Abstract
Chronic obstructive pulmonary disease (COPD), a heterogeneous lung disorder that is characterized by airflow obstruction and the third leading cause of death, globally. COPD is influenced by environmental and genetic factors. Here, we measured the serum level of matrix metalloproteinase-9 (MMP-9), cyclooxygenase-2 (COX-2) and prostaglandin E-2 (PGE-2) and reveal the correlation between their levels in COPD subjects. In this study, we included a total of 79 COPD and 79 healthy controls. We assessed demographic profile, risk factors, respiratory symptoms, clinical history, COPD Assessment Test (CAT) score and spirometry. Further, we determined the serum levels of MMP-9, COX-2 and PGE-2 by enzyme-linked immunosorbent assay (ELISA). The correlation between their serum levels was also determined. Among the studied population age, gender, body mass index and socioeconomic status were comparable. Serum levels of MMP-9, COX-2 and PGE-2 were significantly increased in the COPD group than in healthy controls (P < 0.0001). Moreover, MMP-9, COX-2 and PGE-2 levels were increased with the GOLD grades and CAT score (> 10). Serum levels of MMP-9, COX-2 and PGE-2 was enhanced in patients with larger clinical history (> 20 years) than those with lower clinical history (< 10 years). Serum levels of MMP-9 and COX-2; MMP-9 and PGE-2; COX-2 and PGE-2 showed a positive correlation (P < 0.0001) with the COPD group. Our data demonstrate that serum levels of MMP-9, COX-2 and PGE-2 were correlated with the GOLD grade, CAT score and clinical history of the COPD group, pointing that they can be used as a indicators to understand the disease progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12291-021-00973-2.
Keywords: Biomarkers, Correlation, Enzyme linked immunosorbent assay, Expression, Clinical history
Introduction
Chronic obstructive pulmonary disease (COPD) is an alarming global public health problem. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report 2021, COPD is the top third leading cause of death, worldwide [1]. Approximately 90% of deaths occur in low and middle-income countries [1]. In 2012, 3 million-plus people died of COPD, estimating 6% of all deaths worldwide [1]. COPD, a heterogeneous lung disorder is characterized by airflow obstruction which is not fully reversible. Inflammation as well as structural changes in small airways and lung parenchyma are considered to be the main factors leading to airflow limitation in COPD [2]. Major risk factor of COPD is smoking which is a dominant cause of COPD. COPD is influenced by not only genetic but also environmental factors [3].
Matrix metalloproteinases (MMP) are a class of proteases that are known to regulate extracellular matrix renewal during COPD lung tissue remodeling [2]. MMP-9 is also called gelatinase B, with a molecular weight of 85 kDa secreted by bronchial epithelial cells, neutrophils, eosinophils, mast cells and alveolar macrophages [4]. Albiet, numerous MMPs have been found to play a role in the lung pathology, MMP-9 is the predominant protease in alveolar tissue and because of its easy detection and quantification, it has attracted the attention in MMPs. Inflammation modulates the protease/antiprotease balance leading to progressive airway destruction as well as remodeling. During the inflammatory events of COPD, alveolar type II cells, bronchial epithelial cells, clara cells, endothelial cells, fibroblasts and smooth muscle cells produce MMP-9 and leucocytes in the lung [5, 6]. MMP-9 degrades elastin and promotes further lung damage that is secreted by alveolar macrophages and neutrophils, which can promote inflammation in COPD [6]. Overall, these mechanisms support the role of MMP-9 as a key mediator in COPD.
Cyclooxygenase (COX) is the rate-limiting enzyme that arachidonic acid forms prostaglandin and thromboxane [7, 8]. COX metabolites are released throughout the body at the site of inflammation or after infection. COX is expressed in two isoforms- COX-1 exists constitutively, while COX-2 is mainly expressed after inflammation. COX-1 and COX-2 were primarily responsible for the production of various effective biological mediators (prostaglandins) that regulate homeostasis and disease processes [7]. COX-2 is altered by a variety of aggravation which includes growth factors, cytokines and tumor promoters [8]. Studies suggested that COX-2 plays important role in the production of different types of prostaglandins at the time of inflammatory response [9].
Prostaglandin E2 (PGE-2) is a prostanoid lipid mediator formed by arachidonic acid via the COX pathway. Prostaglandins are key players in the regulation of inflammation. In addition, they are activated by both autocrine and paracrine signaling. PGE-2 is produced by mainly all lung cell types, but the major site is epithelial cells, fibroblasts and macrophages [10]. PGE2 levels were higher in the lungs of COPD patients and correlated with the severity of airflow obstruction [2]. In addition to this, PGE2 plays an essential role in cigarette smoke-induced inflammation [11]. In addition, by partially protecting against allergen-induced inflammation, PGE2 exerts an anti-inflammatory effect in asthmatics [2]. PGE-2 is considered as a pro-inflammatory mediator in airway diseases. Likewise, several studies had stated that COX-2 levels were increased in the airway of COPD subjects. Taken together, COX-2 and PGE-2 may be the important players in the pathogenesis of inflammation in COPD. However, the contribution of COX-2 in airway remodeling in COPD is still vague.
However, the expression levels and possible biological phenomenon of MMP-9, COX-2 and PGE-2 in COPD is not clearly understood. In the present study, we aimed to evaluate and compare the serum levels of MMP-9, COX-2 and PGE-2 in COPD and healthy controls. Also, assessed the demographic profile, clinical characteristics and risk factors of COPD. Moreover, the correlation between expression levels of MMP-9, COX-2 and PGE-2 and clinical characteristics of COPD patients was shown.
Materials and Methods
Study Design and Setting
In this case–control study, we had screened 186 participants. In which 79 COPD (cases) and 79 non-COPD (healthy control) were recruited randomly from the outpatient department (OPD) of the respiratory medicine department of a tertiary care hospital.
Informed written consent was taken from all the participants prior to enrolment and study protocol was approved by the institutional ethics committee. Participants were grouped as ‘control’ and ‘COPD’. To check the role of smoking, we had divided control into two groups viz non-smoker control and smoker control.
Inclusion & Exclusion Criteria
The inclusion criteria for COPD patients were as follows: 1) Age group of 40–75 years; 2) cases were enrolled irrespective of the stages of COPD; 3) patients who had symptoms of a persistent cough, or dyspnea and/or a history of exposure to risk factors of the disease -COPD; 4) the diagnosis of COPD was confirmed by spirometry {as per GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria}. And exclusion criteria of cases were- 1) Age < 40 years and > 75 years; 2) recent respiratory tract infection, exacerbation history of the respiratory disease, or taken course of oral steroids and/ antibiotics in the previous month; 3) lactating and/ pregnant women; 4) asthma, pulmonary tuberculosis, heart disease, diabetes, hypertension and any other associated co-morbidities; 5) patients or their attendant denied giving their consent.
Age and sex-matched controls were enrolled. The age, sex, ethnicity, exposure to tobacco was noted as per the questionnaire. Healthy volunteers who were non-COPD based on their medical history and clinical examination were included.
Diagnosis of COPD and its Severity Grading
Patients who had a chronic cough, dyspnea and history of exposure to risk factors of COPD and the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) < 0.7 and the reversibility of inhaled bronchodilators in FEV1 < 12% or < 200 mL after administering two puff of 200 μg salbutamol using a pressure metered-dose inhaler with spacer was considered in the COPD group. Diagnostic criteria of COPD was confirmed by GOLD guidelines [12]: GOLD-1, the predicted FEV1 percentage was ≥ 80%; GOLD-2, the predicted FEV1 percentage was ≥ 50%, but < 80%; GOLD-3, the predicted FEV1 percentage was ≥ 30%, but < 50%; and GOLD-4, the predicted FEV1 percentage was, < 30%.
Sample Collection and Preparation
The peripheral venous blood of the participants was drawn by a technician into a plain vacutainer tube. Further, collected blood samples were centrifuged at 3000 rpm for 10 min and then serum was separated in micro-centrifuge tubes and stored immediately at − 80 °C until analysis. The stored serum was defrosted at room temperature when needed for the experiment.
Measurement of Serum Levels of MMP-9, COX-2 and PGE-2
The serum levels of MMP-9, COX-2 and PGE-2 was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol. Human MMP-9, COX-2 and PGE-2 ELISA kits were purchased from KINESISDx (Brea, CA, USA). Briefly, all the reagents were brought at room temperature before use. Standard dilutions were done for MMP-9 (Cat No. K12-0937), COX-2 (Cat No. K12-0781) and PGE-2 (Cat No. K12-1010) as per kit instructions.
Statistical Analysis
The data collected from the present study were analyzed statistically to get significant results with GraphPad Prism 6 (GraphPad Software Inc, San Diego, CA, USA). All the data were presented as mean ± standard deviation (SD) and percentage. Data was analyzed by Student t-test, one way ANOVA, Chi-Square test and Pearson correlation test.
Results
Clinico-demographic Profile of Studied Population
In Table 1 demographic profile, clinical characteristics of participants was presented. A total of 79 healthy control and 79 COPD patients were included in the present study. Mean age of control group was 58.44 [± standard deviation (SD): 9.38] and COPD group was 60.76 [± SD:8.6]. In control group 58 (73%) male and 21 (27%) females were enrolled, while in COPD 52 (67%) males and 27 (34%) females were enrolled. Body mass index (BMI) was calculated by the Asian Indian cutoff [13]. In COPD group BMI of 40 participants was normal, 24 were overweight and obese (n = 15). While in control group 24 individuals had normal BMI, 36 were overweight and 19 were obese. In the COPD group 10 patients (13%) were current smoker, 41 (52%) were ex-smokers and 28 (35%) were non-smoker. And in the control group, 11% were current smokers, 18% were ex-smoker and 71% were non-smoker. In the present study ever-smokers and never-smokers were put in the group of non-smokers, as never-smokers were defined as person who had not smoked more than 1 cigarette per day for 1 year (< 1/20 pack-years) in their lifetime [12]. Pack-years (mean ± SD) in COPD was 16.5 ± 6.4 and control was 10.3 ± 6.8.
Table 1.
Clinico-demographic characteristics of the studied population
| S.No | Characteristics | Control (n = 79) | COPD (n = 79) |
|---|---|---|---|
| 1 | Age (mean ± SD) | 58.44 ± 9.38 | 60.76 ± 8.6 |
| 2 |
Gender (n, %) Male Female |
58 (73.42) 21 (26.58) |
52 (65.83) 27 (34.18) |
| 3 |
Body mass index$ (n, %) 18.0–22.9 kg/m2 23.0–24.9 kg/m2 > 25 kg/m2 |
24 (30.38) 36 (45.57) 19 (24.05) |
40 (50.63) 24 (30.38) 15(18.99) |
| 4 |
Smoking history (n, %) Smoker Ex-smoker Non-smoker |
9 (11.39) 14 (17.72) 56 (70.89) |
10 (12.66) 41 (51.9) 28 (35.44) |
| 5 | Pack years (mean ± SD) | 10.3 ± 6.8 | 16.5 ± 6.4 |
| 6 |
CAT Score* (n,%) < 10 ≥ 10 |
17 (21.52) 62 (78.48) |
|
| 7 |
GOLD grading# (n,%) 1 2 3 4 |
9 (11.4) 13 (16.5) 31 (39.24) 26 (32.91) |
|
| 8 |
Clinical history (years) (n,%) < 10 10–20 ≥ 20 |
17 (21.52) 33 (41.77) 29 (40.51) |
|
| 9 |
Socio economic status! (n,%) Upper Upper-Middle Lower-Middle Upper-Lower Lower |
05 (6.33) 18 (22.78) 12 (15.19) 33 (41.77) 11 (13.92) |
06 (7.59) 13 (16.45) 18 (22.78) 20 (25.32) 22 (27.85) |
Notes: Data expressed in mean ± standard deviation, number, percentage. $Body mass index was calculated by Asian Indian cutoff [13]. GOLD Grade[12]: GOLD-1, the predicted FEV1 percentage was ≥ 80%; GOLD-2, the predicted FEV1 percentage was ≥ 50%, but < 80%; GOLD-3, the predicted FEV1 percentage was ≥ 30%, but < 50%; and GOLD-4, the predicted FEV1 percentage was, < 30%. !Socioeconomic status was calculated by Kuppuswamy’s Scale [15]
Abbreviations: n, number of participants; %, percentage; SD, standard deviation; COPD, chronic obstructive pulmonary disease; CAT Score, COPD Assessment Test Score; GOLD, Global Initiative for Chronic Obstructive Lung Disease
COPD Assessment Test (CAT) score was determined for each COPD patients, 17 (21.52%) have CAT score < 10 and ≥ 10 score was found in 62 (78.48%). The socioeconomic status of the participants was calculated by revised Kuppuswamy’s Scale [15]; it was comparable between the control and COPD group (Chi-square test, P = 0.35). Diagnosis of COPD was confirmed by spirometry and all the COPD patients were divided into GOLD 1, 2, 3, 4 grade [12]. A total of 9, 13, 31 and 26 patients were grouped in GOLD-1, 2, 3 and 4 grade, respectively. Clinical history of COPD was categorized into three sections: less than 10 years (n = 17), between 10–20 years (n = 33) and more than 20 years (n = 29).
Serum Levels of MMP-9
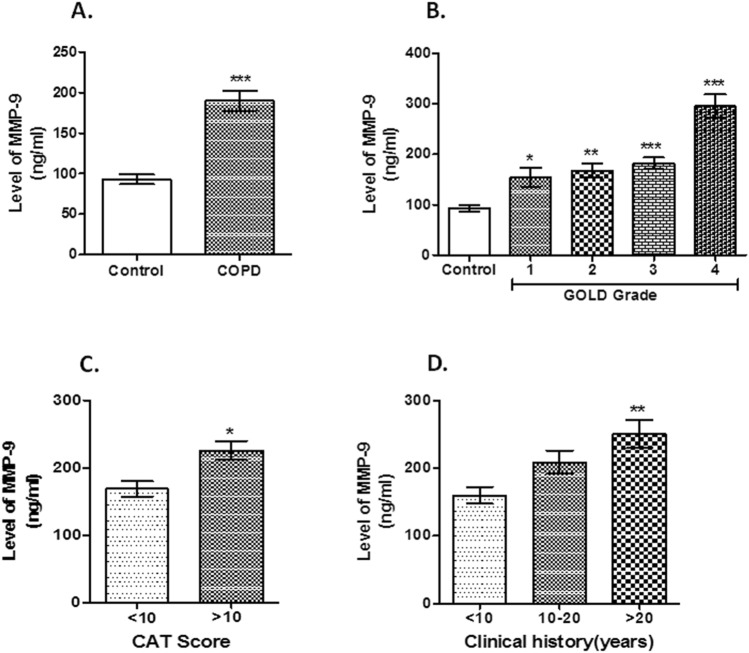
Serum levels of MMP-9 (mean ± SD, ng/ml) was increased in COPD group (213.79 ± 100.52 ng/ml) compared to control (93.03 ± 53.30 ng/ml) {P < 0.0001, Fig. 1 A}. Also, we had seen higher MMP-9 levels in COPD group among non-smoker controlb vs. COPD and smoker controlc vs. COPD (Supplementary Fig. 1 A). MMP-9 serum level was increased from GOLD 1 to 4 {1(154.45 ± 56.43 ng/ml, P < 0.05), 2 (167.34 ± 50.39 ng/ml, P < 0.01), 3 (181.89 ± 65.64 ng/ml, P < 0.001), 4 (295.59 ± 116.220, ng/ml P < 0.001), Fig. 1 B}. COPD patients with CAT score of > 10 (226.06 ± 107.80 ng/ml) showed higher serum levels of MMP-9 than those with CAT score of < 10 (169.03 ± 47.35 ng/ml) {P = 0.04, Fig. 1 C}. Additionally, COPD subjects with long (> 20 years) clinical history (250.8 ± 118.76 ng/ml) displayed a higher levels of serum MMP-9 (P = 0.01, Fig. 1 D) than those with short history (160.13 ± 50.07 ng/ml for clinical history of < 10 years).
Fig. 1.
Serum levels of MMP-9. Notes: The serum MMP-9 levels was increased in COPD subjects when compared with control (***P < 0.001, Fig. 1a). Increased serum MMP-9 level positively correlated with GOLD grade (*P < 0.05, **P < 0.01, ***P < 0.001, Fig. 1b). COPD subjects with CAT score (> 10) displayed higher serum levels of MMP-9 than those with < 10 CAT score (*P = 0.037, Fig. 1c). COPD subjects with long clinical history showed elevated level of serum MMP-9 than those with a short history (*P = 0.01, Fig. 1 d). Abbreviations: MMP-9, matrix metalloproteinase-9; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT Score, COPD Assessment Test Score
Serum Levels of COX-2
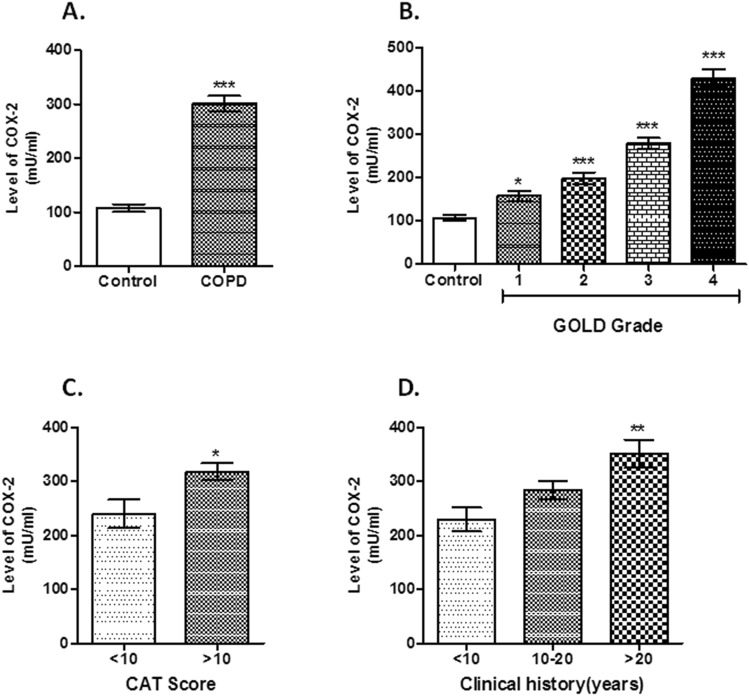
Serum levels of COX-2 ((mean ± SD, mU/ml) was elevated in COPD group (301.62 ± 125.79 mU/ml) compared to control (106.48 ± 61.22 mU/ml) {P < 0.0001, Fig. 2 A}. Also, we had found higher COX-2 levels among non-smoker controlb vs. COPD and smoker controlc vs. COPD (Supplementary Fig. 1 B). Serum level of COX-2 was increased from GOLD 1 to 4 {1(157.94 ± 37.04 mU/ml, P < 0.05), 2 (198.91 ± 50.44 mU/ml, P < 0.001), 3 (279.03 ± 71.92 mU/ml, P < 0.001), 4 (429.64 ± 104.43 mU/ml, P < 0.001), Fig. 2 B}. In addition, COPD subjects with CAT score of > 10 (318.29 ± 125.64 mU/ml) showed higher serum levels of COX-2 than those with CAT score of < 10 (276.92 ± 99.98 mU/ml) {P = 0.02, Fig. 2 C}. COPD subjects with long clinical history (337.47 ± 144.84 mU/ml for COPD patients with > 20 years clinical history) displayed a higher levels of serum COX-2 (P = 0.005, Fig. 2 D) than those with short history (237.21 ± 97.09 mU/ml for clinical history of < 10 years).
Fig. 2.
Serum levels of COX-2. Notes: An increased level of serum COX-2 was observed in COPD subjects compared with healthy controls (***P < 0.001, Fig. 2a). Upregulated serum COX-2 positively correlated with the GOLD grade in COPD subjects (*P < 0.05, ***P < 0.001, Fig. 2b). COPD subjects with CAT score (> 10) showed increased serum levels of COX-2 than those with < 10 CAT score (*P = 0.023, Fig. 2c). COPD subjects with long clinical history showed elevated COX-2 serum levels than those with a short history (**P = 0.002, Fig. 2d). Abbreviations: COX-2, cyclooxygenase-2, COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT Score, COPD Assessment Test Score
Serum Levels of PGE-2
Serum level of PGE-2 (mean ± SD, pg/ml) was significantly increased in COPD (423.02 ± 179.2 pg/ml) compared to control (146.49 ± 73.39 pg/ml) {P < 0.0001, Fig. 3 a}. Also, we had seen higher COX-2 levels among non-smoker controlb vs. COPD and smoker controlc vs. COPD (Supplementary Fig. 1C). PGE-2 serum level was increased from GOLD 1 to 4 {1(216.28 ± 87.47 pg/ml), 2 (301.83 ± 68.27 pg/ml, P < 0.0001), 3 (367.45 ± 89.40 pg/ml, P < 0.0001), 4 (621.43 ± 179.17 pg/ml, P < 0.0001), Fig. 3 b}. COPD patients with CAT score of > 10 (446.53 ± 177.18 pg/ml) showed higher serum levels of PGE-2 than those with CAT score of < 10 (337.25 ± 164.01 pg/ml) {P = 0.02, Fig. 3 c}. COPD subjects with long clinical history (478.74 ± 218.21 pg/ml for clinical history of > 20 years) displayed a higher levels of serum PGE-2 (P = 0.003, Fig. 3 d) than those with short history (160.13 ± 50.07 ng/ml for clinical history of < 10 years).
Fig. 3.
Serum PGE-2 level in control and COPD subjects. Notes: Patients with COPD displayed higher levels of serum PGE-2 levels than those in the healthy controls (***P < 0.001, Fig. 3a). This upregulated level was increased with the GOLD grade in the COPD group(*P < 0.05, ***P < 0.001, Fig. 3b). COPD subjects with CAT score (> 10) displayed higher serum levels of PGE-2 than those with < 10 CAT score (*P = 0.024, Fig. 3c). COPD subjects with long clinical history showed elevated level of serum PGE-2 than those with a short history (*P = 0.02, Fig. 3d). Abbreviations: PGE-2, prostaglandin E2; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT Score, COPD Assessment Test Score
Correlation between Serum Levels of MMP-9, COX-2 and PGE-2 in Patients with COPD.
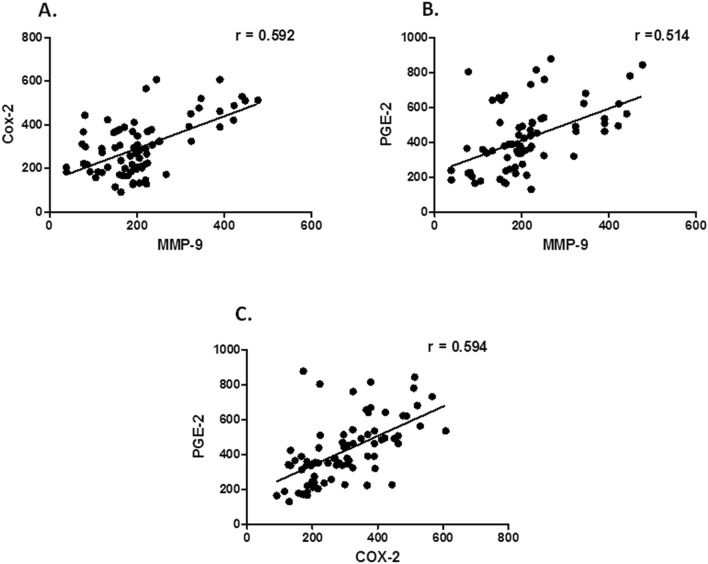
Serum levels of MMP-9 and COX-2 showed a positive correlation [{correlation coefficient of Pearson’s correlation test (r = 0.592)}, P < 0.0001, 95% {confidence interval (CI) = 0.426–0.718}, Fig. 4 a]. Further, serum levels of MMP-9 and PGE-2 showed a positive correlation [{correlation coefficient of Pearson’s correlation test (r = 0.514)}, P < 0.0001, 95% CI = 0.33- 0.66, Fig. 4 b]. Moreover, serum levels of COX-2 and PGE-2 showed a positive correlation [{correlation coefficient of Pearson’s correlation test (r = 0.594)}, P < 0.0001, 95% CI = 0.429- 0.72, Fig. 4 c].
Fig. 4.
Correlation between the serum levels of MMP-9, COX-2 and PGE-2 in patients with COPD. Notes: Positive correlation was found between serum levels of (a) MMP-9 and COX-2 [Pearson correlation test (r = 0.591), P < 0.0001], (b) MMP-9 and PGE-2 in patients with COPD [Pearson correlation test (r = 0.514), P < 0.0001] and (c) COX-2 and PGE-2 in patients with COPD. [Pearson correlation test (r = 0.593), P < 0.0001]. Abbreviations: MMP-9, matrix metalloproteinase-9; COX-2, cyclooxygenase-2; PGE-2, prostaglandin E2; COPD, chronic obstructive pulmonary disease
Discussion
In the present study, we had determined the blood serum levels of MMP-9, COX-2 and PGE-2 in COPD as well as in healthy controls who were free from COPD. Our data demonstrated that MMP-9, COX-2 and PGE-2 serum levels were significantly increased in the COPD group than healthy controls. A pattern of increased levels was found as- control < non-smoker control < smoker control < COPD; it was consistent with MMP-9, COX-2 and PGE-2 levels, individually. Moreover, serum levels of MMP-9, COX-2 and PGE-2 were significantly increased in GOLD grade- 1 to 4, greater CAT score and longer clinical history in the COPD group. The serum levels of MMP-9, COX-2 and PGE-2 showed a positive correlation in COPD patients.
MMP-9 is related to airflow obstruction which suggests that it has a primal role in the pathogenesis of COPD. A previous study suggested that MMP-9 negatively correlated with the severity of airway obstruction [16]. Linder et al. had performed a population-based COPD cohort study and concluded that productive cough and lowered FEV1 (forced expiratory volume in 1 s) were correlated with MMP-9 [17]. Additionally, the elevated serum MMP-9 levels in COPD indicated increased proteolytic activity which is related to the disease severity [17]. A study reported enhanced level of TIMP-1, TIMP-2 and MMP-9 was found in bronchoalveolar lavage (BAL) of acute exacerbation- COPD [18]. Uysal P et al. had investigated plasma concentration of MMP-9, serpina3g and tissue inhibitor of metalloproteinase-1 and 2 (TIMP-1 and 2) were increased in COPD and this increased level were also found with GOLD grading [3]. Additionally, plasma MMP-9 levels and MMP-9/TIMP-1 ratio was elevated in emphysema when compared with other phenotypes and FEV1 was associated with MMP-9/TIMP-1 imbalance, the authors explained [3]. Authors had conducted a study in the Chinese population and concluded that genetic polymorphism in MMP-9 and transforming growth factor-β1 (TGF- β1) correlated with not only pulmonary fibrosis but also emphysema [19]. Likewise, MMP-8, MMP-9 and neutrophil elastase were found to be increased in peripheral blood of COPD [20]. Our data showed that MMP-9 level was increased in the COPD group than healthy control as well as the increased expression was positively correlated with GOLD grading, these findings are in line with many previous studies [3, 17–22].
Previous studies suggested that MMPs are increased in the airways and blood of COPD patients, which contributes to the disease pathogenesis and tissue remodeling [21]. However, it is not clear that MMP levels in airways, blood and urine are related to each other, or MMP levels are associated with disease severity or exacerbation history. To answer this, Cane J L etal. had examined MMP-8 and MMP-9 levels in the airway, blood and urine of 72 hospitalized COPD patients [21]. They had found that serum and urine MMP-9 was slightly increased during an exacerbation, while MMP-8 was not changed. The authors concluded that MMP concentrations in lung, serum and urine are independent of each other and do not reflect disease severity in hospitalized COPD subjects [21].
A study had investigated PGE-2, MMP-2 and COX-2 levels in the sputum of 43 stable COPD patients, 12 smoking control and 10 non-smoking control [2]. Compared with the non-smoking control group, the levels of PGE-2 and MMP-2 in the sputum of the smoking control and COPD subjects not only elevated but also gradually increased with the progress of airflow limitation, authors concluded [2]. Also, COX-2 expression increased in COPD subjects compared with non-smoking controls. In addition to this, the concentrations of PGE-2 and MMP-2 in COPD patients are inversely proportional to FEV1%. Moreover, sputum PGE-2 level was positively correlated with MMP-2 in the COPD subjects [2]. Our data also showed an increased level of PGE-2 and COX-2 in the serum samples of COPD patients.
The levels of PGE-2 in exhaled breath condensate was elevated in stable COPD subjects compared with healthy controls [23]. Fibroblast-derived PGE-2 may regulate the angiogenesis and inflammation through the production of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) in the airways of COPD subjects, respectively [24]. In addition, COX-2, E-prostanoid receptor (EP) 2 and EP4 levels were increased in COPD fibroblasts suggesting their role in inflammation [24]. Further, the percentage of COX-2 immunostained cells in stable COPD subjects were significantly higher than that in the control group and the macrophages were the most positively stained cells [25]. Present study showed similar results that the expression of PGE2 and COX-2 in the COPD patients increased significantly, indicating that COX-2 and PGE2 are related to the chronic inflammation of COPD.
In the current investigation, the expression of serum MMP-9, COX-2 and PGE-2 is increased and this pattern was consistent with GOLD grade. Also, all these markers are positively correlated with COPD subjects. These findings indicate that MMP-9, COX-2 and PGE2 is closely related to the pathogenesis of inflammation and airflow limitation in the progression of COPD. Evidence suggests that smoking is a major risk factor in airway diseases so that tobacco users should be encouraged to quit smoking.
This study has some limitations. The number of participants was limited and patients were selected from a single hospital but the hospital has a lot of patient burden. Here, the physical activity and exercise capacity of the participants were not studied. It is clear that COPD is associated with genetic factors. However, a genetic analysis was not done in the present study. We had measured the level of MMP-9, COX-2 and PGE-2 in blood serum only. Moreover, for the clinical reliability of these results, multi-centric studies with a large sample size are required.
Conclusion
The serum level of MMP-9, COX-2 and PGE-2 increased in patients with COPD. The enhanced level of MMP-9, COX-2 and PGE-2 was found with GOLD Grade, CAT score and clinical history which showed the association of these biomarkers with the severity of airflow limitation in COPD. Further, the serum levels of MMP-9, COX-2 and PGE-2 showed a positive correlation in COPD patients. These findings suggest that they may be used as indicators to reach the depth of the progression and management of COPD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank to all the participants who had given their consent to participate in the study.
Authors' contributions
Conceptualization: AKV, AKP; Data analysis:. AKV, AKP, AAM, VP; Funding: AKV; Investigation: AKP, AKV, AS, SK, AAM, VP, KMA, RKD, SCC; Experimental studies: AKV, AKP, AS, KMA; Validation: AKV, AKP, AS, SK, SCC, RKD; Manuscript writing & original draft: AKP, AKV, AS; Writing, review & editing: AKV, AKP, AS, SK, AAM, VP, KMA, RKD, SCC.
Funding
This work was supported by Council of Science and Technology, Uttar Pradesh (No: CST/SERPD/D-2477).
Declarations
Conflict of interest
Authors declare no competing financial interest.
Consent for publication
Informed written consent was obtained from all the participants prior to enrolling in the study.
Ethical Approval
Study protocol was approved by Institutional Ethics Committee of the institution. And study was performed in accordance of approved protocol.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ajay Kumar Verma and Anuj Kumar Pandey - Authors contributed equally
References
- 1.Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2021 Report). 2020 Global Initiative for Chronic Obstructive Lung Disease, Inc. https://goldcopd.org/2021-gold-reports/ (assessed on 15 December 2020).
- 2.Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13(7):1014–21. doi: 10.1111/j.1440-1843.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 3.Uysal P, Uzun H. Relationship between circulating serpina3g, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and-2 with chronic obstructive pulmonary disease severity. Biomolecules. 2019;9(2):62. doi: 10.3390/biom9020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churg A, Zhou S, Wright JL. Matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious and multifaceted. Physiol Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd El-Fatah MF, Ghazy MA, Mostafa MS, El-Attar MM, Osman A. Identification of MMP-9 as a biomarker for detecting progression of chronic obstructive pulmonary disease. Biochem Cell Biol. 2015;93(6):541–547. doi: 10.1139/bcb-2015-0073. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128(6):1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Z, Chen Y, Pei Y, Long Y, Liu C, Cao J, et al. The role of cyclooxygenase-2 in the protection against apoptosis in vascular endothelial cells induced by cigarette smoking. J Thorac Dis. 2017;9(1):30–41. doi: 10.21037/jtd.2017.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K, Lee SH, Kim TH. The biology of prostaglandins and their role as a target for allergic airway disease therapy. Int J Mol Sci. 2020;21(5):1851. doi: 10.3390/ijms21051851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaslona Z, Peters-Golden M. Prostanoids in asthma and COPD: actions, dysregulation and therapeutic opportunities. Chest. 2015;148(5):1300–1306. doi: 10.1378/chest.15-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Lee IT, Yang YL, Lee CW, Kou YR, Yang CM. Induction of COX-2/PGE(2)/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: role of TLR4-dependent NADPH oxidase activation. Free Radic Biol Med. 2010;48:240–254. doi: 10.1016/j.freeradbiomed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Shan F, Zhang Y, Jiang L, Cheng Z. Increased serum IL-17 and decreased serum IL-10 and IL-35 levels correlate with the progression of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2483. doi: 10.2147/COPD.S167192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170. [PubMed] [Google Scholar]
- 14.Tan WC, Sin DD, Bourbeau J, Hernandez P, Chapman KR, Cowie R, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70:822–829. doi: 10.1136/thoraxjnl-2015-206938. [DOI] [PubMed] [Google Scholar]
- 15.Sharma R. Revised Kuppuswamy’s Socioeconomic Status Scale: explained and updated. Indian Pediatr. 2017;54:867–870. doi: 10.1007/s13312-017-1151-x. [DOI] [PubMed] [Google Scholar]
- 16.Beeh KM, Beier J, Kornmann O, Buhl R. Sputum matrix metalloproteinase-9, tissue inhibitor of metalloprotinease-1 and their molar ratio in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and healthy subjects. Respir Med. 2003;97(6):634–639. doi: 10.1053/rmed.2003.1493. [DOI] [PubMed] [Google Scholar]
- 17.Linder R, Ronmark E, Pourazar J, Behndig A, Blomberg A, Lindberg A. Serum metalloproteinase-9 is related to COPD severity and symptoms-cross sectional data from a population based cohort-study. Respir Res. 2015;16(28):1–9. doi: 10.1186/s12931-015-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papakonstantinou E, Karakiulakis G, Batzios S, Savic S, et al. Acute exacerbations of COPD are associated with significant activation of matrix metalloproteinase 9 irrespectively of airway obstruction, emphysema and infection. Respir Res. 2015;16(1):78. doi: 10.1186/s12931-015-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Bian W, Gu XH, Shen C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-β1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J Med Sci. 2017;33(3):124–129. doi: 10.1016/j.kjms.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sng JJ, Prazakova S, Thomas PS, Herbert C. MMP-8, MMP-9 and Neutrophil Elastase in Peripheral Blood and Exhaled Breath Condensate in COPD. COPD. 2017;14(2):238–244. doi: 10.1080/15412555.2016.1249790. [DOI] [PubMed] [Google Scholar]
- 21.Cane JL, Mallia-Millanes B, Forrester DL, Knox AJ, Bolton CE, Johnson SR (2016) Matrix metalloproteinases-8 and-9 in the airways, blood and urine during exacerbations of COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease. 13(1); 26–34. [DOI] [PubMed]
- 22.Xin XF, Zhao M, Li ZL, Song Y, Shi Y. Metalloproteinase-9/tissue inhibitor of metalloproteinase-1 in induced sputum in patients with asthma and chronic obstructive pulmonary disease and their relationship to airway inflammation and airflow limitation. Chinese J Tuberculosis Respir Dis. 2007;30(3):192–196. [PubMed] [Google Scholar]
- 23.Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58:585–8. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonanno A, Albano GD, Siena L, Montalbano AM, Riccobono L, Anzalone G, et al. Prostaglandin E2 possesses different potencies in inducing vascular endothelial growth factor and interleukin-8 production in COPD human lung fibroblasts. Prostaglandins Leukot Essent Fatty Acids. 2016;106:11–18. doi: 10.1016/j.plefa.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Taha R, Olivenstein R, Utsumi T, Ernst P, Barnes PJ, Rodger IW, et al. Prostaglandin H synthase 2 expression in airway cells from patients with asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:636–640. doi: 10.1164/ajrccm.161.2.9811063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.