Abstract
The in vitro activities of buforin II, cecropin P1, and magainin II, alone and in combination with six clinically used antimicrobial agents, against 12 clinical isolates of Stenotrophomonas maltophilia were investigated. Antimicrobial activities were measured by MIC and time-kill studies. The isolates were susceptible to the peptides at concentrations in the range of 0.50 to 16 μg/ml. Synergy was observed when the peptides were combined with polymyxin E, meropenem, ceftazidime, piperacillin, and clarithromycin.
Multidrug resistance is widespread among gram-negative bacteria; indeed, the emergence of new opportunistic pathogens is somehow linked to their multiresistant phenotype, which makes them refractory to the antimicrobial agents commonly used in clinical practice (6, 7, 9, 18). The main reason for this bacterial resistance is thought to be the organism's low outer membrane permeability to antimicrobial agents. One of these multiresistant pathogens is Stenotrophomonas maltophilia, a free-living gram-negative organism closely related to the genus Pseudomonas and with a wide geographic distribution (1, 3). S. maltophilia is an increasingly frequent cause of infection, particularly in debilitated or immunocompromised patients, such as cancer patients, transplant recipients, and patients hospitalized in intensive care units (3, 12, 15, 21). One way to overcome the problems of the emergence of resistance is to use new antimicrobial compounds and/or combination therapy. Such combination therapy is generally used to increase the in vivo activity and to broaden the antimicrobial spectrum (20).
In recent years, many positively charged polypeptides have been isolated from a wide range of animals and plant and bacterial species; they are thought to be major factors in antibacterial defense (2, 10). Recent reports hypothesize that these compounds cross the outer membrane of gram-negative bacteria via the self-promoted uptake pathway. The initial step in this process should be the binding of the peptide to the surface lipopolysaccharide with a high affinity, causing the displacement of divalent cations that stabilize adjacent lipopolysaccharide molecules (5, 8, 17, 22). The displacement of divalent cations is hypothesized to destabilize the outer membrane of gram-negative bacteria and to possibly or likely lead to self-promoted uptake of the destabilizing compound across the outer membrane and to subsequent channel formation in the cytoplasmic membrane, resulting in cell death. The lethal event which occurs at the cytoplasmic membrane is not fully understood; the association of several molecules may form a water-filled pore which would serve as an ion-conducting, anion-selective channel. Recent reports have shown that the peptides may act by inserting into the cytoplasmic membrane and triggering the activity of bacterial murein hydrolases, resulting in damage or degradation of the peptidoglycan and lysis of the cell (8). In this study, we investigated the in vitro activities of buforin II, cecropin P1, and magainin II alone and in combination with 10 clinically used antimicrobial agents against S. maltophilia.
Twelve clinical isolates of S. maltophilia were tested. They were isolated from distinct patients with unrelated sources of infection through a 5-year period. The strains were identified according to the following criteria: gram negativity, rod shape, characteristic colonial morphology and pigmentation, negative oxidase test, and substrate utilization (API-20 NE gallery; Biomérieux, Marcy l'Etoile, France).
Buforin II, cecropin P1, and magainin II were obtained from Sigma-Aldrich (Milan, Italy). The peptides were solubilized in phosphate-buffered saline (pH 7.2), yielding 1-mg/ml stock solutions. The in vitro activities of the following antibiotics were evaluated: chloramphenicol, doxycycline, netilmicin, ofloxacin, piperacillin, polymyxin E, and rifampin (all from Sigma- Aldrich), clarithromycin (Abbott, Rome, Italy), ceftazidime (Glaxo-Wellcome, Verona, Italy), and meropenem (Zeneca, Rome, Italy). Laboratory-grade powders were diluted in accordance with the manufacturers' recommendations, yielding 1-mg/ml stock solutions. Solutions of drugs were made fresh on the day of the assay or stored at −80°C in the dark for short periods. The concentration range assayed for buforin II, cecropin P1, and magainin II was 0.125 to 64 μg/ml, and the range for the other antimicrobial agents was 0.25 to 256 μg/ml.
The MIC of each compound was determined using a broth microdilution method with Mueller-Hinton (MH) broth (Becton Dickinson Italia, Milan, Italy) and an initial inoculum of 5 × 105 CFU/ml (16). Polypropylene 96-well plates (Sigma-Aldrich) were incubated for 18 h at 37°C in air, and since several peptides have a tendency to precipitate, plates were shaken throughout the study. The MIC was considered to be the lowest peptide concentration at which observable growth was inhibited. Experiments were performed in triplicate. The peptides showed different ranges of inhibitory values: the 12 clinical isolates were more susceptible to buforin II than to cecropin P1 and magainin II. The results are summarized in Table 1.
TABLE 1.
MICs of membrane-active peptides and other antimicrobial agents for S. maltophilia
| Agent | MIC (μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Buforin II | 1–16 | 4 | 8 |
| Cecropin P1 | 2–16 | 4 | 16 |
| Magainin II | 1–16 | 4 | 8 |
| Piperacillin | 16–256 | 64 | 256 |
| Ceftazidime | 2–32 | 8 | 32 |
| Meropenem | 8–128 | 32 | 128 |
| Clarithromycin | 8–64 | 32 | 64 |
| Chloramphenicol | 16–256 | 32 | 256 |
| Doxycycline | 2–64 | 16 | 32 |
| Rifampin | 1–128 | 8 | 64 |
| Ofloxacin | 0.25–32 | 2 | 32 |
| Netilmicin | 0.50–64 | 8 | 64 |
| Polymyxin E | 1–16 | 4 | 8 |
Values are geometric means. 50% and 90%, MICs at which 50 and 90% of the isolates are inhibited, respectively.
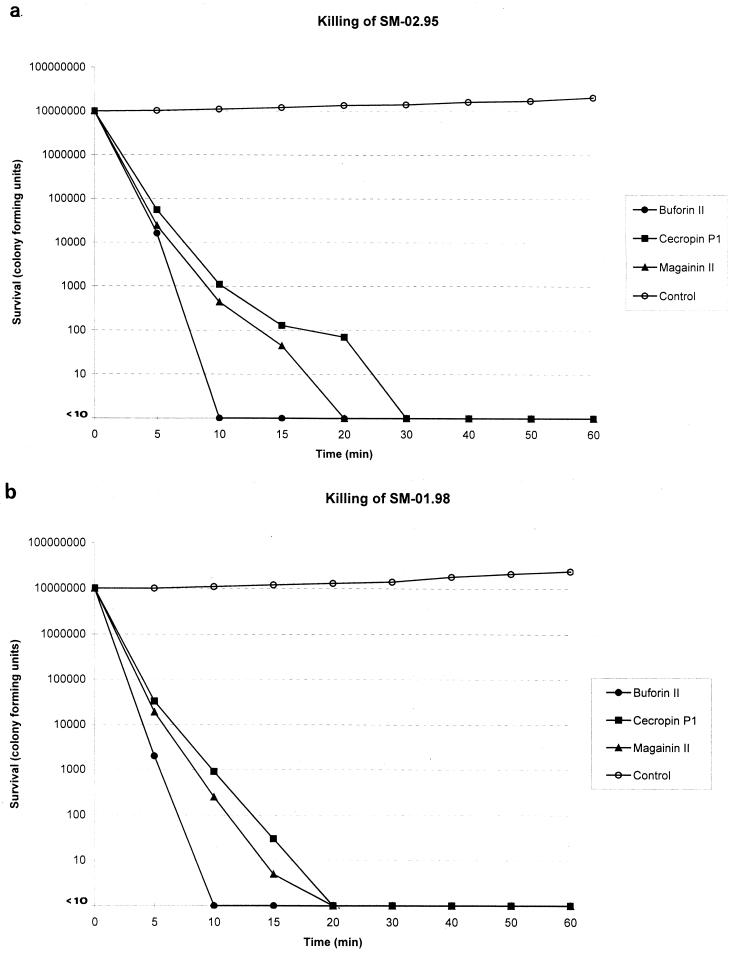
To study the in vitro killing effect of the peptides, two representative strains of S. maltophilia, SM-02.95 and SM-01.98, were selected. MICs of all the peptides were lowest for the former and highest for the latter. Aliquots of exponentially growing bacteria were resuspended in fresh MH broth at approximately 107 cells/ml and exposed to each peptide at four times the MIC for 0, 5, 10, 15, 20, 30, 40, 50, and 60 min at 37°C. After these times, samples were serially diluted in 10 mM sodium HEPES buffer (pH 7.2) to minimize the carryover effect and were plated onto MH agar plates to obtain viable colonies. As shown in Fig. 1, killing was complete after a 10- to 30-min exposure period.
FIG. 1.
Time-kill kinetics of membrane-active peptides against S. maltophilia SM-02.95 and S. maltophilia SM-01.98. Peptides were tested at concentrations of four times the MIC: buforin II, 4 (a) and 64 (b) μg/ml; cecropin P1, 8 (a) and 64 (b) μg/ml; and magainin II, 4 (a) and 64 (b) μg/ml.
In interaction studies, the above-mentioned strains, SM-02.95 and SM-01.98, were used to test the antibiotic combinations by a checkerboard titration method using 96-well polypropylene microtiter plates. Chloramphenicol, doxycycline, netilmicin, ofloxacin, polymyxin E, rifampin, clarithromycin, piperacillin, ceftazidime, and meropenem were tested in combination with each peptide. The ranges of drug dilutions used were 0.125 to 64 μg/ml for buforin II, cecropin P1, and magainin II and 0.25 to 256 μg/ml for clinically used antibiotics. The fractionary inhibitory concentration (FIC) index for combinations of two antimicrobials was calculated as follows: FIC index = FICA + FICB, FICA = [A]/MICA, and FICB = [B]/MICB, where [A] is the concentration of drug A in the well that has the lowest inhibitory concentration in its dilution row and MICA is the MIC of drug A alone for the organism (4). Synergistic combinations are defined as having FIC indices of ≤0.5. Overall, only combinations of peptides with clarithromycin, polymyxin E, and beta-lactams proved synergistic (Table 2).
TABLE 2.
Results of studies of interaction between cationic peptides and other drugs
| Agenta | FIC index
|
|||||
|---|---|---|---|---|---|---|
|
S. maltophilia SM-02.95
|
S. maltophilia SM-01.98
|
|||||
| Buforin II | Cecropin P1 | Magainin II | Buforin II | Cecropin P1 | Magainin II | |
| PIP | 0.375 | 0.250 | 0.250 | 0.375 | 0.375 | 0.250 |
| CAZ | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 |
| MEM | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 | 0.375 |
| CLR | 0.312 | 0.312 | 0.312 | 0.250 | 0.250 | 0.250 |
| C | 2.0 | 2.0 | 2.0 | 1.5 | 2.0 | 2.0 |
| D | 0.750 | 0.750 | 1.0 | 0.750 | 0.750 | 0.750 |
| RA | 1.5 | 1.0 | 1.5 | 1.0 | 1.0 | 1.5 |
| OFX | 1.5 | 2.0 | 1.5 | 1.25 | 1.5 | 1.5 |
| NET | 1.5 | 2.0 | 2.0 | 1.5 | 1.5 | 1.5 |
| PL-E | 0.187 | 0.187 | 0.187 | 0.250 | 0.250 | 0.250 |
PIP, piperacillin; CAZ, ceftazidime; MEM, meropenem; CLR, clarithromycin; C, chloramphenicol; D, doxycycline; RA, rifampin; OFX, ofloxacin; NET, netilmicin; PL-E, polymyxin E.
Our data are in agreement with recent reports which showed that killing by peptides was very rapid and resulted in log orders of cell death within minutes of peptide addition (14, 19).
Combination studies showed synergism between peptides and polymyxin E, clarithromycin, and beta-lactams. The mechanism of this interaction appears to be complex. The polymyxins are a group of cyclic cationic peptides originally derived from Bacillus polymyxa; they are amphipathic compounds, with a hydrophobic region at their amino terminus. Polymyxins and polymyxin-like peptides act synergistically with lipophilic and amphiphilic agents, such as rifampin, macrolides, fusidic acid, and novobiocin (17, 19). In addition, it has been demonstrated that polymyxin-like peptides allow maximal entry of hydrophobic substrates into the cell (22).
The mechanisms of the positive interaction between peptides and clarithromycin appears to be complex, too. The permeabilization of the outer membrane by hydrophobic molecules, such as the macrolides, might explain this positive interaction. Actually, large hydrophobic antibiotic molecules are usually ineffective against gram-negative bacteria since they cannot diffuse across the outer membrane (11, 13, 23). The peptides might potentiate the anti-Stenotrophomonas activity of clarithromycin by increasing the permeability of the outer membrane of the gram-negative rod.
The positive interaction between peptides and piperacillin, ceftazidime, and meropenem might be due to increased access of these drugs to the cytoplasmic membrane following breakdown of the peptidoglycan by beta-lactams. On the other hand, the peptides, by triggering the activity of bacterial murein hydrolases (10), may cause degradation of the peptidoglycan and enhance the activity of the beta-lactams.
In spite of this speculated mode of peptide interaction, proof of clinical benefits is lacking. Very few in vivo studies of cationic peptide action have been published, and despite several preclinical studies by small biotechnology companies, there are unanswered concerns about in vivo efficacy and unknown toxicities (10). However, the positive interactions demonstrated by several combinations make them potentially useful as compounds that enhance the activity of many clinically used antibiotics.
REFERENCES
- 1.Alonso A, Martínez J L. Multiple antibiotic resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;41:1140–1142. doi: 10.1128/aac.41.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon M. A family of wound healers. Nature. 1987;328:478. doi: 10.1038/328478a0. [DOI] [PubMed] [Google Scholar]
- 3.Denton M, Todd N J, Kerr K G, Hawkey P M, Littlewood J M. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–1958. doi: 10.1128/jcm.36.7.1953-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliopoulos G M, Moellering R C., Jr . Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams & Wilkins; 1996. pp. 330–393. [Google Scholar]
- 5.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:10298–10303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 6.George A M. Multidrug resistance in enteric and other gram-negative bacteria. FEMS Microbiol Lett. 1996;139:1–10. doi: 10.1111/j.1574-6968.1996.tb08172.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanberger H, Garcia-Rodriguez J A, Gobernado M, Goossens H, Nilsson L E, Struelens M J the French and Portuguese ICU Study Groups. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. JAMA. 1999;281:67–71. doi: 10.1001/jama.281.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Hancock R E W. Antibacterial peptides and the outer membranes of gram-negative bacilli. J Med Microbiol. 1997;46:1–3. doi: 10.1099/00222615-46-1-1. [DOI] [PubMed] [Google Scholar]
- 9.Hancock R E W. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27:S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 10.Hancock R E W, Chapple D S. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe R A, Spencer R C. Macrolides for the treatment of Pseudomonas aeruginosa infections? J Antimicrob Chemother. 1997;40:153–155. doi: 10.1093/jac/40.2.153. [DOI] [PubMed] [Google Scholar]
- 12.Khardori N, Elting L, Wong E, Schable B, Bodey G P. Nosocomial infections due to Xanthomonas maltophilia (Pseudomonas maltophilia) in patients with cancer. Rev Infect Dis. 1990;12:997–1003. doi: 10.1093/clinids/12.6.997. [DOI] [PubMed] [Google Scholar]
- 13.Molinari G, Guzman C A, Pesce A, Schito G C. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J Antimicrob Chemother. 1993;31:681–688. doi: 10.1093/jac/31.5.681. [DOI] [PubMed] [Google Scholar]
- 14.Moore A J, Beazley W D, Bibby M C, Devine D A. Antimicrobial activity of cecropins. J Antimicrob Chemother. 1996;37:1077–1089. doi: 10.1093/jac/37.6.1077. [DOI] [PubMed] [Google Scholar]
- 15.Munter R G, Yinnon A M, Schlesinger Y, Hershko C. Infective endocarditis due to Stenotrophomonas (Xanthomonas) maltophilia. Eur J Clin Microbiol Infect Dis. 1998;17:353–356. doi: 10.1007/BF01709460. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Approved standard M7-A5. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Piers K L, Hancock R E W. The interaction of a recombinant cecropin/mellitin hybrid peptide with the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1994;12:951–958. doi: 10.1111/j.1365-2958.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 18.Quinn J P. Clinical problems posed by multiresistant nonfermenting gram-negative pathogens. Clin Infect Dis. 1998;27:S117–S124. doi: 10.1086/514912. [DOI] [PubMed] [Google Scholar]
- 19.Vaara M, Porro M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob Agents Chemother. 1996;40:1801–1805. doi: 10.1128/aac.40.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vartivarian S, Anaissie E, Bodey G, Sprigg H, Rolston K. A changing pattern of susceptibility of Xanthomonas maltophilia to antimicrobial agents: implications for therapy. Antimicrob Agents Chemother. 1994;38:624–627. doi: 10.1128/aac.38.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor M A, Arpi M, Bruun B, Jonsson V, Hansen M M. Xanthomonas maltophilia bacteremia in immunocompromised hematological patients. Scand J Infect Dis. 1994;26:163–170. doi: 10.3109/00365549409011780. [DOI] [PubMed] [Google Scholar]
- 22.Viljanen P, Matsunaga H, Kimura Y, Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot. 1991;44:517–523. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob Agents Chemother. 1993;37:1749–1755. doi: 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]