiency, therefore, leads to several pathological states, the primary one being anaemia. Iron deficiency is diagnosed when the serum iron levels are < 50 µg/dL in males and < 40 µg/dL in females [8, 18].
It would be pertinent here to mention that the mainstay of iron absorption from the duodenum and upper jejunum depends on competitive uptake through divalent metal transporters which are shared by iron, zinc and lead [19]. Iron in the ferric form (trivalent) is not absorbed and, therefore, needs to be reduced to the divalent form before absorption can occur. Since the transporter is shared, iron absorption can be hindered by excessive dietary content of zinc and lead [20]. In a study done in our laboratory (communicated for publication) on 200 patients with anaemia, high serum zinc along with low serum iron levels was observed in 61% of the subjects. Though a normal diet may not contain an excess of zinc, yet currently there is a heightened knowledge amongst the general public about vitamin and micronutrient supplementations which are available over the counter, and, therefore, are frequently consumed in excess. Also, these often contain zinc in concentrations above the prescribed upper limit of daily intake [21, 22]. As for lead, dietary content depends on the source of water and use of colouring agents in edible and nonedible commodities. Recently, there has been an increased awareness and demonstration of high prevalence of lead toxicity in our population, with one study observing a prevalence of 44% in the paediatric age group [23].
The clinical implication of vitamin B12 deficiency starts in utero and continues through infancy, childhood and adulthood. It is imperative for neural tissue development, especially myelination, and for red cell maturation. Its deficiency has been associated with impaired cognitive and motor functions in infancy, anaemia and delayed cognitive development in childhood, and anaemia and peripheral neuropathy in adulthood [24].
It is well established that a large proportion of our daily requirement of cobalamin is derived from the synthesis of vitamin B12 by our intestinal microbes. The remaining is provided by non-vegetarian diet like meat, meat products, fish, shell-fish, and eggs. The only vegetarian foods that contains this vitamin are milk, dairy products and fortified cereals (in countries where fortification is done).
The absorption of vitamin B12 is complex and occurs in several stages through the alimentary canal, the actual absorption occurring in the distal ileum through receptors on the mucosal surface which bind to the Intrinsic Factor-B12 complex. It involves haptocorrin (R protein or transcobalamin I) from the saliva, intrinsic factor (IF) from the stomach, pancreatic enzymes in the proximal ileum and receptors in the distal ileum. In addition, 1–5% of the free cobalamin from diet is absorbed along the entire intestines by passive diffusion. Typically, the liver stores 1–1.5 mg of the vitamin which is the equivalent of 2000–3000 days’ daily requirement, and hence deficiency does not manifest for a long time, sometimes as long as 5–10 years [25, 26].
Hence, deficiency of this vitamin (due to dietary insufficiency or defects in absorption) does not become evident for almost 5–10 years.
Like vitamin B12, folate, also known as folic acid or vitamin B9, is a water-soluble vitamin of the B group. Again like vitamin B12, clinical implication of its deficiency is evidenced throughout development—intra-uterine as well as childhood. From neural tube defects to autism, folate deficiency has been implicated in a myriad of conditions including vascular disease and even progeria. Its deficiency is defined as a serum folate concentration < 3.0 µg/dL [27].
It is interesting to note that folate is available in common dietary substances like green leafy vegetables, mushrooms and fruits; also, that its absorption is mainly by passive diffusion through a non-saturable high-affinity proton-coupled folate transporter (PCFT) [28].
Since our population show signs of only minimal folate deficiency, it would seem that the NNACP has been partially successful; however, iron deficiency persists. This latter may be attributed to altered absorption of this micronutrient and the prevalence of heterogenous social, religious, cultural, socioeconomic, and dietary considerations in our population—the possible contributors to vitamin B12 deficiency also, as mentioned above. So our approach may have to be modified now.
For any of the prevalent deficiencies—of iron and vitamin B12—the causative factors are very varied and would be difficult to address individually. Yet, since these impact public health negatively and severely, they need to be addressed by a multipronged approach. Supplements have been used, as in NNACP, but with limited success. We suggest that while the national programme NNACP should be continued and, in fact, be accelerated, it is possible that the public health may be benefitted by food fortification with iron, and vitamins B12 [29].
It would be pertinent to mention here that the first requirement for food supplementation and fortification was identified in India in 1931 by Dr. Lucy Wills [30]. As a result, food fortification with thiamine, riboflavin, niacin and iron was started in USA in the early 1940s; later folate was included and even later vitamin B12 was added to the fortification. In fact, WHO implemented iron and folate supplementation for pregnant women in the developing countries based on Dr.Will’s findings. This supplementation was actually implemented in India, too, through the NNACP which took it beyond the pregnant woman to all women of reproductive age. Yet food fortification was not implemented.
It is possible that iron does not lend itself to uncomplicated fortification in foods, for in subjects who are not deficient, excess of iron in the diet may lead to an overload of iron, though this is very rare [31]. The WHO guidelines on food fortification say “in the long-term, measures for the prevention and control of micronutrient deficiencies should be based on diet diversification and consumer education about how to choose foods that provide a balanced diet, including the necessary vitamins and minerals” [32].
It is the opinion of the authors that food fortification with iron and vitamin B12 is the dire need of the day to effectively address the continued prevalence of these deficiencies. Diet diversification and consumer education should also be implemented simultaneously, so that in the long run, the population learns to acquire appropriate nutrition from their choice of foods. However, as mentioned in the WHO report, achieving a change in dietary habits is notoriously difficult.
Supplements or Food Fortification?
Supplements, as are used in the NNACP, have several disadvantages as opposed to food fortification, as detailed below [33].
Fortification uses micronutrients in such a manner as to give the individual an equivalent of the recommended daily requirement; supplements usually contain higher concentrations of the micronutrient.
Supplements reach a limited population in terms of those included in the program, whereas fortification reaches all strata of society and has a widespread benefit.
Fortification is not dependent on individual compliance whereas supplements can be.
As a consequence, fortification is more likely to maintain the body stores as well as breast milk stores of the fortified micronutrients, giving advantage to the postpartum women as well as the infant.
Multiple micronutrient deficiencies often co-exist. Fortification with multiple micronutrients is desirable, whereas supplementing the same can become daunting.
Fortification—with a single micronutrient or multiple ones—is cost-effective; the same is not true of supplements. Moreover, the diversity of available combinations of supplements and the fact that they are available over the counter makes it very difficult for the consumer to choose the appropriate one.
The possible disadvantages of food fortification are:
Though fortified foods contain an adequate concentration of multiple micronutrients, they cannot replace a wholesome diet.
They may not be consumed by all members of the population and, hence, may not benefit the population as a whole. This is especially so for infants and young children, who consume small amounts and, hence, may not get the required daily dose of the micronutrients.
Fortified foods are more commonly available in the urban areas. Therefore, they are less likely to be consumed by the rural people who are more likely to consume home-grown or locally produced foods.
Though here we are alluding to fortification with iron, folate (to maintain body stores), and vitamin B12, some of the micronutrients interactions are not completely understood though some are known; for e.g. calcium may inhibit iron absorption, but vitamin C would enhance it; on the other hand, fortification with vitamin D is ideal in the presence of a rich store of calcium, thus making simultaneous fortification with iron and vitamin D undesirable.
Having enunciated the basic considerations given in the WHO guidelines for food fortification, it remains to be ascertained which methodology would best benefit our population. Again, as detailed in this WHO document, in the underdeveloped countries where poverty is also a public concern, food fortification programs must go hand-in-hand with other poverty-reducing programs. Our population, hence, would also benefit by a similar approach.
Our first step should be to ascertain the prevalence of all micronutrient deficiencies in our population. Then, for a start, on basis of the prevalence defined by this study and several others, fortification with iron and vitamin B12 would be mandatory. Vitamin C may be added to improve absorption of iron (since our population has a high prevalence of gastrointestinal infections and malabsorptive states). Folate may be added to maintain body stores. The medium for fortification with iron, folate and vitamin B12 would ideally be food grains (rice and wheat flour) which are the staple diet throughout the country. It would, then, be imperative that these fortified foods become available to all socioeconomic strata at no extra cost, or maybe even at a government-subsidised rate for the lower socioeconomic strata who barely get one square meal a day.
Conclusion
In our population, there is a high prevalence of micronutrient deficiencies, namely iron and B12. The most plausible first step towards mitigating these deficiencies would be through fortification of food grains (rice and wheat flour) with iron, B12 and folate (to maintain current status of adequacy of this vitamin in our population), with continuation of the NNACP for women in the reproductive age group. Simultaneously, the science of diet diversification may be included in education curricula (school as well as graduate and post-graduate courses) for added benefit towards mitigation of these micronutrient deficiencies.
Also, there needs to be some assessment of the efficacy of these corrective measures by way of population studies on prevalence of micronutrient deficiencies, probably, every five years.
Acknowledgements
The authors would like to thank Ms.Jyoti Punjabi, Ms. Naushba Parveen, Mr.Virender Singh, Mr. Deepak Tuteja and Ms.Vinita Rawat for their contribution in extraction, compilation and tabulation of data. The authors also thank Dr.Satish Saluja for his advice in statistical analysis.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhargava S, Ali A, Bhargava EK, Manocha A, Kankra M, Das S, et al. Lowering homocysteine and modifying nutritional status with folic acid and vitamin B12 in Indian patients of vascular disease. J Clin Biochem Nutr. 2012;50(3):222–226. doi: 10.3164/jcbn.11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005. WHO Global Database on Anaemia; 2008. pp. 21–31.
- 3.Kumar A. National nutritional anaemia control program in India. Indian J Public Health. 1999;43(1):3–5. [PubMed] [Google Scholar]
- 4.Upadhyay RP, Palanivel C, Kulkarni V. Unrelenting burden of anaemia in India: Highlighting possible prevention strategies. Int J Med Public Health. 2012;2(4):1–6. doi: 10.5530/ijmedph.2.4.1. [DOI] [Google Scholar]
- 5.Toteja GS, Singh P. Policy and Program implications in Micronutrient profile of Indian population. Published by Indian Council of Medical Research; 2004.
- 6.Global Data. 2018 https://www.globaldata.com/india-highest-prevalence-anaemia-among-16-major-pharma-markets-finds-globaldata/. Accessed Sep 2019.
- 7.International Institute for Population Sciences and ICF. National Family Health Survey (NFHS-4), 2015–16: India; 2017 Mumbai: IIPS.
- 8.Kotecha PV. Nutritional anaemia in young children with focus on Asia and India. Indian J Community Med. 2011;36(1):8–16. doi: 10.4103/0970-0218.80786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO, UNICEF, UNU. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. Geneva, World Health Organization; 2001. Available from http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html
- 10.Pathak P, Kapil U, Yajnik CS, Kapoor SK, Dwivedi SN, Singh R. Iron, folate and vitamin B12 stores among pregnant women in a rural area of Haryana state, India. Food Nutr Bull. 2007;28(4):435–438. doi: 10.1177/156482650702800409. [DOI] [PubMed] [Google Scholar]
- 11.García-Casal MN, Osorio C, Landaeta M, Leets I, Matus P, Fazzino F, et al. High prevalence of folic acid and vitamin B12 deficiencies in infants, children, adolescents and pregnant women in Venezuela. Eur J Clin Nutr. 2005;59(9):1064–1070. doi: 10.1038/sj.ejcn.1602212. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed F, Khan MR, Banu CP, Qazi MR, Akhtaruzzaman M. The coexistence of other micronutrient deficiencies in anaemic adolescent schoolgirls in rural Bangladesh. Eur J Clin Nutr. 2008;62(3):365–372. doi: 10.1038/sj.ejcn.1602697. [DOI] [PubMed] [Google Scholar]
- 13.Bhardwaj A, Kumar D, Raina SK, Bansal P, Bhushan S, Chander V. Rapid assessment of coexistence of vitamin B12 and iron deficiency anaemia among adolescent males and females in a Northern Himalayan state of India. Anaemia. 2013 doi: 10.1155/2013/959605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Refsum M, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–241. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 15.Antony AC. Prevalence of cobalamin (vitamin B12) and folate deficiency in India—audi alteram partem (Editorial) Am J Clin Nutr. 2001;74:157–159. doi: 10.1093/ajcn/74.2.157. [DOI] [PubMed] [Google Scholar]
- 16.Balaji LN, Dustagheer A. Nutrition scenario in India—implications for clinical practice. J Indian Med Assoc. 2000;98(536–8):542. [PubMed] [Google Scholar]
- 17.Mathan VI. Tropical sprue in Southern India. Trans R Soc Trop Med Hyg. 1988;82:10–14. doi: 10.1016/0035-9203(88)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari R, Bharti RK, Singh K, Sinha A, Kumar S, Saran A, et al. Prevalence of iron deficiency and iron deficiency anaemia in adolescent girls in a tertiary care hospital. J Clin Diagn Res. 2017;11(8):04–06. doi: 10.7860/JCDR/2017/26163.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir A, Hopfer U. Regional specificity of iron uptake by small intestinal brush-boarder membranes from normal and iron deficient mice. Am J Physiol. 1985;248:G376–G379. doi: 10.1152/ajpgi.1985.248.3.G376. [DOI] [PubMed] [Google Scholar]
- 20.WHO/CDC (2004). Expert consultation agrees on best indicators to assess iron deficiency, a major cause of anaemia. http://www.who.int/mediacentre/news/notes/2004/anaemia/en. Accessed Dec 2020.
- 21.Chugh PK, Lamo Y. An assessment of vitamin supplements in the Indian market. Indian J Pharm Sci. 2012;74(5):469–473. doi: 10.4103/0250-474X.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the national health and nutrition examination survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhary S, Firdaus U, Ali SM, Mahdi AA. Factors Associated with elevated blood lead levels in children. Indian Pediatr. 2018;55:38–40. doi: 10.1007/s13312-018-1225-4. [DOI] [PubMed] [Google Scholar]
- 24.Black MM. Nutrition and brain development. In: Walker WA, Duggan C, Watkins JB, editors. Nutrition in pediatrics. New York: BC Decker; 2003. pp. 386–396. [Google Scholar]
- 25.Andrès E, Loukili NH, Esther Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. Can Med Assoc J. 2004;171(3):251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viola-Villegas N, Rabideau AE, Bartholomä M, Zubieta J, Doyle RP. Targeting the cubilin receptor through the vitamin B(12) uptake pathway: cytotoxicity and mechanistic insight through fluorescent Re(I) delivery. J Med Chem. 2009;52(16):5253–5261. doi: 10.1021/jm900777v. [DOI] [PubMed] [Google Scholar]
- 27.Dali-Youcef N, Andres E. An update on cobalamin deficiency in adults. QJ Med. 2009;102:17–28. doi: 10.1093/qjmed/hcn138. [DOI] [PubMed] [Google Scholar]
- 28.Bhargava S, Tyagi SC. Nutriepigenetic regulation by folate-homocysteine-methionine axis: a review. Mol Cellular Biochem. 2014;387(1–2):55–61. doi: 10.1007/s11010-013-1869-2. [DOI] [PubMed] [Google Scholar]
- 29.Shenkin A, Roberts NB. Vitamins and trace elements. In: Burtis A, Bruns S, editors. Tietz textbook of clinical chemistry and molecular diagnostics. Philadelphia: Elsevier; 2006. pp. 895–983. [Google Scholar]
- 30.Wills L. Treatment of “pernicious anaemia of pregnancy” and “tropical anaemia” with special reference to yeast extract as a curative agent. Br Med J. 1931;1:1059–1064. doi: 10.1136/bmj.1.3676.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins JM. Universal iron fortification of foods: the view of a haematologist. Rev Bras Hematol Hemoter. 2012;34(6):459–463. doi: 10.5581/1516-8484.20120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Report of Joint FAO/WHO Expert Consultation on vitamin and mineral requirement in human nutrition: Bangkok 1998. Second Edition FAO Rome, 2004. http://whqlibdoc.who.int/publications/2004/9241546123.pdf. Accessed Dec 2019.
- 33.World Health Organisation Team Nutrition. The role of food fortification in the control of micronutrient malnutrition. In ‘Guidelines on food fortification with micronutrients’ 2004. https://www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf. Accessed Jan 2020.