Abstract
Frailty is a powerful prognostic tool in cirrhosis. Claims- based frailty scores estimate the presence of frailty without the need for in- person evaluation. These algorithms have not been validated in cirrhosis. Whether they measure true frailty or perform as well as frailty in outcome prediction is unknown. We evaluated 2 claims- based frailty scores— Hospital Frailty Risk Score (HFRS) and Claims- Based Frailty Index (CFI)— in 3 prospective cohorts comprising 1100 patients with cirrhosis. We assessed differences in neuromuscular/neurocognitive capabilities in those classified as frail or nonfrail based on each score. We assessed the ability of the indexes to discriminate frailty based on the Fried Frailty Index (FFI), chair stands, activities of daily living (ADL), and falls. Finally, we compared the performance of claims- based frailty measures and physical frailty measures to predict transplant- free survival using competing risk regression and patient- reported outcomes. The CFI identified neuromuscular deficits (balance, chair stands, hip strength), whereas the HFRS only identified poor chair- stand performance. The CFI had areas under the receiver operating characteristic curve (AUROCs) for identifying frailty as measured by the FFI, ADL, and falls of 0.57, 0.60, and 0.68, respectively; similarly, the AUROCs were 0.66, 0.63, and 0.67, respectively, for the HFRS. Claims- based frailty scores were associated with poor quality of life and sleep but were outperformed by the FFI and chair stands. The HFRS, per 10- point increase (but not the CFI) predicted survival of patients in the liver transplantation (subdistribution hazard ratio [SHR], 1.08; 95% confidence interval [CI], 1.03– 1.12) and non– liver transplantation cohorts (SHR, 1.13; 95% CI, 1.05– 1.22). Claims- based frailty scores do not adequately associate with physical frailty but are associated with important cirrhosis- related outcomes.
Frailty is an emerging indicator of poor outcomes in cirrhosis and liver transplantation.(1–4) It is a multidimensional construct informed by physical, cognitive, and psychosocial factors, which together quantify physiologic reserve. Although many nonphysical factors influence the development of frailty in cirrhosis,(1,5,6) the most validated frailty tools—the Fried Frailty Index (FFI) and the Liver Frailty Index(3,4)—include multiple physical performance measures such as hand grip, chair stands, and walking speed.(7) These require in- person evaluation, effort, and time. As such, there is mounting interest in diagnosis code–based algorithms that could leverage administrative data in place of physical measurements.(8) The 2 most studied candidate algorithms (Claims- Based Frailty Index [CFI] and Hospital Frailty Risk Score [HFRS]) have been developed from community-dwelling elders aged >70 years.(9,10) If validated in people with cirrhosis, these algorithms will enable research on frailty at the population level.
Two crucial steps are required to validate frailty algorithms in cirrhosis. First, we must understand what frailty algorithms measure. To be considered a frailty index, they must discriminate deficits in the neuromuscular or neurocognitive capacities underlying frailty in cirrhosis. Second, as frailty predicts clinical outcomes, so too must the algorithms. In cirrhosis, frailty is linked to survival, liver transplantation outcomes, and health-related quality of life (HRQOL).
Herein we evaluated frailty algorithms with 3 aims using 3 separate cohorts of patients with cirrhosis. First, we evaluated whether the algorithms capture neuromuscular and neurocognitive deficits. Second, we assessed the test characteristics of each algorithm to discern gold standard frailty and disability measures. Third, we compared the performance of each algorithm with in-person measures of frailty for the prediction of mortality and poor HRQOL.
Patients and Methods
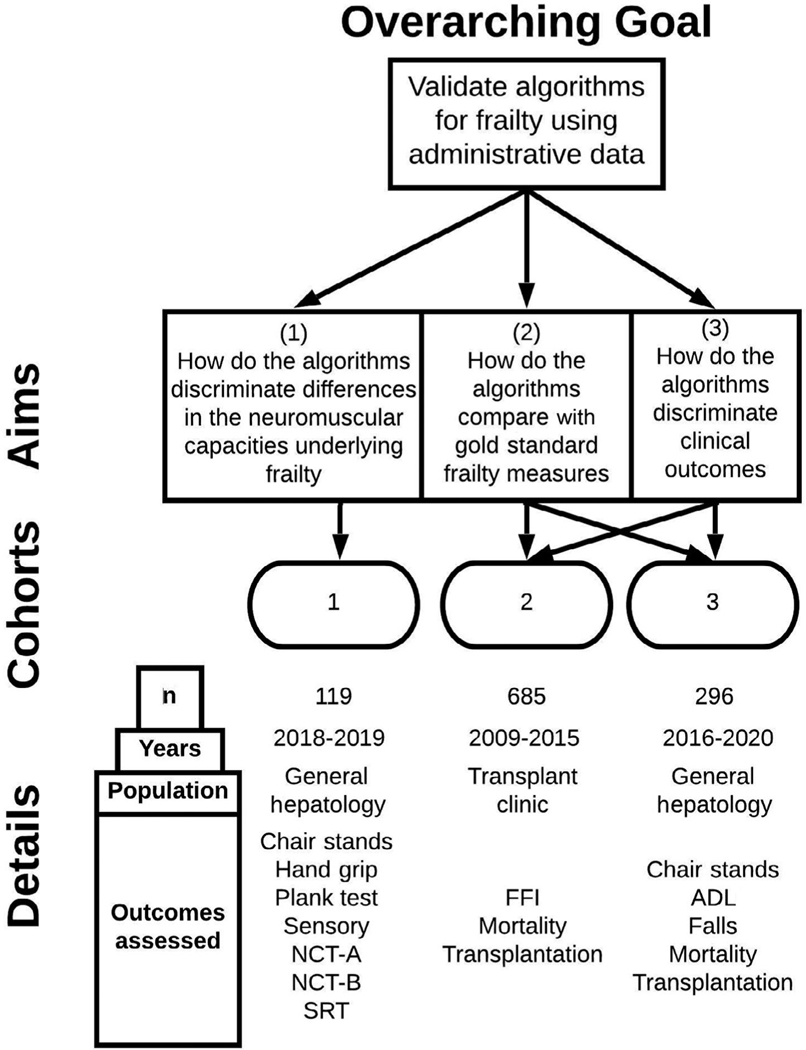
We conducted our study using 3 previously published prospective cohorts of patients with a confirmed diagnosis of cirrhosis from the University of Michigan Hepatology and Liver Transplant Clinics. The first cohort (cohort 1), conducted from August 2018 to April 2019, was used to define the differences in neuromuscular capacities according to frailty algorithm classification. The second cohort (cohort 2), conducted from July 2009 to February 2015, was used to compare the performance of each claims- based frailty measure against the FFI as well as to assess outcomes of HRQOL and transplant-free survival. The third cohort (cohort 3), enrolled from July 2016 to August 2018 and followed through February 2020, was used to define the performance of frailty algorithms against the Katz scale of activities of daily living (ADL) and for the prediction of transplant- free survival. Details of each cohort are presented in the Supporting Methods, and a conceptual overview of how they were used in the current study is presented in Fig. 1.
Fig. 1.
Description of the 3 cohorts of patients included in this study and the specific aims each cohort was used to address.
FRAILTY INDEXES
The FFI is the most widely used frailty tool in patients with organ failure awaiting transplantation.(11) The FFI includes subjective reports of exhaustion, weight loss, and physical activity as well as objective measures of walking speed and hand grip.(12) We used the FFI as the gold standard for frailty.
A total of 2 frailty algorithms based on administrative billing codes were studied. The CFI was developed and validated based on a multivariable regression model of 52 International Classification of Diseases, Ninth Revision (ICD- 9), 16 Healthcare Common Procedure Coding System, and 25 Current Procedural Terminology codes to predict deficit accumulation-based frailty.(9) The CFI is calculated based on the presence or absence of these codes in the preceding 12 months with robust, prefrail, mildly frail, and moderate to severely frail defined by scores of <0.15, 0.15 to 0.24, 0.25 to 0.34, and ≥0.35, respectively.(13) For the purposes of the current study, frailty was defined as a CFI score of ≥0.35. For each patient, codes were included in the algorithm from the preceding 12 months from their date of evaluation.
The HFRS was developed and validated based on a multivariable logistic regression model of International Classification of Diseases, Tenth Revision (ICD- 10) codes found to be prevalent in hospitalized patients.(10) The score is calculated based on the presence or absence of 109 ICD- 10 codes in the preceding 24 months with patients categorized as low, intermediate, and high risk based on HFRSs of <5, 5 to 15, and >15, respectively. In a cohort of patients aged >75 years, the HFRS significantly predicted 30- day mortality, length of stay, and 30- day readmissions after hospitalization.(10) In the current study, HFRS frailty was defined as scores >15. For each patient, codes were included in the algorithm from the preceding 24 months from their date of evaluation.
OUTCOMES
Aim 1
We first compared the differences in neuromuscular and neurocognitive capacities for persons classified as frail or nonfrail according to the CFI and HFRS indexes. Details of the neuromuscular assessments are presented in the Supporting Information. In brief, we assessed frailty using a 30- second chair-stand test, grip strength, unipedal stance time, and hip strength using the lateral plank test. To assess neurocognition, we used the Number Connection Test A (NCT- A), Number Connection Test B (NCT- B), and simple reaction time (SRT) measured using the ReactStick (University of Michigan Department of Physical Medicine and Rehabilitation).
Aim 2
We evaluated the test characteristics of each claims- based frailty algorithm, CFI, and HFRS against the FFI according to their receiver operating characteristics. We also evaluated test characteristics of the CFI and HFRS for disability using the Katz ADL scale, chair stands, and fall history. Any ADL dependency, falls in the preceding 6 months, or chair stands less than the median for the cohort was considered frail.
Aim 3
We evaluated each frailty measure with respect to their ability to discriminate the clinical outcomes of transplant- free survival and HRQOL. The main analysis was conducted in cohort B, a longitudinal cohort of patients evaluated for liver transplantation whose HRQOL was assessed using the Short Form 36 (SF- 36). We repeated this analysis in cohort C, a cohort of patients with Child- Turcotte- Pugh A or B cirrhosis who were enrolled in a study to determine the cumulative incidence of hepatic encephalopathy (HE) and whose HRQOL was assessed using the Short Form 8 (SF- 8) and the Pittsburgh Sleep Quality Index (PSQI).
DATA ANALYSES
Descriptive data are presented as number (percentage) for categorical data and mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous data. Means for continuous neuromuscular and neurocognitive data were compared using the Student t test. Test performance was assessed using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). A CFI score ≥0.25 and an HFRS ≥5 can also be considered as defining frailty(10,13); therefore, a sensitivity analysis was performed using these cutoff values (Supporting Tables 1 and 2). The area under the receiver operating characteristic curve (AUROC) was calculated using continuous values of the frailty predictor variables.
The ability of the frailty scores (FFI, CFI, and HFRS) to predict mortality on the transplant waiting list in cohort 2 was assessed using Fine and Gray competing risk regression, presented as subdistribution hazard ratios (SHRs), with transplantation as a competing event.(14) A priori, we determined we would assess the relationship between each frailty measure controlled for Model for End- Stage Liver Disease–sodium (MELD-Na), HE, ascites, and the Charlson Comorbidity Index (CCI). Survival in cohort 3 was also analyzed using competing risk regression with transplantation as a competing event. Here, frailty measures included the administrative frailty measures (CFI and HFRS) in addition to the physical frailty measures of ADL dependency and chair stands. A priori, we determined we would control for the MELD-Na score, Child- Turcotte- Pugh class, and CCI. These variables were selected given their clinical importance in predicting survival in chronic liver disease.
This study was approved by the University of Michigan Medical School institutional review board. Statistical analysis was performed using RStudio (R Foundation for Statistical Computing). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Baseline characteristics for each study cohort are presented in Table 1. As cohort 2 included patients evaluated in the liver transplantation clinic, they had a higher median MELD- Na score of 13 (IQR, 10– 18) and a higher prevalence of decompensating events (55% with ascites and 39% with a history of HE) at enrollment. The prevalence of ICD-10 scores in cohort 2 used in the calculation of the CFI and HFRS are presented in Supporting Tables 3 and 4. The correlation between the HFRS and CFI was 0.59, 0.63, and 0.63 in cohorts 1, 2, and 3, respectively.
TABLE 1.
Demographics and Clinical Characteristics of the Cohorts
| Variable | Cohort 1: Patients With Cirrhosis Evaluated for Neurocognitive and Muscular Capacities (n = 119) | Cohort 2: Longitudinal Cohort of Patients With Cirrhosis Referred for Liver Transplantation Evaluation (n = 685) | Cohort 3: Longitudinal Cohort of Patients With Cirrhosis Without HE at Enrollment (n = 296) |
|---|---|---|---|
|
| |||
| Age, years, median (IQR) | 62 (58–68) | 56 (49–61) | 60 (52–66) |
| Sex, female, n (%) | 59 (49.6) | 270 (39) | 128 (43) |
| Non-White patients, n (%) | 6(5) | 96(14.0) | — |
| Body mass index, kg/m2, median (IQR) | 30.7 (26.3–35.7) | 28.6 (24.4–33.6) | 29.3 (25.7–34.0) |
| Etiology of cirrhosis, n (%) | |||
| NAFLD | 41 (34) | 157 (23) | 97 (33) |
| Alcohol | 28 (24) | 166 (24) | 65(22) |
| HCV | 18 15) | 156 (23) | 89 (30) |
| Other | 32 (27) | 206 (30) | 45(15) |
| CCI score, median (IQR) | — | 2(1–3) | 4(1–4) |
| Child-Turcotte-Pugh class | |||
| A/B/C, n | 95/21/2* | — | 207/89/0 |
| Ascites, n (%) | — | 374 (55) | 120 (41) |
| HE, n (%) | — | 268 (39) | 0 |
| MELD-Na score, median (IQR) | 9 (8–13) | 13(10–18) | 9(7–13) |
| Albumin, g/dL, median (IQR) | — | 3.2 (2.7–3.7) | 4.0 (3.6–4.3) |
| CFI score, median (IQR) | 0.348(0.315–0.377) | 0.160 (0.109–0.276) | 0.338 (0.287–0.371) |
| Robust, % | 2.5 | 47.9 | 2.0 |
| Prefrail, % | 7.6 | 23.4 | 14.5 |
| Mildly frail, % | 43.7 | 18.5 | 43.9 |
| Moderate to severely frail, % | 46.2 | 10.2 | 39.5 |
| HFRS, median (IQR) | 45.9(26.2–81.3) | 20.2 (6.3–45.9) | 39.3 (21.5–66.8) |
| Low risk, % | 3.4 | 21.2 | 2.4 |
| Intermediate risk, % | 6.7 | 18.1 | 13.9 |
| High risk, % | 89.9 | 60.7 | 83.8 |
NOTE: Race data not collected for cohort 3.
Of the patients, 1 had unknown Child-Turcotte-Pugh class.
PHYSIOLOGICAL MEASURES
We first evaluated the neuromuscular and neurocognitive capacities of patients identified as frail or not according to the CFI and HFRS in cohort 1 (Table 2). Those classified as not frail by the CFI were able to complete more chair stands (11.6 versus 8.2; P < 0.001) and maintain a unipedal stance and lateral plank positions longer (15.2 versus 9.7 seconds [P = 0.002] and 21.7 versus 10.1 seconds [P < 0.001], respectively). Only chair stands were significantly better in those considered to be not frail by the HFRS (13.2 versus 9.8; P = 0.04). For neurocognitive performance (NCT- A, NCT- B, and SRT), only the NCT- B was significantly associated with HFRS frailty.
TABLE 2.
Neurocognitive and Muscular Capacities in Cohort 1 Patients With and Without Frailty Based on CFI Score and HFRS
| Mean Value | CFI |
HFRS |
||||
|---|---|---|---|---|---|---|
| Not Frail (n = 64) | Frail (n = 55) | P Value | Not Frail (n = 12) | Frail (n = 107) | P Value | |
|
| ||||||
| Unipedal stance (seconds) | 15.2 | 9.7 | 0.002 | 18.3 | 12.0 | 0.09 |
| Chair stands in 30 seconds | 11.6 | 8.2 | <0.001 | 13.2 | 9.8 | 0.04 |
| Hand grip (average of 6 lbs) | 55.6 | 49.8 | 0.20 | 56.7 | 52.4 | 0.63 |
| Lateral plank test (seconds) | 21.7 | 10.1 | <0.001 | 19.9 | 15.7 | 0.47 |
| Melbourne visual contrast (decibel) | 22.1 | 21.2 | 0.22 | 22.8 | 21.7 | 0.04 |
| Vibratory sense (seconds) Neurocognitive | 10.7 | 8.0 | 0.01 | 13.7 | 9.0 | 0.007 |
| NCT-A (seconds) | 37.8 | 43.5 | 0.07 | 33.8 | 41.1 | 0.09 |
| NCT-B (seconds) | 96.2 | 112.6 | 0.07 | 82.4 | 105.9 | 0.04 |
| SRT (milliseconds) | 232.4 | 219.2 | 0.13 | 236.3 | 225.1 | 0.50 |
NOTE: For both frailty risk scores, we used the cutoff for severe frailty as CFI scores ≥0.35 or HFRSs >15.
CLAIMS-BASED FRAILTY MEASURES AND PHYSICAL FRAILTY
We next determined the ability of the CFI and HFRS to correctly group patients into frail and nonfrail based on physical frailty measures (Table 3). We also examined whether the CFI and HFRS would discriminate disability (ADL) or a history of falls. The CFI had an AUCROC for identifying frailty as measured by FFI, ADL dependency, and falls of 0.57, 0.70, and 0.68, respectively. For the HFRS, the corresponding AUROC values were 0.66, 0.63, and 0.67 respectively. Overall, the HFRS tended to be a very sensitive and less- specific test for identifying physical frailty, and this difference increased on sensitivity analysis (HFRS frail cutoff of ≥5; Supporting Table 2).
TABLE 3.
Test Performance of CFI Measures and Physical Frailty Measures in Cohorts 2 and 3
| Variable | Frailty Outcomes |
|||
|---|---|---|---|---|
| Cohort 2: Longitudinal Cohort of Patients With Cirrhosis Referred for Liver Transplantation Evaluation |
Cohort 3: Longitudinal Cohort of Patients With Cirrhosis Without HE at Enrollment |
|||
| Frail FFI Performance | Dependent for Performance of ADL | Frail Chair-Stand Performance | Falls Within 6 Months | |
|
| ||||
| CFI | ||||
| Sensitivity | 0.13 | 0.65 | 0.54 | 0.62 |
| Specificity | 0.92 | 0.63 | 0.72 | 0.67 |
| NPV | 0.60 | 0.95 | 0.67 | 0.87 |
| PPV | 0.51 | 0.15 | 0.60 | 0.33 |
| AUC | 0.57 | 0.70 | 0.62 | 0.68 |
| HFRS | ||||
| Sensitivity | 0.73 | 0.96 | 0.88 | 0.95 |
| Specificity | 0.48 | 0.17 | 0.46 | 0.19 |
| NPV | 0.72 | 0.98 | 0.67 | 0.94 |
| PPV | 0.49 | 0.10 | 0.46 | 0.24 |
| AUC | 0.66 | 0.63 | 0.63 | 0.67 |
NOTE: Physical frailty was defined as an FFI ≥3, any ADL dependency, chair stands below the median (<10), and any falls within the preceding 6 months. Claims- based frailty was defined as a CFI score ≥0.35 or HFRS >15. AUC was performed using continuous values of the CFI and HFRS.
FRAILTY MEASURES AND SURVIVAL AND LIVER TRANSPLANTATION
In cohort 2, we evaluated the ability of frailty measures to predict mortality or transplantation in patients evaluated for liver transplantation (Table 4). Median follow- up was 589 days (IQR, 150–1430), during which 201 (29.3%) patients died and 150 (21.9%) patients received a liver transplant. For each 1- point increase in the FFI score, there was a 43% increase in mortality (SHR, 1.43; 95% confidence interval [CI], 1.29–1.59). This remained significant when controlling (individually) for MELD- Na score, the presence of HE, the presence of ascites, or CCI. A higher FFI score was significantly associated with a decreased likelihood of transplantation when adjusted for MELD- Na (SHR, 0.82; 95% CI, 0.73– 0.93). A higher HFRS was associated with an increased risk of mortality (SHR, 1.08; 95% CI, 1.03– 1.12) even after adjustment for other clinical factors, but the HFRS was not predictive of transplantation. The CFI predicted neither death nor transplantation in this cohort. The ability to discriminate 1-year transplant- free survival was highest for the FFI (AUROC 0.68), then the HFRS (AUROC 0.61), and lastly the CFI (AUROC 0.51). Only the FFI score increased the predictive ability of the MELD-Na score (AUROC from 0.76 to 0.79).
TABLE 4.
Association Between Frailty Measures or Algorithms and Mortality Using Competing Risk Regression in Cohorts 2 and 3 (Adjusted Models)
| Cohort 2 |
Cohort 3 |
||||
|---|---|---|---|---|---|
| Longitudinal Cohort of Patients With Cirrhosis Referred for Liver Transplantation Evaluation |
Longitudinal Cohort of Patients With Cirrhosis Without HE at Enrollment |
||||
| Variable | Death |
Transplant |
Death |
||
| SHR (95% CI) | SHR (95% CI) | SHR (95% CI) | |||
|
| |||||
| CFI, per 0.01-point increase | 1.00 (0.99–1.02) | 0.99 (0.97–1.01) | CFI, per 0.01-point increase | 1.13 (1.08–1.19) | |
| + MELD-Na | 1.00 (0.99–1.02) | 0.99 (0.97–1.00) | + MELD-Na | 1.13 (1.07–1.19) | |
| + HE | 1.00 (0.99–1.02) | 0.99 (0.97–1.01) | + Child-Turcotte-Pugh class B | 1.14 (1.08–1.20) | |
| + Ascites | 1.01 (0.99–1.02) | 0.99 (0.97–1.00) | + CCI | 1.13 (1.07–1.19) | |
| + CCI | 1.00 (0.99–1.02) | 0.99 (0.97–1.01) | |||
| HFRS, per 10-point increase | 1.08 (1.03–1.12) | 1.04 (0.98–1.10) | HFRS, per 10-point increase | 1.13 (1.05–1.22) | |
| +MELD-Na | 1.05 (1.00–1.10) | 1.00 (0.93–1.06) | + MELD-Na | 1.11 (1.03–1.20) | |
| +HE | 1.07 (1.03–1.12) | 1.04 (0.98–1.10) | + Child-Turcotte-Pugh class B | 1.14 (1.06–1.23) | |
| +Ascites | 1.08 (1.04–1.13) | 1.04 (0.98–1.09) | + CCI | 1.11 (1.03–1.20) | |
| + CCI | 1.07 (1.03–1.12) | 1.05 (0.99–1.10) | |||
| FFI, per 1-point increase | 1.43 (1.29–1.59) | 0.91 (0.81–1.02) | ADL, any disability | 3.39 (1.60–7.19) | |
| + MELD-Na | 1.37 (1.22–1.53) | 0.82 (0.73-.93) | + MELD-Na | 3.59 (1.67–7.72) | |
| + HE | 1.41 (1.27–1.57) | 0.90 (0.81–1.01) | + Child-Turcotte-Pugh class B | 3.46 (1.64–7.31) | |
| + Ascites | 1.40 (1.26–1.57) | 0.92 (0.82–1.04) | + CCI | 3.41 (1.61–7.20) | |
| + CCI | 1.41 (1.27–1.57) | 0.92 (0.82–1.03) | |||
| Chair stands, per 1-point increase* | 0.88 (0.84–0.93) | ||||
| + MELD-Na | 0.89 (0.84–0.94) | ||||
| + CTP-B | 0.89 (0.84–0.94) | ||||
| + CCI | 0.89 (0.84–0.94) | ||||
NOTE: We used Fine and Gray competing risk regression to test the association between each frailty metric and the outcome of death or transplantation for our 2 cohorts with longitudinal data. The univariable SHRs are presented for each frailty metric and then adjusted by sequentially adding other factors (bivariable analysis). In cohort 2, we adjusted for MELD-Na, HE, ascites, and CCI. In cohort 3, we added MELD-Na followed by Child- Turcotte- Pugh class B and CCI. Bold values are statistically significant.
Increasing number of chair stands reflects a robust state.
In cohort 3, 42 (14.2%) patients died and 12 (4.1%) underwent liver transplantation during a median follow- up of 980 days (IQR, 791– 1122). Because of the small number of patients who underwent liver transplantation, the prediction of this outcome was not analyzed. Mortality was significantly predicted by ADL disability (SHR, 3.39; 95% CI, 1.60– 7.19) and chair stands (SHR, 0.88; 95% CI, 0.84– 0.93). Mortality was also predicted by both the CFI score (SHR, 1.13; 95% CI, 1.08–1.19) and the HFRS (SHR, 1.13; 95% CI, 1.05–1.22). The prediction of mortality using both physical and claims- based frailty measures remained significant after adjusting for clinical factors.
FRAILTY MEASURES AND QUALITY OF LIFE
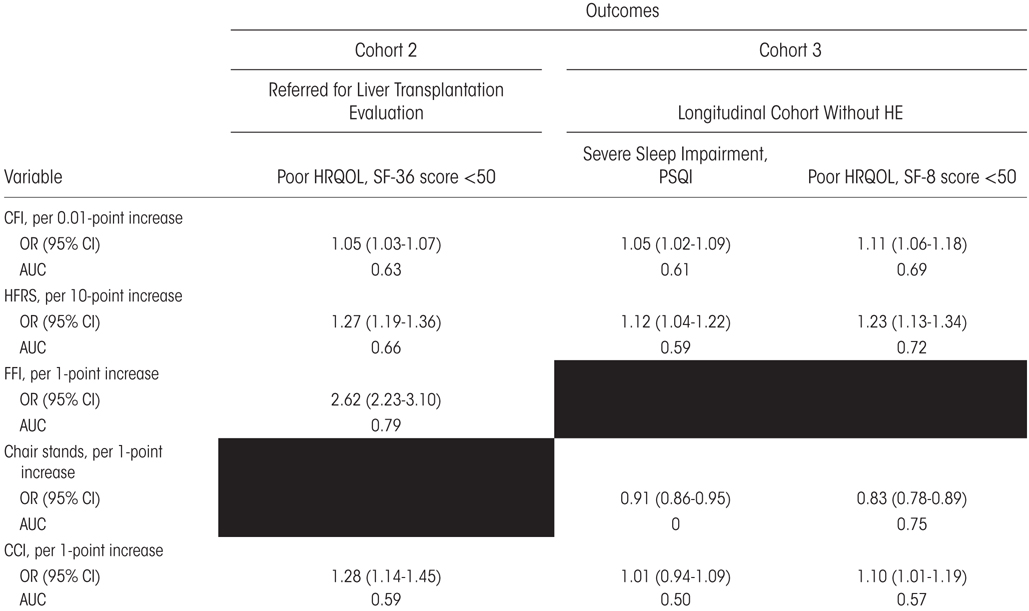
In cohort 2, an SF- 36 score <50 was significantly associated with the CFI (odds ratio [OR], 1.05; 95% CI, 1.03– 1.07), the HFRS (OR, 1.27; 95% CI, 1.19– 1.36), and the FFI (OR, 2.62; 95% CI, 2.23– 3.10). The FFI had the best ability to discriminate between those with SF-36 scores less than or greater than 50 (AUROC 0.79) followed by the HFRS (AUROC 0.66) and then the CFI (AUROC 0.63).
In cohort 3, poor HRQOL was determined based on poor sleep (PSQI >5) and low SF- 8 scores (SF- 8 <50) and was best predicted by chair stands (AUROCs 0.65 and 0.75, respectively), followed by the CFI and HFRS. To a lesser extent, the CCI predicted SF- 8 scores (AUROC 0.57; Table 5).
TABLE 5.
Association Between HRQOL and Frailty Measures or Algorithms
|
NOTE: We evaluated 2 measures of global HRQOL— the SF- 36 and SF- 8. Both are considered “poor” when the score is <50. We also examined sleep quality using severe sleep impairment on the PSQI as an outcome. Blacked-out cells reflect a lack of such data for the cohort.
Discussion
Among patients with cirrhosis, frailty is strongly associated with diminished transplant- free survival,(1) hospitalization,(15) and posttransplant outcomes.(16) Although it reflects deficits in neuromuscular and neurocognitive capacities, the concept of frailty is operationalized as poor performance on specific physical tests. However, these tests are rarely performed in clinical practice. Claims- based algorithms for frailty can be applied to populations provided they are validated to identify frailty and predict frailty- related outcomes.
In this study, we sought to validate the use of 2 claims- based frailty algorithms, the HFRS and CFI, for use in patients with cirrhosis. Both algorithms were associated with survival and poor HRQOL, albeit to a lesser degree than conventional physical frailty measures. Neither algorithm offered good discrimination or PPVs for physical frailty. However, the HFRS offered excellent sensitivity (and NPVs) for disability.
FRAILTY AND OUTCOMES IN CHRONIC LIVER DISEASE
Physical frailty is known to predict mortality and poor HRQOL independent of Model for End-Stage Liver Disease (MELD) score.(1–4,17) The value of claims- based frailty indexes should be defined in part by their ability to reproduce similar associations. We found that claims- based frailty indexes predicted mortality even after adjusting for MELD score and cirrhotic decompensations (Table 3). However, the FFI was better able to discriminate 1-year transplant- free survival compared with the CFI and HFRS. Furthermore, only the addition of FFI improved the MELD score prediction of transplant-free survival. Both the CFI and HFRS were associated with poor HRQOL and poor sleep but performed less well relative to physical measures such as the FFI and chair stands.
In the cohort of patients evaluated in the liver transplantation clinic, transplantation was not predicted by the claims- based scores or the FFI (except when adjusted for MELD score). Although frailty is associated with mortality on the liver transplant waiting list, it is but 1 of many factors weighted in transplantation decision making.
CLAIMS-BASED MEASURES POORLY DISCRIMINATE FRAILTY
Identifying frailty in persons with cirrhosis is essential to subsequently implement targeted frailty-modifying interventions (nutritional, prehabilitation) that may improve outcomes.(3) Although the CFI was associated with worsened neuromuscular performance, we found that both claims- based indexes (CFI and HFRS) failed to adequately discriminate physical frailty. Our findings are consistent with those of the HFRS derivation cohort where there was poor agreement (κ = 0.30 and 0.22) between the HFRS with the Rockwood and FFI measures of frailty.(10) Compared with prior studies using the CFI, we found a lower discrimination ability of the CFI for FFI (AUROC 0.57 for our data versus 0.78 for others), but similar values in predicting ADL dependency (AUROC 0.70 for our data versus 0.66 for others).(13,18) An important difference between the cohorts used to derive the claims- based frailty indexes and our cohort is the markedly higher proportion of patients in our cohort classified as frail using administrative data. In prior studies, those identified as frail based on the CFI (≥0.35) or the HFRS (>15) comprised 2.2% and 20.0%, respectively.(10,13) Using these cutoffs, 10.2% to 46.2% (based on the CFI) and 60.7% to 89.9% (based on the HFRS) of patients in the current study were categorized as frail.
THE CFI AND HFRS ARE LIKELY EXPANDED MEASURES OF COMORBIDITY
Our data show that claims-based frailty indexes derived from the general population cannot be generalized to patients with cirrhosis. The reason that the algorithms cannot discriminate frailty in cirrhosis is because of the diagnostic codes that compose these scores (Supporting Table 1). For example, abnormal results of function studies (R94), other disorders of kidney and ureter (N28), unspecified renal failure (N19), and other disorders of fluid, electrolyte, and acid- base balance (E87) were found in more than half of cohort 2 (55% with ascites). Combined these codes would classify a patient as intermediate risk frail based on the HFRS. These factors may identify frailty in the general population but are so common among patients with cirrhosis that they are not discriminatory. This renders the claims- based frailty indexes overly sensitive and nonspecific among patients with cirrhosis. Beyond that, established contributors to the frail state in cirrhosis are missing from claims- based frailty indexes. These conditions include sarcopenia, malnutrition, decompensating events, and hepatic dysfunction.(3,16,19)
In sum, the claims- based frailty algorithms appear to serve as weighted comorbidity counts that outperform conventional comorbidity indexes such as the CCI.(20) We found that the CFI and HFRS are both associated with clinical and patient-reported outcomes to a greater extent than the CCI. Kochar et al. showed that patients with inflammatory bowel disease categorized as frail using an adapted HFRS had a median CCI of 6 compared with 2 in those not categorized as frail.(8)
THE WAY FORWARD FOR CLAIMS-BASED INDEXES
Whether these algorithms track with frailty or comorbidity, they are associated with survival and patient-reported outcomes. As such, there is value in developing and refining these resources for the cirrhosis population. Inputs into a potential algorithm should include appropriately weighted codes known to contribute to the frailty phenotype in cirrhosis, and these algorithms should predict frailty (and respond to frailty- modifying interventions) in cirrhosis. This will be challenging for 2 reasons. First, in a large retrospective study using natural language processing and ICD- 10 codes to identify sarcopenia, frailty, and cachexia, 86% of patients with these conditions did not have the associated ICD- 10 code and were identified only by natural language processing.(21) Even codes for HE or ascites are insensitive.(22,23) The major limitation of claims-based frailty indexes is its dependence on a provider’s recognition of the frail state with subsequent accurate coding. This adds both measurement error and subjectivity into the assessment of frailty. This runs counter to the trend of using more objective and standardized measures of frailty (eg, Liver Frailty Index).(3,16)
Second, changes in frailty over time have important implications on cirrhosis-related outcomes.(24) The CFI and HFRS, however, are calculated from 1 to 2 years of prior data, respectively. This renders these scores relatively static and less likely to be meaningful in assessing the trajectory of the frail phenotype.
Finally, there remains value in the direct in-person assessment of frailty that cannot be accounted for in the use of administrative data. Involvement of the patient in the frailty evaluation allows for their direct observation and understanding of their current physical condition.
CONTEXTUAL FACTORS
Our data must be interpreted in the context of the study design. First, we assessed the ability of physical and claims- based frailty measures to predict long- term survival. Although the CFI has been validated to predict 1-year and 2-year mortality,(9,13) the HFRS was developed and externally validated to predict short- term (30- day) mortality.(10,25) Second, the CFI uses ICD- 9 codes, whereas the HFRS uses ICD- 10 codes. In the current study, ICD-9 codes were mapped to their corresponding ICD-10 codes. Third, the cohorts were different with regard to their stage of liver disease; however, this was an advantage that allowed for the assessment of the claims- based algorithms in distinct populations. Fourth, frailty is a construct with multiple accepted tools and surveys used to diagnose its presence. Our selection of a subset of frailty tools to which to compare the performance of the claims- based indexes introduced bias, but these are generally accepted measures (or components) of frailty in cirrhosis.(3) Lastly, as discussed previously, traditional cutoffs for each claims-based frailty measure applied to our cohorts resulted in a high proportion being classified as frail. We did not attempt to derive new cutoffs. Rather, we assessed major outcomes using the continuous values of these scores to better evaluate their ability to rank the outcomes of interest.
CONCLUSION
Claims-based data are useful tools for the evaluation of outcomes at the population level and have been used to develop indexes for frailty. For patients with cirrhosis, the CFI and HFRS claims-based indexes did not adequately discriminate physical frailty. Instead, they likely function as comorbidity indexes. These tools are associated with mortality and patient-reported outcomes, but to a substantially lower degree than gold standard measures of frailty or disability. Future attempts to identify frailty in cirrhosis for population- based studies using claims-based indexes would benefit from a derivation cohort composed of patients with cirrhosis.
Supplementary Material
Acknowledgments
This study was funded in part by National Institutes of Health Grant K23DK117055/T32DK062708 to Elliot B. Tapper and Jeremy Louissaint.
Elliot B. Tapper has served as a consultant to Novartis, Kaleido, and Allergan; has served on advisory boards for Takeda, Mallinckrodt, Rebiotix, and Bausch Health; and has received unrestricted research funding from Gilead.
Abbreviations:
- ADL
activities of daily living
- AUROC
area under the receiver operating characteristic curve
- CCI
Charlson Comorbidity Index
- CFI
Claims- Based Frailty Index
- CI
confidence interval
- FFI
Fried Frailty Index
- HCV
hepatitis C virus
- HE
hepatic encephalopathy
- HFRS
Hospital Frailty Risk Score
- HRQOL
health- related quality of life
- ICD- 9
International Classification of Diseases, Ninth Revision
- ICD- 10
International Classification of Diseases, Tenth Revision
- IQR
interquartile range
- MELD
Model for End- Stage Liver Disease
- MELD- Na
Model for End- Stage Liver Disease– sodium
- NAFLD
nonalcoholic fatty liver disease
- NCT- A
Number Connection Test A
- NCT- B
Number Connection Test B
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- PSQI
Pittsburgh Sleep Quality Index
- SD
standard deviation
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1).Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant 2018;18:2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Tapper EB, Baki J, Parikh ND, Lok AS. Frailty, psychoactive medications, and cognitive dysfunction are associated with poor patient-reported outcomes in cirrhosis. Hepatology 2019;69:1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Murphy SL, Tapper EB, Blackwood J, Richardson JK. Why do individuals with cirrhosis fall? A mechanistic model for fall assessment, treatment, and research. Dig Dis Sci 2019;64:316–323. [DOI] [PubMed] [Google Scholar]
- 6).Murphy SL, Richardson JK, Blackwood J, Martinez B, Tapper EB. Neurocognitive and muscular capacities are associated with frailty in adults with cirrhosis. Dig Dis Sci 2020;65:3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol 2016;111:1768–1775. [DOI] [PubMed] [Google Scholar]
- 8).Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Pre-treatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology 2020;158:2104–2111. e2. [DOI] [PubMed] [Google Scholar]
- 9).Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant 2019;19:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 13).Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, Schneeweiss S. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci 2019;74:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496–509. [Google Scholar]
- 15).Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J. Gastroenterol 2017;23:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tapper EB. Frailty and outcomes after liver transplantation. Curr Transplant Rep 2019;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring frailty in administrative claims data: comparative performance of four claims-based frailty measures in the U.S. medicare data. J Gerontol A Biol Sci Med Sci 2020;75:1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Louissaint J, Tapper EB. A claims-based Frailty Risk Score is associated with hospitalization for acute on chronic liver failure—but is it frailty? Liver Transpl 2021;27:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Eckart A, Hauser SI, Haubitz S, Struja T, Kutz A, Koch D, et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open 2019;9:e026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Moorthi RN, Liu Z, El-Azab SA, Lembcke LR, Miller MR, Broyles AA, et al. Sarcopenia, frailty and cachexia patients detected in a multisystem electronic health record database. BMC Musculoskelet Disord 2020;21:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Bengtsson B, Askling J, Ludvigsson JF, Hagström H. Validity of administrative codes associated with cirrhosis in Sweden. Scand J Gastroenterol 2020;55:1205–1210. [DOI] [PubMed] [Google Scholar]
- 23).Tapper EB, Korovaichuk S, Baki J, Williams S, Nikirk S, Waljee AK, Parikh ND. Identifying patients with hepatic encephalopathy using administrative data in the ICD-10 era. Hepatol Clin Gastroenterol 2019;19:604–606. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol 2020;73:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol 2019;4:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.