OBJECTIVES:
Nosocomial pneumonia (NP) remains a costly complication of hospitalization fraught with subsequent complications and augmented resource utilization. Consisting of ventilated hospital-acquired bacterial pneumonia (vHABP), nonventilated hospital-acquired bacterial pneumonia (nvHABP), and ventilator-associated bacterial pneumonia (VABP), each may respond differently to inappropriate empiric treatment (IET). We explored whether IET affects the three pneumonia types differently.
DESIGN:
A multicenter, retrospective cohort study within the Premier Research database.
SETTING:
Acute care hospitals in the United States.
PATIENTS:
Patients with three types of NP were identified based on a previously published International Classification of Diseases, 9th Edition/International Classification of Diseases, 10th Edition Clinical Modification algorithm.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We compared the impact of IET on hospital costs, length of stay (LOS), and development of Clostridium difficile infection (CDI), extubation failure (EF), and reintubation (RT). Marginal effects were derived from multivariable regression analyses. IET was present if no drug covering the organism recovered from the index culture was administered within 2 days of the culture date. Among 17,819 patients who met the enrollment criteria, 26.5% had nvHABP, 25.6% vHABP, and 47.9% VABP. Compared with non-IET, IET was associated with increased mean unadjusted hospital LOS across all NP types: nvHABP 12.5 versus 21.1, vHABP 16.7 versus 19.2, and VABP 18.6 versus 21.4 days. The adjusted marginal hospital LOS (4.9 d) and costs ($13,147) with IET were the highest in nvHABP. Incident CDI was rare and similar across NP types (2.4% nvHABP to 3.6% VABP). Both EF and RT were more common with IET in VABP (EF, 15.4% vs 19.2%; RT, 6.2% vs 10.4%), but not vHABP (EF, 15.1% vs 17.7%; RT, 8.1% vs 9.1%).
CONCLUSIONS:
Although IET is relatively uncommon, it affects resource utilization and the risk of complications differently across NP types. The impact of IET is greatest on both LOS and costs in nvHABP and is greater on VABP than vHABP in terms of EF and RT.
Keywords: complications, costs, hospital-acquired bacterial pneumonia, nosocomial pneumonia, pneumonia, ventilator-associated bacterial pneumonia
Hospital-acquired complications (HACs) remain a source of considerable morbidity and costs in the U.S. healthcare system. A 2016 IBM Watson Health analysis found that nearly 49,000 HACs occur annually in the United States, costing the system over $2 billion (1). Although not included as one of the 12 conditions explored in the IBM analysis, ventilator-associated bacterial pneumonia (VABP) represents one of the costliest HACs. According to a recent report from the Agency for Healthcare Research and Quality, a single case of VABP costs over $47,000 (2). Although there are no current estimates for the total incidence of VABP in the United States, older analyses suggest that there are between 250,000 and 300,000 cases each year (3). If these numbers remain correct, then the current burden of VABP results in an aggregate annual cost of over $12 billion. Unfortunately, even this exorbitant price tag fails to capture completely all the costs of nosocomial pneumonia (NP), which, in addition to VABP, also includes hospital-acquired bacterial pneumonia (HABP) that develops in nonventilated patients.
Prolonged hospitalization is one of the central risk factors for HACs in general and prolonged time on mechanical ventilation (MV) for VABP in particular. Less well explored is the impact of HACs themselves upon prolonging hospitalization. For instance, whether NP, itself an HAC, predisposes a patient to another common HAC, such as C. difficile infection (CDI), has not been adequately studied. Yet there is biological reason to think this is the case, given a high propensity for broad-spectrum antimicrobials use in NP and their well-documented association with CDI. Furthermore, since appropriate empiric treatment represents the single most important potentially modifiable factor that might improve outcomes in severe infections, including NP, its impact on these potential downstream complications in NP is unclear (4–14). Finally, several reports indicate that HABP that requires ventilation (ventilated hospital-acquired bacterial pneumonia [vHABP]) is a distinct entity from HABP that does not (nonventilated hospital-acquired bacterial pneumonia [nvHABP]) (15–19). This begs the question whether these different types of NP—VABP, vHABP, and nvHABP—also differ in their risks for downstream HACs and their associations with empiric treatment. To answer these questions, we conducted a retrospective cohort study examining a large, multi-institutional database.
MATERIALS AND METHODS
Ethics Statement
Because this study used already-existing fully deidentified data, it was exempt from ethics review under U.S. 45 Code of Federal Regulations 46.101(b)4 (20). Thus, an Institutional Review Board review was not sought.
Data Source
The data source was the Premier Research database, an electronic laboratory, pharmacy, and billing data repository, for years 2014 through the third quarter of 2019. The database has been described in detail previously (18, 19, 21–26). Approximately 200 U.S. institutions submitted microbiology data during the study time frame. The details of the current cohort can be found in (18) and (19).
Study Design and Patient Population
We conducted a multicenter retrospective cohort study of hospitalized patients with culture-positive nvHABP, vHABP, or VABP to explore their microbiology, empiric treatment patterns, and the impact of receiving inappropriate empiric treatment (IET) on hospital outcomes. The case-identification approach relied on a slight modification of a previously published algorithm (21). The details of the study methods can be found in (18), an analysis conducted in the same cohort, and an additional analysis focusing on the microbiology and antimicrobial treatments of these infections in (19).
Briefly, patients were included if they were adults (age > 18 yr) whose pneumonia appeared as a secondary diagnosis, whose index respiratory and/or blood culture had to be obtained on hospital day 3 or later for HABP, or on MV day 3 or later for VABP, and who were treated with an antibiotic on the day of the index culture and for the next greater than 3 consecutive days. We excluded patients who fit the definition for either a complicated urinary tract infection (cUTI) or a complicated intra-abdominal infection in order to reduce misclassification (22, 23).
Pneumonia Classification
Pneumonia was defined as HABP if, at the time of the index culture, the patient was not on MV and VABP if, at the time of the index culture, the patient had been on MV for 3+ days. HABP was further subdivided into vHABP and nvHABP. Specifically, vHABP designation was given for patients who needed MV less than 5 days following the onset of index HABP episode and nvHABP if MV was not required.
Microbiology and Empiric Treatment
Details of organism classification and distribution can be found in (19). We examined both Gram-positive and Gram-negative pathogens that cause bacterial NP.
Antimicrobial coverage was considered appropriate if a drug administered within 2 days of the index culture being obtained covered the recovered organism. All other treatment was defined as IET or as indeterminate, the latter if there were no results of testing of the isolated pathogen for susceptibility to the administered drug (19).
Outcome Variables
The outcomes examined in the current analysis were: 1) incident CDI, 2) treatment (or extubation) failure, defined as the need to reintubate less than 3 days of index extubation, and 3) clinical deterioration, identified as reintubation 4+ days after extubation. The impact of IET on hospital costs and components of postinfection onset length of stay (LOS) (MV duration, ICU LOS and hospital LOS) was examined stratified by pneumonia type. Incident CDI was defined by the start of oral metronidazole or oral vancomycin or fidaxomicin treatment between infection day 3 and discharge from the hospital or hospital day 30, whichever came first.
Statistical Analyses
Continuous variables are reported as means with values of sd and as medians with interquartile ranges. Differences between mean values across pneumonia types were tested via a one-way analysis of variance test, and between medians using the Kruskal-Wallis test. Categorical data are summarized as proportions, with the chi-square test used to examine intergroup differences unless a cell count was less than 5, wherein the Fisher exact test was used.
To examine the impact of IET on hospital outcomes, we employed multivariable logistic regression for modeling the binary outcomes of CDI onset, extubation failure, and reintubation, and generalized linear regression models for the continuous variables of MV duration and LOS. All models derived robust standard errors based on clustering at the hospital level. Variables in the models included hospital LOS prior to the onset of pneumonia; demographic characteristics; comorbidity burden; acute illness severity as measured by the need for ICU admission, dialysis, and vasopressor use; whether the admission was due to medical or surgical diagnosis; diagnosis of acute trauma or neurologic insult; a variety of common treatments provided to patients in the ICU (e.g., nutritional support and inotropes); and hospital structural characteristics (census region, size, teaching status, and urbanicity) (18). Interaction effects between IET status and pneumonia type were explored in each model and as a subanalysis, and separate regression models were rerun for each outcome for each of the three patient groups. p values of less than 0.05 were considered statistically significant.
RESULTS
Among 17,819 patients meeting enrollment criteria, 4,728 (26.5%) had nvHABP, 4,561 (25.6%) vHABP, and 8,530 (47.9%) VABP (Table 1). Patients with nvHABP and vHABP were older than those with VABP, and those with vHABP had the highest acute and chronic illness severity. The majority of the isolated pathogens were Gram-negative (55.1% nvHABP, 53.4% vHABP, and 56.7% VABP), and most common organisms were Staphylococcus aureus (~40% in each pneumonia type, methicillin resistant 16.9% nvHABP, 14.8% vHABP, and 11.2% VABP), and Pseudomonas aeruginosa (18.5% nvHABP, 16.4% vHABP, and 16.8% VABP) (19). The prevalence of carbapenem resistance was 7.6% in nvHABP, 6.8% in vHABP, and 9.1% in VABP (19). The rates of IET were generally low across all pneumonia groups, lowest in vHABP (5.6%), and highest in nvHABP (8.5%). Although nearly all VABP and vHABP patients required it, only 58.0% of nvHABP group required an ICU stay within 2 days of pneumonia onset (Table 1).
TABLE 1.
Selected Baseline, Infection, and Empiric Treatment Characteristicsa
| Characteristic | Nonventilated HABP | Ventilated HABP | Ventilator-Associated Bacterial Pneumonia | ||||
|---|---|---|---|---|---|---|---|
| n = 4,728 | % | n = 4,561 | % | n = 8,530 | % | p | |
| Mean age, yr (sd) | 66.7 (15.1) | 65.7 (14.0) | 59.7 (16.6) | < 0.001 | |||
| Gender: male | 2,845 | 60.17 | 2,921 | 64.04 | 5,557 | 65.15 | < 0.001 |
| Charlson Comorbidity Score | |||||||
| Mean (sd) | 3.9 (2.8) | 4.1 (2.8) | 3.2 (2.5) | < 0.001 | |||
| Median (interquartile range) | 3 (2–5) | 4 (2–6) | 3 (1–5) | < 0.001 | |||
| Illness severity measures by day 2 from infection onset | |||||||
| ICU admission | 2,742 | 57.99 | 4,287 | 93.99 | 8,162 | 95.69 | < 0.001 |
| Vasopressors | 365 | 7.72 | 1,770 | 38.81 | 2,103 | 24.65 | |
| Severe sepsis | 627 | 13.26 | 1,166 | 25.56 | 1,393 | 16.33 | < 0.001 |
| Septic shock | 557 | 11.78 | 1,472 | 32.27 | 1,603 | 18.79 | < 0.001 |
| Empiric treatment appropriateness | |||||||
| Non-IET | 3,856 | 81.56 | 3,929 | 86.14 | 7,178 | 84.15 | < 0.001 |
| IET | 403 | 8.52 | 254 | 5.57 | 615 | 7.21 | |
| Indeterminate | 469 | 9.92 | 378 | 8.29 | 737 | 8.64 | |
The unadjusted outcomes stratified by IET and pneumonia type are shown in Tables 2 and 3.
TABLE 2.
Unadjusted Hospitalization Outcomes Compared by Inappropriate Empiric Therapy Status Across Pneumonia Types
| Outcome | Nonventilated Hospital-Acquired Bacterial Pneumonia | Ventilated Hospital-Acquired Bacterial Pneumonia | Ventilator-Associated Bacterial Pneumonia | ||||
|---|---|---|---|---|---|---|---|
| n = 4,728 | % | n = 4,561 | % | n = 8,530 | % | p | |
| Hospital costs overall, $ | |||||||
| Mean (sd) | 59,002 (67,448) | 82,372 (80,624) | 101,386 (106,229) | < 0.001 | |||
| Median (IQR) | 39,911 (24,142–69,129]) | 62,464 (39,149–99,323) | 77,657 (50,823–122,419) | < 0.001 | |||
| IET | |||||||
| Mean (sd) | 76,659 (96,831) | 98,073 (91,361) | 117,925 (100,695) | < 0.001 | |||
| Median (IQR) | 48,422 (27,681–89,726) | 72,867 (48,075–113,655) | 89,620 (56,916–141,762) | < 0.001 | |||
| Non-IET | |||||||
| Mean (sd) | 57,477 (64,412) | 81,728 (80,771) | 100,255 (108,059) | < 0.001 | |||
| Median (IQR) | 38,978 (23,967–67,569) | 62,318 (39,057–98,213) | 77,526 (51,004–121,095) | < 0.001 | |||
| Postinfection hospital LOS, (d) | |||||||
| Mean (sd) | 13.2 (20.0) | 17.0 (17.6) | 18.8 (21.6) | < 0.001 | |||
| Median (IQR) | 8 (4–15) | 13 (7–21) | 14 (8–23) | < 0.001 | |||
| IET | |||||||
| Mean (sd) | 21.1 (37.9) | 19.2 (21.1) | 21.4 (24.8) | 0.574 | |||
| Median (IQR) | 11 (6–20) | 14 (8–21) | 14 (8–25) | < 0.001 | |||
| Non-IET | |||||||
| Mean (sd) | 12.5 (17.6) | 16.7 (17.1) | 18.6 (21.4) | < 0.001 | |||
| Median (IQR) | 8 (4–15) | 13 (7–21) | 14 (8–22) | < 0.001 | |||
| Postinfection onset ICU LOS for survivors, d | |||||||
| Mean (sd) | 3.7 (6.9) | 10.9 (9.3) | 12.2 (10.7) | < 0.001 | |||
| Median (IQR) | 2 (0–5) | 8 (5–14) | 9 (5–16) | < 0.001 | |||
| IET | |||||||
| Mean (sd) | 5.1 (9.3) | 13.2 (13.8) | 12.3 (9.6) | < 0.001 | |||
| Median (IQR) | 2 (0–6) | 9 (5–15) | 10 (6–17) | < 0.001 | |||
| Non-IET | |||||||
| Mean (sd) | 3.6 (6.7) | 10.8 (9.1) | 12.2 (10.9) | < 0.001 | |||
| Median (IQR) | 2 (0–5) | 8 (5–14) | 10 (5–16) | < 0.001 | |||
| Postinfection onset mechanical ventilation duration, da | |||||||
| Mean (sd) | 7.2 (7.3) | 9.3 (10.4) | < 0.001 | ||||
| Median (IQR) | 5 (3–9) | 7 (3–12) | < 0.001 | ||||
| IET | |||||||
| Mean (sd) | 8.0 (6.9) | 16.1 (12.7) | < 0.001 | ||||
| Median (IQR) | 7 (3–11) | 13 (8–20) | < 0.001 | ||||
| Non-IET | |||||||
| Mean (sd) | 7.4 (7.5) | 14.4 (12.2) | < 0.001 | ||||
| Median (IQR) | 5 (3–9) | 12 (8–18) | < 0.001 | ||||
| Incident Clostridium difficile, % | 114 | 2.4 | 146 | 3.2 | 304 | 3.6 | 0.001 |
| IET | 10 | 2.5 | 11 | 4.3 | 18 | 2.9 | 0.392 |
| Non-IET | 90 | 2.3 | 118 | 3.0 | 259 | 3.6 | 0.001 |
| Extubation failure, %a | 688 | 15.1 | 1,351 | 15.8 | 0.010 | ||
| IET | 45 | 17.7 | 118 | 19.2 | 0.614 | ||
| Non-IET | 592 | 15.1 | 1,105 | 15.4 | 0.647 | ||
| Reintubation, %a | 380 | 8.3 | 573 | 6.7 | < 0.001 | ||
| IET | 23 | 9.1 | 64 | 10.4 | 0.546 | ||
| Non-IET | 318 | 8.1 | 444 | 6.2 | < 0.001 | ||
IET = inappropriate empiric therapy, IQR = interquartile range, LOS = length of stay.
aVentilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia only.
TABLE 3.
Unadjusted Hospitalization Outcomes Compared by Inappropriate Empiric Therapy Status Within Pneumonia Types
| Outcomes and Pneumonia Types | IET | Non-IET | p |
|---|---|---|---|
| Hospital costs overall, $ (mean [sd]) | |||
| nvHABP (n = 4,728) | 76,659 (96,831) | 57,477 (64,412) | < 0.001 |
| vHABP (n = 4,561) | 98,073 (91,361) | 81,728 (80,771) | 0.002 |
| VABP (n = 8,530) | 117,925 (100,695) | 100,255 (108,059) | < 0.001 |
| Postinfection hospital LOS, (d) (mean, [sd]) | |||
| nvHABP (n = 4,728) | 21.1 (37.9) | 12.5 (17.6) | <0.001 |
| vHABP (n = 4,561) | 19.2 (21.1) | 16.7 (17.1) | 0.030 |
| VABP (n = 8,530) | 21.4 (24.8) | 18.6 (21.4) | 0.002 |
| Postinfection onset ICU LOS for survivors, d (mean [sd]) | |||
| nvHABP (n = 4,728) | 5.1 (9.3) | 3.6 (6.7) | 0.004 |
| vHABP (n = 4,561) | 13.2 (13.8) | 10.8 (9.1) | 0.002 |
| VABP (n = 8,530) | 12.3 (9.6) | 12.2 (10.9) | 0.876 |
| Postinfection onset mechanical ventilation duration, da (mean [sd]) | |||
| vHABP (n = 4,561) | 8.0 (6.9) | 7.4 (7.5) | 0.207 |
| VABP (n = 8,530) | 16.1 (12.7) | 14.4 (12.2) | 0.001 |
| Incident Clostridium difficile, n (%) | |||
| nvHABP (n = 4,728) | 10 (2.5) | 90 (2.3) | 0.853 |
| vHABP (n = 4,561) | 11 (4.3) | 118 (3.0) | 0.236 |
| VABP (n = 8,530) | 118 (2.9) | 259 (3.6) | 0.381 |
| Extubation failure, n (%)a | |||
| vHABP (n = 4,561) | 45 (17.7) | 592 (15.1) | 0.255 |
| VABP (n = 8,530) | 118 (19.2) | 1,105 (15.4) | 0.013 |
| Reintubation, n (%)a | |||
| vHABP (n = 4,561) | 23 (9.1) | 318 (8.1) | 0.587 |
| VABP (n = 8,530) | 64 (10.4) | 444 (6.2) | < 0.001 |
IET = inappropriate empiric therapy, LOS = length of stay, nvHABP = nonventilated hospital-acquired bacterial pneumonia, VABP = ventilator-associated bacterial pneumonia, vHABP = ventilated hospital-acquired bacterial pneumonia.
avHABP and VABP only.
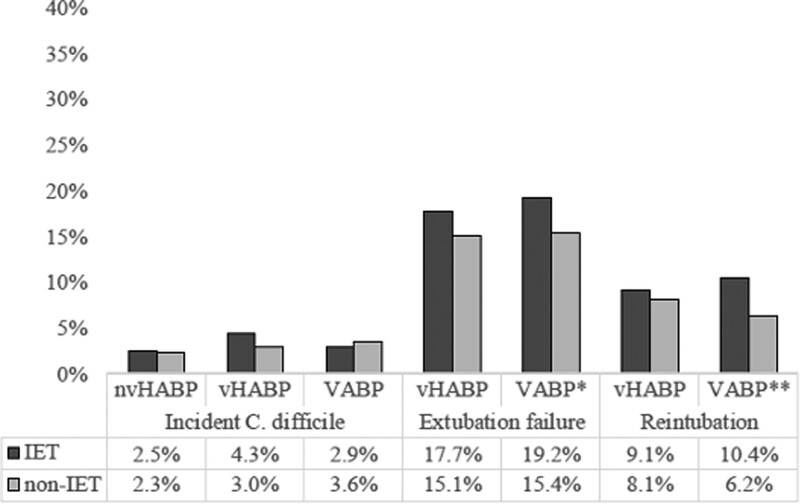
The impact of IET varied across pneumonia types on some of the outcomes, though not on all. Although the overall hospital costs were highest in the VABP group and lowest in nvHABP, IET was associated with the greatest jump in mean cost among nvHABP patients, where it rose from a mean (sd) of $57,477 ($64,412) in those treated appropriately to $76,659 ($96,831) in the setting of IET. In the other two groups, the gap was more modest ($81,728 [$80,771] vs $98,073 [$91,361] in vHABP, and $100,255 [108,059] vs $117,925 [$100,695] in VABP), though still substantial. The prolongation of each of the components of postinfection onset LOS followed a similar pattern. For example, although mean hospital LOS went up by less than 3 days in vHABP and VABP, it rose by nearly 9 days in nvHABP (Tables 2 and 3). CDI was rare across all pneumonia types, and the risks of extubation failure and reintubation were more pronounced in VABP than in vHABP (Fig. 1).
Figure 1.
Patient events among nonventilated hospital-acquired bacterial pneumonia (nvHABP), ventilated hospital-acquired bacterial pneumonia vHABP), and ventilator-associated bacterial pneumonia (VABP) pneumonia in receipt of inappropriate empiric therapy (IET) or non-I(ET.
In multivariable models adjusting for confounders, pneumonia type remained a significant determinant of the magnitude of IET’s impact on many of the outcomes of interest (Table 4). Among the three groups, relative to non-IET, IET was associated with the greatest marginal adjusted cost ($13,147; 95% CI, $3,009–23,284) and postinfection onset hospital LOS (4.9; 95% CI, 3.0–6.9 d) in nvHABP. However, these differences did not persist for the postinfection onset ICU LOS or MV duration (Table 4). In fact, in the vHABP group, there was no difference in either hospital costs or postinfection LOS between the IET and non-IET groups, whereas the effect was present, but small relative to that in nvHABP and VABP, on postinfection ICU LOS. Similar to the unadjusted results, reintubation and extubation failures were significantly more likely in the setting of IET than non-IET in VABP, but not in vHABP (Table 4).
TABLE 4.
Impact of Inappropriate Empiric Therapy on the Outcomes Stratified by Pneumonia Type
| Outcome | Nonventilated Hospital-Acquired Bacterial Pneumonia | Ventilated Hospital-Acquired Bacterial Pneumonia | Ventilator-Associated Bacterial Pneumonia | |||
|---|---|---|---|---|---|---|
| Point Estimate (95% CI) | p | Point Estimate (95% CI) | p | Point Estimate (95% CI) | p | |
| Postinfection onset hospital LOS, d | 4.9 (3.0–6.9) | < 0.001 | 0.6 (–1.3 to 2.5) | 0.559 | 1.5 (–0.2 to 3.2) | 0.075 |
| Postinfection onset ICU LOS, d | 1.1 (0.1–2.2) | 0.029 | 1.4 (0.0–2.9) | 0.056 | 0.4 (–0.6 to 1.4) | 0.406 |
| Hospital costs, $ | $13,147 (3,009–23,284) | < 0.001 | $4,658 (–2,424 to 11,750) | 0.198 | $6,161 (928–11,394) | 0.021 |
| Postinfection onset mechanical ventilation duration, d | 0.5 (–0.3 to 1.2) | 0.259 | 0.8 (–0.2 to 1.9) | 0.104 | ||
| Clostridium difficile | OR = 1.25 (0.58–2.71) | 0.566 | OR = 1.70 (0.86–3.34) | 0.125 | OR = 0.95 (0.58–1.55) | 0.824 |
| Extubation failure | OR = 1.20 (0.84–1.72) | 0.323 | OR = 1.34 (1.05–1.71) | 0.017 | ||
| Reintubationa | OR = 1.11 (0.69–1.81) | 0.66 | OR = 1.61 (1.17–2.20) | 0.003 | ||
LOS = length of stay, OR = odds ratio.
aVentilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia groups only
DISCUSSION
We demonstrate that although IET is an uncommon event in the contemporary population of hospitalized patients with culture-positive bacterial NP, how it impacts outcomes differs by the type of NP. Namely, although VABP is still the costliest NP with the longest overall postinfection onset LOS, the most drastic marginal increase in both the costs and LOS arising in the setting of IET is clearly among patients with nvHABP. Indeed, the adjusted contributions of IET to the total hospital LOS and cost in the setting of nvHABP are nearly 5 days and over $13,000 relative to non-IET. Additionally, IET is associated with worse extubation outcomes in VABP but not in vHABP. Whether IET has any impact on the development of CDI is less obvious. Though the current rates of CDI in all groups are by no means low, their small numbers may obscure the potential impact of IET on its development.
Much of the literature on NP has traditionally differentiated between the ventilator-associated and hospital-acquired, the latter occurring among patients who are not on MV when the infection develops, without subdividing HABP into that which does and that which does not require subsequent MV. Yet more recent studies suggest that there are distinct features associated with each of these types of HABP (15–19). For example, in a single-center study from Spain, Esperatti et al (15) observed that of all pneumonia acquired in the ICU, approximately ½ is VABP, and of the remaining HABP, another ~½ requires subsequent MV, a breakdown similar to ours. They further reported that mortality in vHABP is nearly double that in nvHABP, which is also consistent with our observations, although the overall mortality rates we report are somewhat lower across the board owing likely to regional and patient mix differences (15, 18). Vallecoccia et al (17) confirmed this hierarchy of mortality among the three types of pneumonia in their review of the literature focused on these entities. Our study builds on these previous efforts and expands our understanding of other important outcomes, such as complications and hospital resource utilization, providing further rationale for classifying vHABP and nvHABP as distinct syndromes.
The issue of appropriate empiric treatment has been addressed extensively in many infectious syndromes. Although in such infections as cUTIs and skin and soft-tissue infections the impact of IET on mortality is minimal, it is substantial in pneumonia. Investigations have converged on a two- to three-fold rise in the adjusted risk of death with IET in patients with NP (4–6, 8). Interestingly, in a prior analysis of this same cohort, we reported no detectable association of IET with mortality in any of the pneumonia types (19). This may reflect the fact that contemporary practice features relatively little IET, making it difficult to detect whatever increase in mortality risk it contributes during hospitalization. Alternatively, because we could not distinguish true infection from colonization, what could explain the lack of detected mortality effect is if colonization were overrepresented. However, IET has also been consistently reported to increase resource utilization as well, irrespective of its association with mortality (10, 27, 28). Our current analysis suggests that, even in the absence of increased hospital mortality, IET is not without penalty. That is, it adds substantially to the price tag for a hospitalization that includes NP, most notably in the nvHABP group. This begs the question of whether more attentive and targeted approach to empiric therapy in NP overall and nvHABP more specifically is required.
Our study has a number of limitations and strengths. As an observational study, it is subject to multiple threats to validity, particularly selection bias. Defining the enrollment criteria prospectively mitigates this bias. Misclassification is of particular concern when using administrative data. To deal with this, we used a previously published, though not clinically validated, algorithm that identifies the first episode of pneumonia. We also excluded other potential sources of infection, such as cUTIs and complicated intra-abdominal infections. Although these criteria improved the specificity of our case finding approach, they reduced its sensitivity, and thus likely led to undercounting of cases. Including microbiology specimens from specific sources, pharmacy data, and dates of cultures and treatments further improved the specificity at the expense of sensitivity. Although this strategy likely misclassified some patients, it was nondifferential across the comparator groups and, thus, would have driven the differences between groups toward null. At the same time, it is possible that only the most severe cases of both, HABP and VABP, were coded as pneumonia, in which case the outcomes we report may be worse across the three groups than those seen in clinically defined NP. However, the agreement in mortality rates with other literature lends face validity to our methods (29). The data did not allow us to differentiate between infection and colonization. As stated above, high rates of colonization rather than infection would decrease differences between IET and non-IET groups. Although confounding is present in all observational studies, we developed multivariable models to adjust it away. However, despite a large number of confounders examined, residual confounding remains a concern. As a large multicenter geographically representative database, it is only minimally prone to lack of generalizability. At the same time, our results capture only the events that occur in the hospital and lack such data points as postdischarge death. Because we preferred to err on the side of specificity and required a positive culture and no evidence of cUTIs or complicated intra-abdominal infections, our results may not generalize to the excluded groups. We also did not focus on results of rapid diagnostic testing, thus potentially missing data pertinent to centers that preferentially use this diagnostic modality. Despite these limitations, this is the largest and most contemporary multicenter cohort study to examine NP in the United States.
CONCLUSION
In summary, we have further confirmed that vHABP and nvHABP are distinct from each other and also dissimilar from VABP. We further provide data on the marginal impact of IET in each type of culture-positive NP, including its associated hospital costs. Although the current volume of NP in the United States is not known, if the VABP numbers have not changed over the past 2 decades, it may be reasonable to assume that, in addition to the 300,000 annual VABP cases, there are approximately 150,000 nvHABP and 150,000 vHABP (3). At the current rate of IET and its marginal hospital costs, the aggregate annual costs to the U.S. economy relative to IET in all forms of NP may be as high as $340 million. This estimate excludes both human and financial costs of unnecessarily occupied ICU beds, particularly as our ICUs are already strained by the current pandemic. Our results support the call by Vallecoccia et al (17) for renewed effort to study these inter-NP group differences in future prospective studies.
Footnotes
Drs. M. D. Zilberberg, Puzniak, N. W. D. Zilberberg, and Shorr contributed substantially to the study design, data interpretation, and the writing of the article. Dr. Nathanson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He contributed substantially to the study design, data analysis, and the writing of the article.
Supported, in part, by a grant from Merck Sharp & Dohme, a subsidiary of Merck, Kenilworth, NJ.
Portions of these data have been presented as posters at the IDWeek, September 29–October 3, 2021, virtual conference, and published or accepted for publication in Critical Care Medicine (reference 18) and Infection Control & Hospital Epidemiology (reference 19).
Dr. M. D. Zilberberg’s employer, EviMed Research Group, LLC, has received research grant support from Merck Sharp & Dohme, a subsidiary of Merck, Kenilworth, NJ. Dr. Nathanson’s employer, OptiStatim, LLC, has received support from EviMed Research Group, LLC, Goshen, MA. Dr. Puzniak is an employee of Merck Sharp & Dohme, a subsidiary of Merck, and a stockholder in Merck. Dr. Zilberberg has no conflicts to report. Dr. Shorr is a consultant to and has received research grant support from of Merck Sharp & Dohme, a subsidiary of Merck. Drs. M. D. Zilberberg and Shorr have received grant support and/or have served as consultants to Lungpacer, Melinta, Tetraphase, Pfizer, Astellas, Shionogi, The Medicines Company, Spero, and Theravance.
No party other than the listed authors was involved in the design, conduct, or reporting of the study.
The data used in this study derive from Premier Research database, a proprietary third-party database available to researchers through a specific agreement with Premier.
REFERENCES
- 1.IBM Watson Health. Hospital-Acquired Conditions Lead to Avoidable Cost and Excess Deaths. 2016. Available at: https://www.ibm.com/downloads/cas/X97QXLER. Accessed August 25, 2021
- 2.Agency for Healthcare Research and Quality: Estimating the Additional Hospital Inpatient Cost and Mortality Associated With Selected Hospital-Acquired Conditions. 2017. Available at: https://www.ahrq.gov/hai/pfp/haccost2017.html. Accessed August 20, 2021
- 3.Koenig SM, Truwit JD: Ventilator-associated pneumonia: Diagnosis, treatment, and prevention. Clin Microbiol Rev 2006; 19:637–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iregui M, Ward S, Sherman G, et al. : Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 2002; 122:262–268 [DOI] [PubMed] [Google Scholar]
- 5.Zilberberg MD, Shorr AF, Micek ST, et al. : Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: A single-center experience. Chest 2008; 134:963–968 [DOI] [PubMed] [Google Scholar]
- 6.Micek ST, Kollef KE, Reichley RM, et al. : Health care-associated pneumonia and community-acquired pneumonia: A single-center experience. Antimicrob Agents Chemother 2007; 51:3568–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zilberberg MD, Shorr AF, Micek ST, et al. : Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Lerma F: Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med 1996; 22:387–394 [DOI] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Carlet JM, et al. ; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine: Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36:296–32718158437 [Google Scholar]
- 10.Shorr AF, Micek ST, Welch EC, et al. : Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 2011; 39:46–51 [DOI] [PubMed] [Google Scholar]
- 11.Kollef MH, Sherman G, Ward S, et al. : Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest 1999; 115:462–474 [DOI] [PubMed] [Google Scholar]
- 12.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, et al. : Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med 2003; 31:2742–2751 [DOI] [PubMed] [Google Scholar]
- 13.Harbarth S, Garbino J, Pugin J, et al. : Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 2003; 115:529–535 [DOI] [PubMed] [Google Scholar]
- 14.Ferrer R, Artigas A, Suarez D, et al. ; Edusepsis Study Group: Effectiveness of treatments for severe sepsis: A prospective, multicenter, observational study. Am J Respir Crit Care Med 2009; 180:861–866 [DOI] [PubMed] [Google Scholar]
- 15.Esperatti M, Ferrer M, Theessen A, et al. : Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med 2010; 182:1533–1539 [DOI] [PubMed] [Google Scholar]
- 16.Talbot GH, Das A, Cush S, et al. ; Foundation for the National Institutes of Health Biomarkers Consortium HABP/VABP Project Team: Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J Infect Dis 2019; 219:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallecoccia MS, Dominedo C, Cutuli SL, et al. : Is ventilated hospital-acquired pneumonia a worse entity then ventilator-associated pneumonia? Eur Resp Rev 2020; 29:200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Nathanson BH, Puzniak LA, et al. : Descriptive epidemiology and outcomes of nonventilated hospital-acquired, ventilated hospital-acquired, and ventilator-associated bacterial pneumonia in the United States, 2014-2019. Crit Care Med 2022; 50:460-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilberberg MD, Nathanson BH, Puzniak LA, et al. : Microbiology, empiric therapy and its impact on the outcomes of nonventilated hospital-acquired, ventilated hospital-acquired, and ventilator-associated bacterial pneumonia in the United States, 2014-2019. Infect Control Hosp Epidemiol 2022; 43:277-283 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services Office for Human Research Protections. Human Subject Regulations Decision Charts. 2020.. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts/index.html. Accessed February 3, 2021.
- 21.Zilberberg MD, Nathanson BH, Sulham K, et al. : A novel algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: A retrospective cohort study. Chest 2019; 155:1119–1130 [DOI] [PubMed] [Google Scholar]
- 22.Zilberberg MD, Nathanson BH, Sulham K, et al. : Antimicrobial susceptibility and cross-resistance patterns among common complicated urinary tract infections in U.S. hospitals, 2013 to 2018. Antimicrob Agents Chemother 2020; 64:e00346–e00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zilberberg MD, Nathanson BH, Ditch K, et al. : Carbapenem treatment and outcomes among patients with culture-positive complicated intra-abdominal infections in US hospitals: A retrospective cohort study. Open Forum Infect Dis 2019; 6:ofz504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothberg MB, Pekow PS, Priya A, et al. : Using highly detailed administrative data to predict pneumonia mortality. PLoS One 2014; 9:e87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothberg MB, Haessler S, Lagu T, et al. : Outcomes of patients with healthcare-associated pneumonia: Worse disease or sicker patients? Infect Control Hosp Epidemiol 2014; 35(Suppl 3):S107–S115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagu T, Stefan MS, Haessler S, et al. : The impact of hospital-onset Clostridium difficile infection on outcomes of hospitalized patients with sepsis. J Hosp Med 2014; 9:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zilberberg MD, Nathanson BH, Sulham K, et al. : 30-day readmission, antibiotics costs and costs of delay to adequate treatment of Enterobacteriaceae UTI, pneumonia, and sepsis: A retrospective cohort study. Antimicrob Resist Infect Control 2017; 6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zilberberg MD, Shorr AF, Micek ST, et al. : Hospitalizations with healthcare-associated complicated skin and skin structure infections: Impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540 [DOI] [PubMed] [Google Scholar]
- 29.Kalil AC, Metersky ML, Klompas M, et al. : Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]