This randomized clinical trial compares the clinical response to Gram stain–guided vs guideline-based antibiotic therapy in patients with ventilator-associated pneumonia.
Key Points
Question
Does Gram stain–guided antibiotic therapy restrict the administration of broad-spectrum antibiotic agents for ventilator-associated pneumonia without detrimental effects on patient outcomes?
Findings
In this randomized clinical trial that included 206 patients with ventilator-associated pneumonia in the intensive care unit, the clinical response to Gram stain–guided antibiotic therapy was noninferior to that of guideline-based antibiotic therapy (76.7% vs 71.8%). Gram stain–guided antibiotic therapy reduced the use of antipseudomonal agents and anti–methicillin-resistant Staphylococcus aureus agents.
Meaning
The findings of this trial suggest that Gram staining can be used in the critical care setting to ameliorate the spread of multidrug-resistant pathogens.
Abstract
Importance
Gram staining should provide immediate information for detecting causative pathogens. However, the effect of Gram staining on restricting the initial antibiotic choice has not been investigated in intensive care units (ICUs).
Objective
To compare the clinical response to Gram stain–guided restrictive antibiotic therapy vs guideline-based broad-spectrum antibiotic treatment in patients with ventilator-associated pneumonia (VAP).
Design, Setting, and Participants
This multicenter, open-label, noninferiority randomized clinical trial (Gram Stain-Guided Antibiotics Choice for VAP) was conducted in the ICUs of 12 tertiary referral hospitals in Japan from April 1, 2018, through May 31, 2020. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included. The primary analysis was based on the per-protocol analysis population.
Interventions
Patients were randomized to Gram stain–guided antibiotic therapy or guideline-based antibiotic therapy (based on the 2016 Infectious Disease Society of America and American Thoracic Society clinical practice guidelines for VAP).
Main Outcomes and Measures
The primary outcome was the clinical response rate; clinical response was defined as completion of antibiotic therapy within 14 days, improvement or lack of progression of baseline radiographic findings, resolution of signs and symptoms of pneumonia, and lack of antibiotic agent readministration, with a noninferiority margin of 20%. Secondary outcomes were the proportions of antipseudomonal agents and anti–methicillin-resistant Staphylococcus aureus (MRSA) agents as initial antibiotic therapies; 28-day mortality, ICU-free days, ventilator-free days; and adverse events.
Results
In total, 206 patients (median [IQR] age, 69 [54-78] years; 141 men [68.4%]) were randomized to the Gram stain–guided group (n = 103) or guideline-based group (n = 103). Clinical response occurred in 79 patients (76.7%) in the Gram stain–guided group and 74 patients (71.8%) in the guideline-based group (risk difference, 0.05; 95% CI, –0.07 to 0.17; P < .001 for noninferiority). Reduced use of antipseudomonal agents (30.1%; 95% CI, 21.5%-39.9%; P < .001) and anti-MRSA agents (38.8%; 95% CI, 29.4%-48.9%; P < .001) was observed in the Gram stain–guided group vs guideline-based group. The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain–guided group vs 17.5% (n = 18) in the guideline-based group (P = .39). Escalation of antibiotics according to culture results was performed in 7 patients (6.8%) in the Gram stain–guided group and 1 patient (1.0%) in the guideline-based group (P = .03). There were no significant differences between the groups in ICU-free days, ventilator-free days, and adverse events.
Conclusions and Relevance
Results of this trial showed that Gram stain–guided treatment was noninferior to guideline-based treatment and significantly reduced the use of broad-spectrum antibiotics in patients with VAP. Gram staining can potentially ameliorate the multidrug-resistant organisms in the critical care setting.
Trial Registration
ClinicalTrials.gov Identifier: NCT03506113
Introduction
The rapid spread of multidrug-resistant (MDR) organisms in intensive care units (ICUs) is recognized as a worldwide health threat,1,2 but development of new pharmaceutical agents is lagging, leaving a decreasing stock of effective antibiotics against these organisms.3,4 Therefore, a global action plan to address antimicrobial resistance was devised by the World Health Organization to optimize the use of antibiotic agents.5
Patients in the ICU are among the largest consumers of antibiotics in the hospital because they are at an increased risk for infection owing to underlying conditions, impaired immunity, and exposure to multiple invasive devices.6 Several studies found that 50% to 70% of patients in the ICU had infections and were given antibiotics during their ICU stay.7,8,9 Among the threats of infection in ICUs, ventilator-associated pneumonia (VAP) is an important complication that necessitates antibiotics in approximately 10% of patients undergoing mechanical ventilation and that results in an estimated attributable mortality of 15% to 50%.10,11,12 The 2016 clinical practice guidelines for VAP published by the Infectious Disease Society of America and American Thoracic Society recommend coverage for both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment.13 However, overuse of broad-spectrum antibiotic agents because of these guidelines could be associated with the accelerated emergence of antimicrobial-resistant organisms.14 Thus, establishing methods to safely reduce overuse of broad-spectrum antibiotic agents for VAP is a pressing challenge.
Gram staining of respiratory samples, an inexpensive, simple examination that is used worldwide, including in lower-income countries, may guide the appropriate use of initial antibiotic therapy. Several studies evaluated the accuracy of Gram staining in detecting causative pathogens, but their results were conflicting.15,16,17,18,19 Furthermore, the question of whether Gram staining results are accurate enough to safely restrict the use of broad-spectrum antibiotics remains controversial. Therefore, we conducted the Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP) trial to compare the clinical response to Gram stain–guided restrictive antibiotic treatment vs guideline-based broad-spectrum antibiotic treatment in patients with VAP.
Methods
Design, Setting, and Patients
The GRACE-VAP trial was a multicenter, open-label, noninferiority randomized clinical trial with blinded end point assessment that was conducted from April 1, 2018, through May 31, 2020. The trial protocol (provided in Supplement 1 and a protocol paper20) was concordant with the Declaration of Helsinki21 and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan and was approved by the institutional review boards of Osaka General Medical Center and each of the participating institutions. Written informed consent was obtained from all participants or their representatives. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
The trial recruited patients from ICUs of 12 tertiary referral hospitals in Japan (Chukyo Hospital, Nagoya, Aichi; Ebina General Hospital, Ebina, Kanagawa; Hitachi General Hospital, Hitachi, Ibaraki; Kansai Medical University Hospital, Hirakata, Osaka; Kansai Medical University Medical Center, Moriguchi, Osaka; Nagasaki University Hospital, Nagasaki; Osaka General Medical Center, Osaka; Saga University Hospital, Saga; Sapporo City General Hospital, Sapporo, Hokkaido; Tajima Emergency and Critical Care Medical Center, Toyooka, Hyogo; University of the Ryukyus Hospital, Nakagami-gun, Okinawa; and Wakayama Medical University Hospital, Wakayama) (eTable 1 in Supplement 2). An independent data center and Data and Safety Monitoring Board provided trial oversight (eTable 2 in Supplement 2).
Patients were eligible if they were 15 years or older; had undergone mechanical ventilation for at least 48 hours; and had been diagnosed with VAP, defined as a modified Clinical Pulmonary Infection Score (mCPIS) of 5 or higher (score range: 0-10, with higher scores indicating greater likelihood of VAP).22 The exclusion criteria were known allergy to study medications, pregnancy, discharge from ICU, heart failure or atelectasis diagnosis, receipt of antibiotics for more than 24 hours after meeting the inclusion criteria, withdrawal or withholding of life support, or the discretion of the site investigator. We also excluded patients who were diagnosed with COVID-19 infection as an ex-post criterion when COVID-19 became pandemic in February 2020.
Data on race and ethnicity were not collected. It was presumed that only Japanese citizens would participate in the trial.
Randomization and Blinding
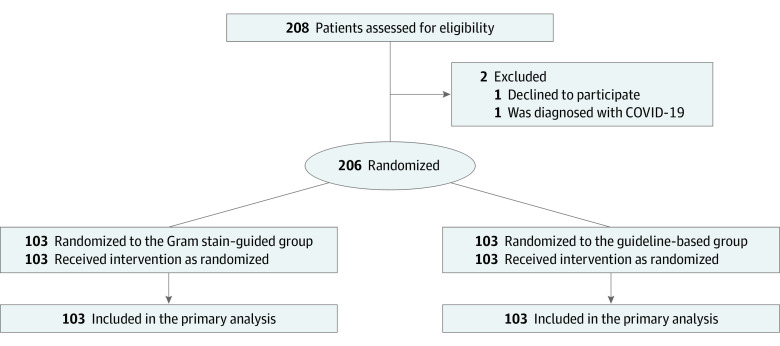
Patients were randomized to receive either Gram stain–guided antibiotic treatment or guideline-based standard treatment (Figure 1). Randomization was performed with a stochastic minimization procedure and was stratified by study center; presence of chronic obstructive pulmonary disease, traumatic brain injury (TBI), and postcardiopulmonary arrest syndrome; and previous antibiotic therapy during the hospitalization. We used an electronic data capture system to conduct randomization and data collection. Site investigators were not blinded to the group randomization.
Figure 1. Study Flow Diagram.
Interventions
Patients in the Gram stain–guided group received antibiotics according to the results of Gram staining of endotracheal aspirate (eFigure 1 in Supplement 2).15,16,23 Gram staining was performed in the laboratory in each ICU or the microbiology laboratory in each institution just after respiratory samples for sputum culture were collected. Bronchoalveolar lavage was not performed to obtain respiratory samples. Gram stain was performed by the Favor method, in which heat-fixed smears on slides were flooded with 0.2% Victoria blue for 30 seconds and washed with tap water, smears were decolorized with 2% picric acid ethanol, and cells were counterstained with 0.004% fuchsin for 30 seconds and then washed with tap water.15 Respiratory sample quality was assessed by Miller and Jones classification and Geckler classification. We categorized the Gram stain results as gram-positive cocci (GPC) chains, GPC clusters, gram-positive bacilli, gram-negative rods (GNR), or a combination thereof.15,16 A nonpseudomonal β-lactam antibiotic was administered when Gram stain results showed only GPC chains and/or gram-positive bacilli. An anti-MRSA agent was administered when Gram stain results showed GPC clusters without GNR. An antipseudomonal agent was administered when Gram stain results showed GNR without GPC clusters. The combination of an antipseudomonal agent and anti-MRSA agent was administered when Gram stain results showed both GPC clusters and GNR. The most broad-spectrum antibiotic agent among the categories was administered when Gram stain results showed 2 or more categories. We administered the combination of an antipseudomonal agent and an anti-MRSA agent when the Gram stain did not show any microorganisms.
Patients in the guideline-based group received the combination of an antipseudomonal agent and an anti-MRSA agent according to the 2016 VAP guidelines.13 We did not administer 2 antipseudomonal agents from different classes as initial antibiotic therapy in either group because P. aeruginosa remained susceptible to antipseudomonal β-lactam (80%-90%), and the frequency of MDR GNR was low (<10%) in all participating institutions (eTable 3 in Supplement 2).
In both groups, escalation of the initial treatment selection was performed to cover all pathogens that were isolated from respiratory samples before the onset of VAP.16 We selected specific antibiotic agents according to previously recorded antimicrobial resistance patterns in each ICU (eTable 3 in Supplement 2).
The study medication could be de-escalated or escalated to a definitive treatment level according to the pathogens that were isolated from respiratory samples. The site investigator adjusted dose regimens according to individual patient status. Study medications were continued for at least 7 days and discontinued at the discretion of the site investigator.
Outcomes
We performed clinical assessments at baseline and daily throughout the study treatment, at the end of therapy, and at the follow-up or test-of-cure visit (7 days after the end of therapy). The primary outcome was the clinical response rate at the follow-up or test-of-cure visit. Clinical response was defined as meeting the following 4 outcomes: completion of antibiotic therapy within 14 days, improvement or lack of progression of baseline radiographic findings at the end of therapy, resolution of signs and symptoms of pneumonia (exacerbation of fever, increased oxygen dose, and cough) at the follow-up or test-of-cure visit, and lack of antibiotic agent readministration owing to pneumonia by the follow-up or test-of-cure visit.
The secondary outcomes were 28-day mortality; 28-day ICU-free days; 28-day ventilator-free days; proportions of antipseudomonal agents and anti-MRSA agents as initial antibiotic therapies; coverage rate of initial antibiotic therapies; de-escalation rate; antibiotic therapy days; and adverse events, including kidney impairment, thrombocytopenia, diarrhea, Clostridioides difficile infection, skin rash, and seizure. Kidney impairment was defined as both an increase in creatinine level to at least 1.5 times over baseline within the follow-up or test-of-cure period, with the worst creatinine level being 1.0 mg/dL or higher. We considered the therapy appropriate when all pathogens that were isolated with at least 1 semiquantitative growth from respiratory samples were covered by the selected antibiotic agents.
The site investigators collected information that was relevant to the outcomes. Each member of the event adjudication committee, who was blinded to the group randomization, independently judged the primary and secondary outcomes according to the individual data. When any disagreements occurred among the committee members, a consensus was reached by discussion.
Statistical Analysis
The primary efficacy analysis assessed the noninferiority of the clinical response in the Gram stain–guided group compared with the guideline-based group. Details of the statistical analyses were described previously,20 and the statistical analysis plan was fixed before the enrollment of the final patient.
Sample size was based on an assumed clinical response rate of 67.8% to both the guideline-based treatment and the Gram stain–guided treatment, as determined in a previous study,16 and a 20% noninferiority margin. This trial required 86 patients per group to achieve 80% power with a 1-sided α = 2.5%. Assuming a 10% rate of nonevaluable patients, we decided to enroll 100 patients per group.
Categorical variables were expressed as frequencies with percentages, and continuous variables were expressed as medians with IQRs. Because the noninferiority of the primary outcome was assessed, the primary analysis was performed in the per-protocol analysis population, which consisted of all randomized patients who were not lost to follow-up and had no major protocol deviations. We verified the noninferiority of the primary outcome using the normal theory test for binomial proportions. We performed a sensitivity analysis for primary efficacy analysis in the patients with an mCPIS of 5 or higher because some patients who were considered eligible at randomization were found to have an mCPIS of 4 after data monitoring.
Secondary outcomes were analyzed under a superiority assumption, which was based on the intention-to-treat principle. The cumulative incidence of mortality at 28 days was estimated with the Kaplan-Meier method, and the differences between groups were assessed by log-rank test. The hazard ratios and 95% CIs for mortality in the Gram stain–guided group vs the guideline-based group were estimated with a Cox proportional hazards regression model. Other secondary outcomes were compared using the χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables.
Predefined subgroup analyses for the primary outcome were conducted with the same method used in the primary efficacy analysis. The subgroup factors, which affected a priori the bacterial flora of a patient’s oral cavity and airway or were related to patient severity, included the presence of previous antibiotic therapy during hospitalization, length of ICU stay before randomization (≥5 or <5 days), comorbidity of chronic obstructive pulmonary disease, TBI, postcardiopulmonary arrest syndrome, presence of sepsis, development of acute kidney injury, and Acute Physiology and Chronic Health Evaluation II score (≥20 or <20; score range: 0-71, with higher scores indicating a higher risk of death). Because few patients had septic shock in the trial, we changed the predefined subgroup from septic shock to sepsis.
All P values were 2-sided, and P < .05 was considered to be significant except in the noninferiority test for clinical response, for which a 1-sided P < .025 was considered to be significant. All statistical analyses were performed with Stata, version 16.1 (StataCorp LLC) and SAS, version 9.4 (SAS Institute Inc).
Results
Of the 208 patients with VAP who were assessed for eligibility, 2 were excluded (1 for declining participation, and 1 for a COVID-19 diagnosis). As a result, we randomized 206 patients, 103 of whom were randomized to the Gram stain–guided group and 103 to the guideline-based group (Figure 1). The sample had a median (IQR) age of 69 (54-78) years and was composed of 141 men (68.4%) and 65 women (31.6%). The median (IQR) hospital stay from ICU admission to randomization was 4 (4-6) days. Demographic data, reason for ICU admission, morbidities, and severity of illness were well balanced between the 2 groups (Table 1). Staphylococcus aureus (n = 103 [50.0%]) was the most frequently isolated bacteria from endotracheal aspirate, followed by Klebsiella spp (34 [16.5%]) and Haemophilus influenza (20 [9.7%]) (eTable 4 in Supplement 2). Summaries of the Gram staining results and selected antibiotic agents are shown in eTables 5 and 6 in Supplement 2.
Table 1. Baseline Characteristics of Patientsa.
| Characteristic | No. (%) | |
|---|---|---|
| Gram stain–guided group (n = 103) | Guideline-based group (n = 103) | |
| Age, median (IQR), y | 69 (54-78) | 69 (54-78) |
| Female sex | 37 (35.9) | 28 (27.2) |
| Male sex | 66 (64.1) | 75 (72.8) |
| Diagnosis on ICU admission | ||
| Trauma | 28 (27.2) | 27 (26.2) |
| Postcardiopulmonary arrest syndrome | 25 (24.3) | 24 (23.3) |
| Stroke | 11 (10.7) | 10 (9.7) |
| Other | 39 (37.9) | 42 (40.8) |
| mCPIS, median (IQR)b | ||
| Overall | 6 (5-7) | 6 (5-7) |
| Temperature | 1 (0-2) | 1 (0-2) |
| Leukocytes | 0 (0-1) | 0 (0-2) |
| Pao2/Fio2 | 0 (0-2) | 0 (0-2) |
| Chest radiograph | 2 (2-2) | 2 (2-2) |
| Tracheal secretions | 2 (2-2) | 2 (2-2) |
| Comorbidities | ||
| Diabetes | 27 (26.2) | 26 (25.2) |
| Chronic | ||
| Heart failure | 22 (21.4) | 16 (15.5) |
| Respiratory disorder | 6 (5.8) | 6 (5.8) |
| Hemodialysis | 5 (4.9) | 5 (4.9) |
| Liver cirrhosis | 4 (3.9) | 1 (1.0) |
| Immunocompromised | 3 (2.9) | 4 (3.9) |
| Previous antibiotic therapy | 30 (29.1) | 27 (26.2) |
| Length of ICU stay before randomization, d | ||
| ≥5 | 53 (51.5) | 49 (47.6) |
| <5 | 50 (48.5) | 54 (52.4) |
| Sepsis | 36 (35.0) | 33 (32.0) |
| Septic shock | 6 (5.8) | 3 (2.9) |
| Acute kidney injuryc | 20 (19.4) | 13 (12.6) |
| APACHE II score, median (IQR)d | 18 (14-24) | 19 (15-23) |
| SOFA score, median, (IQR)e | 7 (5-9) | 5 (7-9) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; Fio2, fraction of inspired oxygen; mCPIS, modified Clinical Pulmonary Infection Score; Pao2, partial pressure of oxygen; SOFA, Sequential Organ Failure Assessment.
Percentages may not total to 100 because of rounding.
The mCPIS ranges from 0 to 10, with higher scores indicating greater likelihood of VAP.
Acute kidney injury was diagnosed according to the Kidney Disease: Improving Global Outcomes definition.
APACHE II scores range from 0 to 71, with higher scores indicating a higher risk of death.
SOFA scores range from 0 to 24 (from 0 to 4 for each of the 6 organ systems), with higher scores indicating more severe organ dysfunction.
Outcomes
Per-protocol analysis showed that, at the follow-up or test-of-cure visit, 79 patients (76.7%) in the Gram stain–guided group had a clinical response compared with 74 patients (71.8%) in the guideline-based group (risk difference, 0.05; 95% CI, –0.07 to 0.17; P < .001 for noninferiority) (Table 2). Sensitivity analysis after excluding 12 patients with an mCPIS of 4 (5 in the Gram stain–guided group and 7 in the guideline-based group) yielded results that were similar to that of the primary analysis (risk difference, 0.06; 95% CI, –0.07 to 0.18; P < .001 for noninferiority). Clinical response rates at the participating institutions are shown in eTable 7 in Supplement 2.
Table 2. Primary and Secondary Outcomes.
| Outcome | No. (%) | P value | |
|---|---|---|---|
| Gram stain–guided group (n = 103) | Guideline-based group (n = 103) | ||
| Primary outcome | |||
| Clinical response rate | 79 (76.7) | 74 (71.8) | <.001a |
| Completion of antibiotic therapy within 14 db | 98 (95.1) | 94 (91.3) | NA |
| Improvement or lack of progression of baseline radiographic findingsb | 85 (82.5) | 78 (75.7) | NA |
| Resolution of signs and symptoms of pneumoniab | 87 (84.5) | 85 (82.5) | NA |
| Lack of antibiotic agent readministrationb | 85 (82.5) | 85 (82.5) | NA |
| Secondary outcomes | |||
| 28-d mortality | 14 (13.6) | 18 (17.5) | .44 |
| 28-d ventilator-free days, median (IQR) | 22 (15-24) | 22 (18-25) | .21 |
| 28-d ICU-free days, median (IQR) | 19 (15-22) | 20 (16-23) | .42 |
| Administration of antibiotic therapy | |||
| Antipseudomonal agents | 72 (69.9) | 103 (100) | <.001 |
| Anti-MRSA agents | 63 (61.2) | 103 (100) | <.001 |
| Coverage rate of initial antibiotic therapy | 89 (86.4) | 95 (92.2) | .18 |
| Escalationb | 7 (6.8) | 1 (1.0) | .03 |
| De-escalation | 67 (65.0) | 79 (76.7) | .07 |
| Antibiotic therapy days until de-escalation, median (IQR) | 3 (2-4) | 3 (2-4) | .22 |
| Antibiotic therapy days, median (IQR) | 8 (7-11) | 8 (7-11) | .09 |
Abbreviations: ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
The noninferiority of the primary outcome was analyzed using the normal theory test for binomial proportions.
Added post hoc.
The cumulative incidence of mortality at 28 days was 13.6% (n = 14) in the Gram stain–guided group and 17.5% (n = 18) in the guideline-based group (P = .39) (eFigure 2 in Supplement 2). The hazard ratio of the Gram stain–guided group was 0.74 (95% CI, 0.37-1.48; P = .39). We observed reduced use of antipseudomonal agents (30.1%; 95% CI, 21.5%-39.9%; P < .001) and anti-MRSA agents (38.8%; 95% CI, 29.4%-48.9%; P < .001) in the Gram stain–guided group vs the guideline-based group (Table 2). Gram staining showed a sensitivity of 83.5% and specificity of 75.7% in detecting S. aureus (κ = 0.59) and a sensitivity of 83.0% and specificity of 60.7% in detecting gram-negative pathogens (κ = 0.43). Concordance between the results of Gram staining and culture is shown in eTable 8 in Supplement 2. There was no significant difference in coverage rates of initial antibiotic therapies between the groups (86.4% [89 of 103] vs 92.2% [95 of 103]; P = .18). Escalation of antibiotics according to culture results was performed in 7 patients (6.8%) in the Gram stain–guided group and 1 patient (1.0%) in the guideline-based group (P = .03). We observed no significant differences between the groups for all other secondary outcomes (Table 2).
Subgroup Analyses
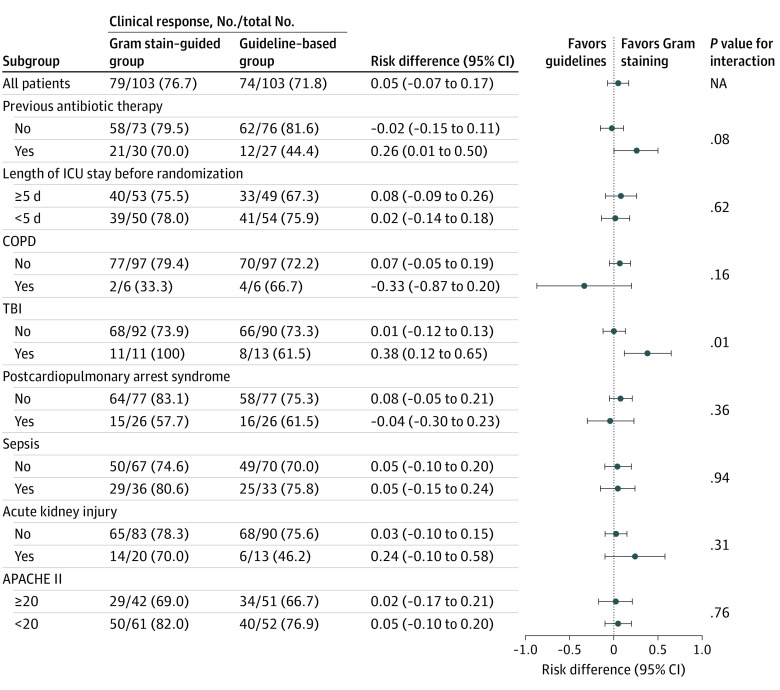
Among patients with TBI, the clinical response rate was 100% (n = 11) in the Gram stain–guided group and 61.5% (n = 13) in the guideline-based group. There was substantial heterogeneity in the effect of Gram stain–guided therapy on the clinical response in patients with TBI (risk difference, 0.38; 95% CI, 0.12-0.65; P = .01 for interaction). However, none of the prespecified subgroups showed substantial heterogeneity other than patients with TBI (Figure 2). Among patients with previous antibiotic therapy, the clinical response rate tended to be more favorable with the Gram stain–guided treatment than the guideline-based treatment (risk difference, 0.26; 95% CI, 0.01-0.50; P = .08 for interaction), although the interaction between an intervention effect and the existence of previous antibiotic therapy did not reach statistical significance. According to the length of ICU stay before randomization, the occurrence of a clinical response was similar between the 2 groups among both the patients with an ICU stay of 5 or more days and those with an ICU stay of fewer than 5 days.
Figure 2. Clinical Response Rate in Prespecified Subgroups.
APACHE indicates Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; NA, not applicable; and TBI, traumatic brain injury.
Adverse Events
There were 69 adverse events in the Gram stain–guided group and 79 in the guideline-based group (Table 3). The most common adverse event was diarrhea (27 of 103 [26.2%] vs 38 of 103 [36.9%]), followed by kidney impairment (17 [16.5%] vs 19 [18.4%]) and thrombocytopenia (16 [15.6%] vs 11 [10.7%]). Among patients with diarrhea, C. difficile infection was noted in 4 patients (1 [1.0%] vs 3 [2.9%]).
Table 3. Adverse Events.
| Adverse event | No. (%) | |
|---|---|---|
| Gram stain–guided group (n = 103) | Guideline-based group (n = 103) | |
| Kidney impairment | 17 (16.5) | 19 (18.4) |
| Thrombocytopenia | ||
| Moderate | 12 (11.7) | 4 (3.9) |
| Severe | 4 (3.9) | 7 (6.8) |
| Diarrhea | 27 (26.2) | 38 (36.9) |
| Clostridioides difficile infection | 1 (1.0) | 3 (2.9) |
| Skin rash | 3 (2.9) | 3 (2.9) |
| Seizure | 5 (4.9) | 5 (4.9) |
Discussion
The GRACE-VAP trial addressed the effectiveness of Gram staining to safely restrict overuse of broad-spectrum antibiotic agents in critically ill patients with VAP. Gram stain–guided restrictive antibiotic therapy was noninferior to guideline-based broad-spectrum antibiotic therapy in patients with VAP in terms of the clinical response rate. Gram staining of endotracheal aspirates optimized the use of broad-spectrum antibiotic agents for VAP without detrimental effects on patient outcomes.
Antimicrobial resistance resulting from the overuse of antibiotics is arguably one of the greatest threats to global human health. Various procedures have been proposed to improve antimicrobial stewardship in patients with suspected pneumonia, including VAP.24,25,26,27,28 A major approach to optimizing antibiotic choice and minimizing unnecessary administration of antibiotics is molecular point-of-care testing. Several randomized clinical trials have been conducted to evaluate the efficacy of such tests, but the results have been conflicting.26,27,28 Moreover, these tests are expensive and require precision measurement instruments, and not all medical facilities can install such expensive equipment. In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide.
Antibiotic choice in the guideline-based group was based on the 2016 VAP guidelines. Alternatively, the European guidelines recommend a different algorithm based on MDR risk factors for selecting antibiotic agents.29 Although this algorithm can certainly limit broad-spectrum antibiotic use, it might lead to an increase in inappropriate antibiotic choice.30 We estimated antibiotic choices and inappropriate coverage rate using the European guidelines for the patients in the guideline-based group. The European guidelines limited broad antibiotic use; however, the appropriate coverage rate also decreased considerably. If we applied the European guidelines to the patients in the guideline-based group, the lower coverage rate might result in worse outcomes for this group. Therefore, the findings should be considered robust when the European guidelines are used, but this estimation should be verified in clinical settings.
Previous antibiotic therapy can influence the results of Gram staining. Antibiotic exposure can change bacterial flora in respiratory samples, making it difficult for physicians to identify causative pathogens. An observational study found that culture positivity was reduced by 20% in blood culture specimens that were obtained during antibiotic therapy.31 However, in the subgroup analysis of the present trial, we did not observe any adverse effects on the clinical response attributable to previous antibiotic therapy.
Limitations
This trial has several limitations. First, the open-label design may have influenced the behavior of the site investigator in charge. However, the schedule of clinical assessments with objective measurements was prefixed, and outcomes were assessed by independent members of the event adjudication committee, who were blinded to the group randomization. Moreover, there was no difference in the secondary end point of 28-day mortality, which was least affected by the open-label design. Second, we set the noninferiority margin at 20%, which was consistent with that in a previous trial,32 but the magnitude of 20% may have been too large for clinicians and the sample size was not large enough to be conclusive. The trial was conducted as planned, and the lower confidence limit of the risk difference of clinical response (−0.07) was sufficiently far from the noninferiority margin of −0.2. Moreover, the incidence of the primary outcome was observed as assumed, and all secondary outcomes and subgroup analyses supported the evidence, suggesting that the findings in the trial could be considered robust. Third, the generalizability of the trial should be considered because of the setting. Namely, the frequency of MDR GNR was low (<10%) at all participating institutions, and nonfermenters, including Pseudomonas aeruginosa and Acinetobacter baumannii, that were isolated from respiratory samples were lower than expected. Moreover, few of the trial participants had septic shock or an extremely low partial pressure of oxygen to fraction of inspired oxygen ratio. Therefore, Gram staining may not be suitable in clinical practice in patients with the most severe VAP or in hospitals or regions in which the frequency of MDR GNR is very high.
Conclusions
This GRACE-VAP trial found that Gram stain–guided treatment compared with guideline-based treatment significantly restricted the use of broad-spectrum antibiotics without detrimental effects and yielded noninferior clinical responses in patients with VAP in the ICU. This finding reinforces the potential use of Gram staining in the critical care setting to ameliorate the spread of MDR pathogens.
Trial Protocol
eTable 1. Participating Institutions and Investigators
eTable 2. Members of the Data and Safety Monitoring Board
eTable 3. Antibiogram of Participating Institutions
eTable 4. Microorganisms Isolated in Respiratory Samples
eTable 5. Summary of the Results of Gram Staining
eTable 6. Summary of Selected Antibiotic Agents
eTable 7. Clinical Response of Participating Institutions
eTable 8. Concordance Between Results of Gram Staining and Culture
eFigure 1. Interventions
eFigure 2. Twenty-Eight-Day Mortality in the Gram Stain–guided Group and Guideline-based Group
Data Sharing Statement
References
- 1.Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241. doi: 10.1177/2042098614554919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. World Health Organization ; 2014. Accessed April 20, 2021. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf
- 3.Wenzel RP. The antibiotic pipeline—challenges, costs, and values. N Engl J Med. 2004;351(6):523-526. doi: 10.1056/NEJMp048093 [DOI] [PubMed] [Google Scholar]
- 4.Cassell GH, Mekalanos J. Development of antimicrobial agents in the era of new and reemerging infectious diseases and increasing antibiotic resistance. JAMA. 2001;285(5):601-605. doi: 10.1001/jama.285.5.601 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Global Action Plan on Antimicrobial Resistance. World Health Organization ; 2015. Accessed April 20, 2021. http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf
- 6.Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47. doi: 10.1186/2110-5820-1-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators . Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353. doi: 10.1097/01.CCM.0000194725.48928.3A [DOI] [PubMed] [Google Scholar]
- 8.Alfandari S, Robert J, Péan Y, et al. ; French Infectious Diseases Society (French acronym SPILF), the French National Observatory for Epidemiology of Bacterial Resistance to Antibiotics (ONERBA), and the SPA2 group . Antibiotic use and good practice in 314 French hospitals: the 2010 SPA2 prevalence study. Med Mal Infect. 2015;45(11-12):475-480. doi: 10.1016/j.medmal.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Rello J, Marshall J, et al. ; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-2329. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Eldridge N, Metersky ML, et al. National trends in patient safety for four common conditions, 2005-2011. N Engl J Med. 2014;370(4):341-351. doi: 10.1056/NEJMsa1300991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184-2193. doi: 10.1097/01.CCM.0000181731.53912.D9 [DOI] [PubMed] [Google Scholar]
- 12.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665-671. doi: 10.1016/S1473-3099(13)70081-1 [DOI] [PubMed] [Google Scholar]
- 13.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. doi: 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057-1098. doi: 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura J, Kinoshita T, Yamakawa K, et al. Impact of Gram stain results on initial treatment selection in patients with ventilator-associated pneumonia: a retrospective analysis of two treatment algorithms. Crit Care. 2017;21(1):156. doi: 10.1186/s13054-017-1747-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura J, Yamakawa K, Kinoshita T, Fujimi S. Gram stain-guided antibiotic choice: a Graceful method to safely restrict overuse of broad-spectrum antibiotic agents. Crit Care. 2018;22(1):338. doi: 10.1186/s13054-018-2270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seligman R, Seligman BG, Konkewicz L, Dos Santos RP. Accuracy of tracheal aspirate gram stain in predicting Staphylococcus aureus infection in ventilator-associated pneumonia. BMC Anesthesiol. 2015;15(1):19. doi: 10.1186/1471-2253-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Horo JC, Thompson D, Safdar N. Is the gram stain useful in the microbiologic diagnosis of VAP? A meta-analysis. Clin Infect Dis. 2012;55(4):551-561. doi: 10.1093/cid/cis512 [DOI] [PubMed] [Google Scholar]
- 19.Blot F, Raynard B, Chachaty E, Tancrède C, Antoun S, Nitenberg G. Value of gram stain examination of lower respiratory tract secretions for early diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 2000;162(5):1731-1737. doi: 10.1164/ajrccm.162.5.9908088 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura J, Yamakawa K, Kinoshita T, Ohta Y, Morimoto T. Gram stain-guided antibiotics choice for ventilator-associated pneumonia (GRACE-VAP) trial: rationale and study protocol for a randomised controlled trial. Trials. 2018;19(1):614. doi: 10.1186/s13063-018-2971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003;31(3):676-682. doi: 10.1097/01.CCM.0000055380.86458.1E [DOI] [PubMed] [Google Scholar]
- 23.Matsushima A, Tasaki O, Shimizu K, et al. Preemptive antibiotic treatment based on gram staining reduced the incidence of ARDS in mechanically ventilated patients. J Trauma. 2008;65(2):309-315. doi: 10.1097/TA.0b013e31817c96f6 [DOI] [PubMed] [Google Scholar]
- 24.Hellyer TP, McAuley DF, Walsh TS, et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir Med. 2020;8(2):182-191. doi: 10.1016/S2213-2600(19)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canadian Critical Care Trials Group . A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355(25):2619-2630. doi: 10.1056/NEJMoa052904 [DOI] [PubMed] [Google Scholar]
- 26.Shengchen D, Gu X, Fan G, et al. Evaluation of a molecular point-of-care testing for viral and atypical pathogens on intravenous antibiotic duration in hospitalized adults with lower respiratory tract infection: a randomized clinical trial. Clin Microbiol Infect. 2019;25(11):1415-1421. doi: 10.1016/j.cmi.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401-411. doi: 10.1016/S2213-2600(17)30120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438-1444. doi: 10.1086/497134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- 30.Restrepo MI, Peterson J, Fernández JF, Qin Z, Fisher AC, Nicholson SC. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care. 2013;58(7):1220-1225. doi: 10.4187/respcare.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheer CS, Fuchs C, Gründling M, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect. 2019;25(3):326-331. doi: 10.1016/j.cmi.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 32.Lopes RD, Heizer G, Aronson R, et al. ; AUGUSTUS Investigators . Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509-1524. doi: 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Participating Institutions and Investigators
eTable 2. Members of the Data and Safety Monitoring Board
eTable 3. Antibiogram of Participating Institutions
eTable 4. Microorganisms Isolated in Respiratory Samples
eTable 5. Summary of the Results of Gram Staining
eTable 6. Summary of Selected Antibiotic Agents
eTable 7. Clinical Response of Participating Institutions
eTable 8. Concordance Between Results of Gram Staining and Culture
eFigure 1. Interventions
eFigure 2. Twenty-Eight-Day Mortality in the Gram Stain–guided Group and Guideline-based Group
Data Sharing Statement