Abstract
Background.
Most information on mucosal and systemic immune response to norovirus infection is derived from human challenge studies, birth cohort studies, or vaccine trials in healthy adults. However, few data are available on immune responses to norovirus in the elderly.
Methods.
To study the mucosal and systemic immune response against norovirus, 43 long-term care facilities were enrolled prospectively in 2010–2014. Baseline saliva samples from 17 facilities, cases and controls up to day 84 from 10 outbreaks, as well as acute and convalescent sera were collected.
Results.
Norovirus-specific immunoglobulin A (IgA) levels in baseline saliva samples were low and increased in both symptomatic patients and asymptomatic shedders at day 5 after onset during outbreaks. Receiver operating characteristics analysis correctly assigned prior norovirus infection in 23 (92%) of 25 participants. Cases and asymptomatic shedders showed seroconversion for IgG (80%), IgA (78%), and blockade antibodies (87%). Salivary IgA levels strongly correlated with increased convalescent serum IgA titers and blockade antibodies.
Conclusions.
Salivary IgA levels strongly correlated with serum IgA titers and blockade antibodies and remained elevated 3 months after a norovirus outbreak. A single salivary sample collected on day 14 could be used to identify recent infection in a suspected outbreak or to monitor population salivary IgA.
Keywords: norovirus, IgA, IgG, salivary IgA, blockade antibodies, immune response, elderly
Noroviruses are recognized as the main cause of epidemic and endemic acute gastroenteritis in all age groups worldwide [1–3] and the primary cause of foodborne illness in the U.S. [4]. Noroviruses are genetically classified into genogroups (G) and further subdivided into genotypes. Although viruses within GI and GII can cause infections in humans, GII.4 viruses are responsible for most norovirus outbreaks [5, 6]. Histo-blood group antigens (HBGAs) expressed on intestinal epithelial cells have been identified as putative attachment factors for norovirus.
Recombinant virus-like particles (VLPs) have been used as antigens for immunological studies and vaccine development [7]. Natural infection primarily generates a genotype-specific immune response [8–10]. To elicit an immune response broad enough to generate cross-genotype blockade antibodies, current vaccine candidates include GI.1 and GII.4c VLPs [11]. Volunteers vaccinated with this formulation showed a broad cross-genotype blockade response and neutralization against GII.4 variant strains that did not circulate at the time of the study, suggesting the vaccine may protect against future emergent GII.4 strains [12, 13].
Additional information on immune response has been obtained from birth-cohort studies involving children <2 years of age [14] or challenge studies in healthy adults [15–18]. Preexisting norovirus-specific salivary immunoglobulin A (IgA) levels, circulating norovirus-specific immunoglobulin G (IgG) memory B cells, and the presence of blockade antibodies (antibodies targeting HBGA epitopes) in serum have been identified as correlates of protection against disease [15, 19]. Norovirus-specific salivary IgA levels correlate with serum IgA levels and block binding of norovirus VLPs to HBGAs [20, 21]. However, 75% of outbreaks reported in the United States occur in long-term care facilities (LTCFs), [22] which often result in an increase of hospitalization and mortality rates for adults >65 years old [23].
We previously reported the epidemiological and virological characteristics of 10 norovirus outbreaks in LTCFs [24]. In this manuscript we describe the humoral (IgA, IgG, and blockade antibody) and mucosal (salivary IgA) immune responses to norovirus using virus-like particles specific to the genotype causing each of these outbreaks.
MATERIALS AND METHODS
Participant Recruitment
Forty-three LTCFs were enrolled in the study from November 2009 through January 2013. Participants were recruited prior to 2 consecutive norovirus seasons (2010 and 2011; baseline; 14 facilities, 370 participants) and when suspected norovirus outbreaks were reported (7 facilities, 114 participants) (Supplementary Figure 1). Participants were classified as cases or controls based on clinical characteristics specific to norovirus [25] and controls were divided into exposed or nonexposed controls [24]. Demographic characteristics, clinical and virological data, and host genetic factors related to this study have been published previously (Supplementary Table 1 and Supplementary information) [24].
Saliva and Serum Collection
Baseline saliva samples were collected from 10 facilities between November 2009 and February 2010 (baseline I) and from 14 facilities between November 2010 and February 2011 (baseline II). During norovirus outbreaks, saliva samples and sera were collected from cases and exposed controls. Saliva samples were collected on the day of symptom onset or exposure (day 0) and on days 1–5, 8, 14, 21, 56, 70, and 84, and acute and convalescent sera were collected on day 0 and 21, respectively. If a specimen could not be obtained on those days, a sample was collected as close as possible to the scheduled day. For nonexposed controls, 1 saliva and 1 serum sample were collected within 7 days after the first symptomatic case in the outbreak was identified.
Virus-Like Particles
Norovirus-like particles (VLPs) were kindly provided by Dr Charles Arntzen (Arizona State University) and Dr Ralph Baric (University of North Carolina). For each serum/saliva sample, only antibodies against the norovirus strain that caused the outbreak were evaluated.
Detection of Total IgA and Norovirus-Specific IgA in Saliva
Total IgA and norovirus-specific salivary IgA titers were determined using GII.4 Den Haag VLPs for baseline I, GII.4 New Orleans VLPs for baseline II, or the outbreak-specific VLP [24]. Briefly, 96-well plates were coated with 1 μg/mL anti-human IgA or 1 μg/mL VLPs. Two-fold serial dilutions of saliva and purified human IgA (Sigma) as a standard were tested in duplicate. Bound IgA was detected using goat anti-human IgA-horseradish peroxidase (HRP) conjugate followed by color development with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Thermo Scientific).
Detection of Norovirus-Specific IgA and IgG in Serum
Norovirus-specific IgA and IgG titers were determined as described previously [26]. Briefly, 96-well plates were coated with 1 μg/mL VLPs. After blocking, 2-fold serial dilutions of serum were tested in duplicate. Bound IgA and IgG were detected using goat anti-human IgA or IgG HRP conjugate followed by color development with TMB substrate.
Detection of Blockade Antibodies
Blockade antibody assays were performed as described previously [26]. Ninety-six–well plates were coated with porcine gastric mucin (PGM) type III (Sigma) for 4 hours at room temperature and blocked overnight at 4°C with 5% dry milk in phosphate-buffered saline with 0.05% Tween-20. Two-fold serial dilutions of each serum sample were preincubated with 1 μg/mL VLP for 1 hour at 37°C and then the mixture was added to PGM-coated plates. Bound VLPs were detected using rabbit anti-norovirus hyperimmune serum and goat anti-rabbit–HRP followed by color development with TMB substrate.
Data Analysis
Total and norovirus-specific salivary IgA titers were extrapolated from the linear portion of the standard curve derived from serial dilutions of purified human IgA. To account for different variables (participants, collection time, and secretion levels), norovirus-specific IgA was normalized to 100 μg of total IgA. When norovirus-specific IgA could not be detected but total IgA was detected, IgA levels were arbitrarily assigned as half of the lower limit of detection, based on a standard curve of purified human IgA [27]. Antibody half maximal effective concentration (EC50) for serum IgA and IgG, and half maximal inhibitory concentration (IC50) for blockade antibodies were determined from log transformed nonlinear, dose-response sigmoidal curves fit data. Geometric mean titers (GMTs) and 95% confidence intervals (CIs) were calculated for each study group and each time point.
Receiver operating characteristics (ROC) analysis was used to evaluate the discriminatory power of norovirus-specific salivary IgA as a potential biomarker for retrospective diagnosis. The day with the highest discriminatory power was determined by the highest AUC (area under the curve), and the optimal threshold value (norovirus-specific/total IgA) that correctly classified participants as cases or controls was set as the maximum Youden index (sensitivity + specificity − 1) for that day. To validate this cutoff, clinically classified cases and controls for which stool was negative or not collected and saliva was available, were analyzed and cross-checked by IgA/IgG seroconversion titers.
Nonparametric data were compared with the Mann-Whitney test. Log-transformed data were analyzed by 1-way ANOVA followed by Tukey comparison test. To assess correlations, Spearman correlation coefficients were computed. Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software), and P values < .05 were considered statistically significant.
Ethics Statement
The study was approved by the institutional review boards of the Oregon State Public Health Division (IRB-08-03) and the Centers for Disease Control and Prevention (protocol number 5051). All residents and staff were eligible for inclusion, excluding those cognitively or decisionally impaired. Written informed consent was obtained from each participant.
RESULTS
Population Salivary IgA Levels Prior to Outbreaks (Baselines)
One hundred and ninety participants from 10 LTCFs were enrolled in baseline I and 180 participants from 14 LTCFs in baseline II (Supplementary Table 2 and Table 3). Seven LTCFs participated in both baseline studies. The number of enrolled participants per LTCF ranged from 7 to 36, and their ages ranged from 21 to 101 years. Eighty percent (298/370) of the participants were female (81.6% and 79.4%, baseline I and II, respectively); 33% (121/370) were residents (41.1% and 23.8%, baseline I and II, respectively); 47% (175/370) were health care workers (35.3% and 60.0%, baseline I and II, respectively); and 20% (23.7% and 16.1%, baseline I and II, respectively) were nonhealth care workers.
Norovirus-specific salivary IgA was determined against GII.4 Den Haag for baseline I samples, and GII.4 New Orleans for baseline II samples. The GMT of norovirus-specific salivary IgA was 489.2 (95% CI, 369.1–648.5) ng/100 μg of total IgA at baseline I. Significant differences were observed between facilities (P < .0001) (Figure 1). For baseline II samples, the GMT norovirus-specific salivary IgA was 42.9 (95% CI, 35.4–51.9) ng/100 μg of total IgA and significantly lower in LTCF XVII compared to other facilities (P < .01) (Figure 1). Furthermore, the GMT of the salivary IgA titers in residents was different, but not statistically significant, from the titer in health care workers in each LTCF (Supplementary Figure 2A and 2B).
Figure 1.
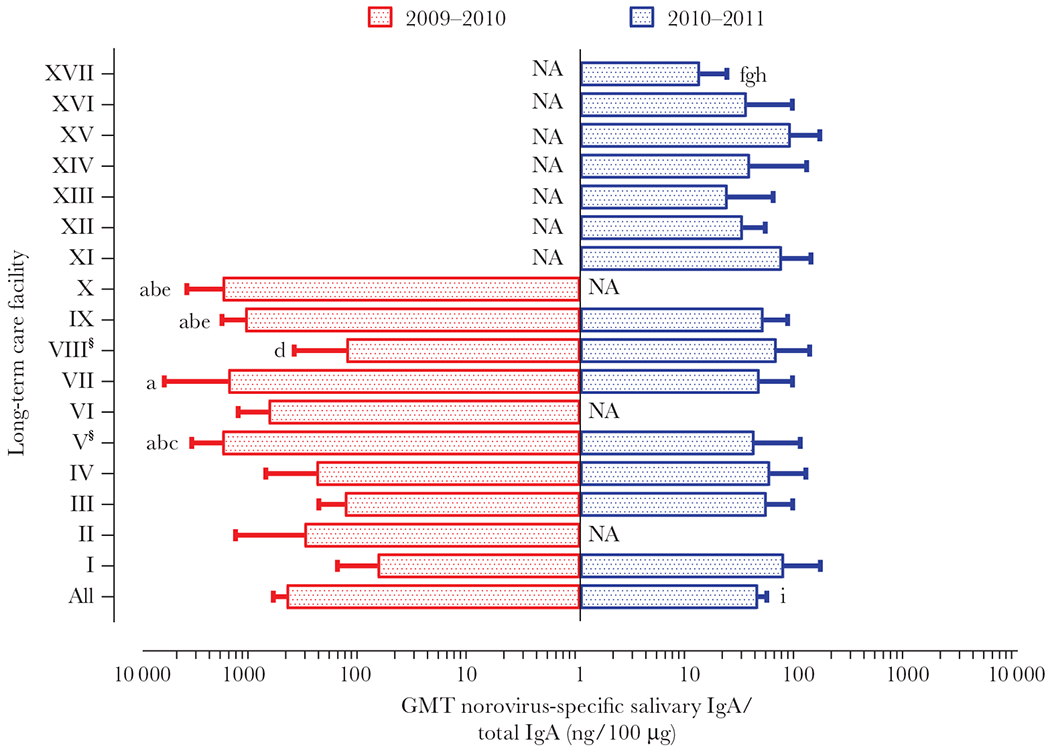
Baseline norovirus-specific salivary IgA. A total of 370 saliva samples were collected before the 2009–2010 (n = 190; baseline I) and 2010–2011 (n = 180; baseline II) winter seasons. Samples collected during baseline I were tested for norovirus-specific salivary IgA against GII.4 Den Haag VLPs, whereas samples collected during in baseline II were tested against GII.4 New Orleans VLPs. The number of saliva samples collected by facility varied between 6 and 37. § Norovirus GII.4 New Orleans and GII.4 Den Haag caused an outbreak at facilities V and VIII, respectively, between baselines. Log-transformed data were analyzed by 1-way ANOVA followed by Tukey comparison test (P < .01). Significant differences were detected on baseline I: a, significantly different from LTCF I; b, significantly different from LTCF III; c, significantly different from LTCF IV; d, significantly different from LTCF V; e, significantly different from VIII, and baseline II: f, g, and h, significantly different from LTCFs I, IX, and XV, respectively; i, significantly different from the all LTCFs during baseline I collection. Abbreviations: ANOVA, analysis of variance; GMT, geometric mean titer; IgA, immunoglobulin A; LTCF, long-term care facility; NA, not available; VLP, virus-like particle. See online version for color figure.
The overall GMT of norovirus-specific salivary IgA against GII.4 New Orleans was significantly lower than against GII.4 Den Haag (P < .0001) (Figure 1). Overall, salivary IgA antibody levels were different among all LTCFs regardless of previous IgA level or outbreak occurrence.
Salivary IgA Levels During Outbreaks
Total follow-up time from onset or exposure for the study population ranged from 3 to 84 (median 77) days. Norovirus-specific salivary IgA titers remained at baseline levels until day 4 after onset, increased between day 5 and 8 and peaked at day 14 for cases (n = 60) and then declined steadily until day 70, after which the titers stayed low but above preoutbreak levels (Figure 2A). Salivary IgA levels of exposed controls without virus shedding (n = 30) remained stable throughout the follow-up period. Significant differences between IgA levels of cases and controls were observed at days 8, 14, 21, 56 (P < .0001); day 70 (P < .01); and day 84 (P < .05) (Supplementary Table 4).
Figure 2.
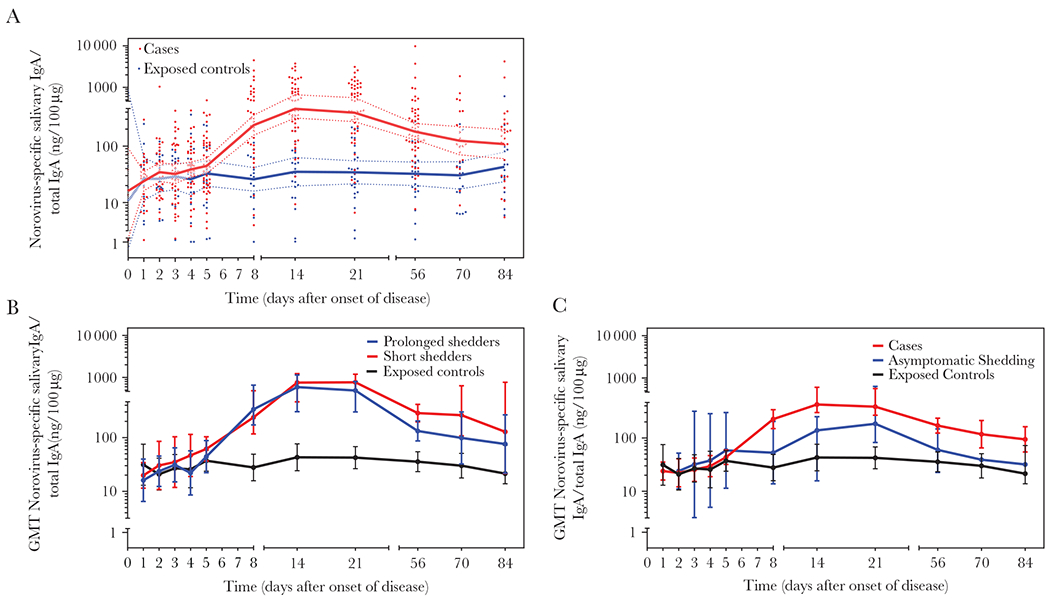
Norovirus-specific salivary IgA antibody levels during outbreaks. A, Norovirus GI.1 and GII.4 variant-specific salivary IgA antibody levels for each time point for 62 cases (red circles; n = 441) and 34 exposed controls (blue circles; n = 208) are shown. Geometrical mean values for salivary IgA antibodies for cases and exposed controls over the course of the study are depicted as red or blue lines with 95% Confidence interval (shadow), respectively. B, Norovirus-specific salivary IgA antibody levels for each time point are presented for participants with norovirus positive acute stool sample (n = 15; red circles; short shedders), with norovirus positive acute and convalescent stool sample (n = 14, blue circles; long shedders), and 35 exposed controls (black circles). C, Norovirus-specific salivary IgA antibody levels for each time point for 33 cases (red circles), 35 exposed controls (black circles), and 3 asymptomatic shedders (blue circles) are shown. Saliva samples were tested using VLPs based on the norovirus strain detected in the outbreak. Abbreviations: GMT, geometric mean titer; IgA, immunoglobulin A; VLPs, virus-like particles. See online version for color figure.
Fifteen (76%) of the 29 cases shed norovirus only in the acute stool (short shedders) whereas the other 14 also had positive convalescent stool (prolonged shedders). Both short and prolonged shedders showed an increase of salivary IgA titers (Figure 2B), which only differed significantly in day 56 saliva samples (P = .0079) (Supplementary Table 4).
Four (14%) of the 29 exposed controls also tested positive for norovirus in their acute stool and had increased norovirus-specific IgA titers at day 14 (Figure 2C) with no significant difference from cases (Supplementary Table 4).
Fluctuations in antibody levels for each genotype were further compared (Supplementary Figure 3). Although with small differences, the overall trend for cases was an increase in IgA beginning at day 5 with a peak at day 14 followed by a steady decline of titer until day 70. Significant differences between salivary IgA levels of individuals infected with different genotypes were found during the first 4 days after infection (P = .003) (Supplementary Table 5).
Norovirus-Specific Salivary IgA as Biomarkers for Retrospective Diagnosis
ROC curves based on logistic regression models for each day were constructed with norovirus-specific salivary IgA data from 39 cases with a norovirus-positive acute stool, as well as 25 controls with a negative acute stool and no IgG seroconversion (Figure 3A). Salivary IgA levels on day 14 showed the highest AUC value of 0.963 (P < .0001). The optimum cutoff to differentiate cases from controls based on salivary IgA levels on day 14 was at 183.76 ng/100 μg of total IgA (2.263 log10 [norovirus-specific salivary IgA/ total IgA]; sensitivity, 97%; specificity, 88%) (Figure 3A and 3B and Supplementary Figure 4). We tested whether saliva collected on day 14 could be used to retrospectively diagnose norovirus infection in participants from whom stool was negative or not collected. Data (symptomatology and norovirus specific-salivary IgA on day 14) were analyzed from 25 participants clinically classified as cases (n = 18) or controls (n = 7) (Figure 3C). In 11 (61%) of the 18 cases and 5 (86%) of 7 controls, the retrospective norovirus diagnosis based on salivary IgA titers agreed with the reported clinical symptoms. However, we found disagreement in 9 (36%) of 25 participants. No IgG/IgA seroconversion or blockade antibodies were shown for 5 (71%) of 7 cases at day 21, in agreement with the low salivary IgA titers on day 14 and the retrospective classification as noninfected. Elevated salivary IgA levels also agreed with norovirus infection in 2 asymptomatic individuals. Overall, retrospective classification of participants based on salivary IgA levels collected on day 14 after onset correctly differentiated infected from noninfected individuals in 23 (92%) of 25 participants regardless of virus detection in stool.
Figure 3.
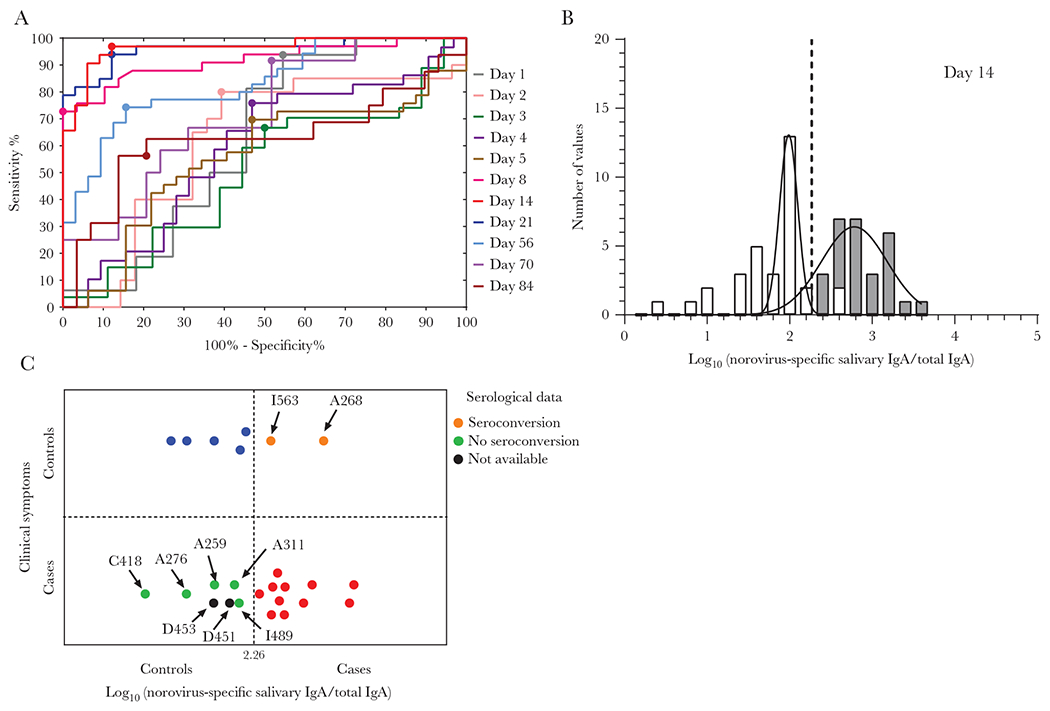
Retrospective analysis of norovirus-specific salivary IgA levels as biomarker for infection. A, Receiver operating curves for each day were constructed with data from 39 cases with positive acute stool, as well as 25 controls with negative acute stool and no IgG seroconversion. The optimal threshold value to differentiate cases from controls was set as the maximum Youden’s index (sensitivity + specificity − 1) represented with a large circle for each curve. B, Frequency distribution of observed norovirus-specific salivary IgA for cases (gray) and controls (white) at day 14 and fitted normal distribution (lines). Vertical line indicates the cutoff value (sensitivity: 96.88%; specificity: 87.88%) based on ROC at day 14 with the highest AUC value of 0.963 (P < .0001). C, Retrospective classification of cases and controls with negative or without acute stool sample, based on norovirus-specific IgA levels on day 14. In 11 (red circles) of the 18 cases, and 5 (blue circles) of 7 controls the retrospective norovirus diagnosis based on salivary IgA agree with the symptomatology. Serological data (as IgA/IgG seroconversion) were used to classify samples when clinical and retrospective IgA results did not match (orange and green circles). Abbreviations: AUC, area under the curve; IgA, immunoglobulin A; IgG, immunoglobulin G; ROC, receiver operating curve. See online version for color figure.
Norovirus-Specific IgG/IgA and Blockade Antibodies During Outbreaks
Seventy-seven acute and convalescent serum pairs were collected from 54 cases and 23 exposed controls (Supplementary Table 1). Among cases, 43 (80%) of 54 showed seroconversion (≥4-fold increase) of IgA, 42 (78%) showed seroconversion for IgG, and 47 (87%) showed seroconversion for blockade antibodies. Four (18%) of 23 exposed controls showed seroconversion (≥4-fold increase) for norovirus-specific IgG and 5 (22%) showed seroconversion for norovirus-specific IgA and blockade antibodies.
Systemic norovirus IgA and IgG were detected in acute sera from all participants (Supplementary Table 1). The GMT for IgG in cases on enrollment day (1019.0; 95% CI, 749.2–1387.0) was similar to the GMT in exposed controls (1141.0; 95% CI, 698.8–1861.0) and nonexposed controls (793.4; 95% CI, 396.7–1587.0; P = .9211). At day 21, 42 (78%) of 54 cases showed ≥ 4-fold rise of IgG (geometric mean fold rise [GMFR], 8.8; 95% CI, 5.8–13.3), whereas a 1.41-fold increase (95% CI, .8–2.4) of IgG was detected in exposed controls, of whom 4 (18%) of 23 showed ≥ 4-fold rise of IgG. Similarly, the GMT for IgA in cases on enrollment day (826.2; 95% CI, 651.6–1048.0) was similar to the GMT in exposed controls (702.4; 95% CI, 441.8–117.0) and nonexposed controls (873.1; 95% CI, 577.9–1319.0) (P = .8667). At day 21, 43 (80%) of 54 cases showed ≥4-fold rise of IgA (GMFR, 8.0; 95% CI, 5.5–11.7), whereas a 1.1-fold increase (95% CI, .7–1.8) in IgA was detected in exposed controls.
The GMT for blockade antibodies in cases during enrollment (21.4; 95% CI, 16.2–28.4) was similar to that in exposed controls (24.5; 95% CI, 15.2–39.7) and nonexposed controls (24.1; 95% CI, 14.6–39.7; P = .4757). At day 21, 47 (87%) of 54 cases showed ≥ 4-fold rise of blockade antibodies (GMFR, 26.7; CI, 17.2–41.4), whereas a 1.9-fold increase (95% CI, 1.0–3.9) was detected among controls, where 5 (22%) of23 showed ≥ 4-fold rise in blockade antibodies (Table 1 and Figure 4). Although slight differences in blockade titers were noted, none of the participants of the GI.1 outbreak showed preexisting blockade antibodies, whereas 29% of individuals in GII.4 outbreaks (cases, 13/54, 24%; exposed controls, 8/23, 35%; nonexposed controls, 6/17, 35%) showed preexisting blockade antibodies against GII.4 strains (Figure 4). No significant differences in GMT blockade antibodies were found between genogroup or GII.4 variants at day 21 after infection.
Table 1.
Systemic (IgA, IgG, and Blockade Antibody) Immune Response to Norovirus Infectiona
| Casesb | Exposed Controlsc | Nonexposed Controlsd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Immunological Marker | N° | GMT (95% CI) |
GMFR (95% CI) | Participants With ≥ 4-Fold rise (%) | N° | GMT (95% CI) |
GMFR (95% CI) | Participants With ≥ 4-Fold rise (%) | N° | GMT (95% CI) |
||
| Acute | Convalescent | Acute | Convalescent | Acute | ||||||||
| IgG | 54 | 1019.0 (749.2–1,387.0) | 8976.0 (5888.0–13 683.0) | 8.8 (5.8–13.3) | 42 (78) | 23 | 1141.0 (698.8–1861.0) | 1606.0 (679.1–3799.0) | 1.41 (.8–2.4) | 4 (18) | 17 | 793.4 (396.7–1,587.0) |
|
| ||||||||||||
| IgA | 54 | 826.2 (651.6–1,048.0) | 6593.0 (4605.0–9441.0) | 8.0 (5.5–11.7) | 43 (80) | 23 | 702.4 (441.8–1,117.0) | 785.3 (441.0–1399.0) | 1.1 (.7–1.8) | 5 (22) | 17 | 873.1 (577.9–1,319.0) |
|
| ||||||||||||
| Blockade antibodies | 54 | 20.6 (15.5–27.3) | 572.8 (374.6–875.9) | 26.7 (17.2–41.4) | 47 (87) | 23 | 24.5 (15.2–39.7) | 47.9 (21.6–106.5) | 1.9 (1.0–3.9) | 5 (22) | 17 | 24.1 (14.6–39.7) |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; IgA, immunoglobulin A; IgG, immunoglobulin G; GMT, geometric mean titer.
Table includes only those cases and exposed controls from which acute and convalescent sera were available.
Classification based on symptoms. Case group includes participant with positive or negative acute stool or without acute stool.
Classification based on symptoms. Control group includes asymptomatic (positive shedding, nonsymptoms) and noninfected (no shedding, nonsymptoms) with or without acute stool.
Only acute serum sample was collected.
Figure 4.
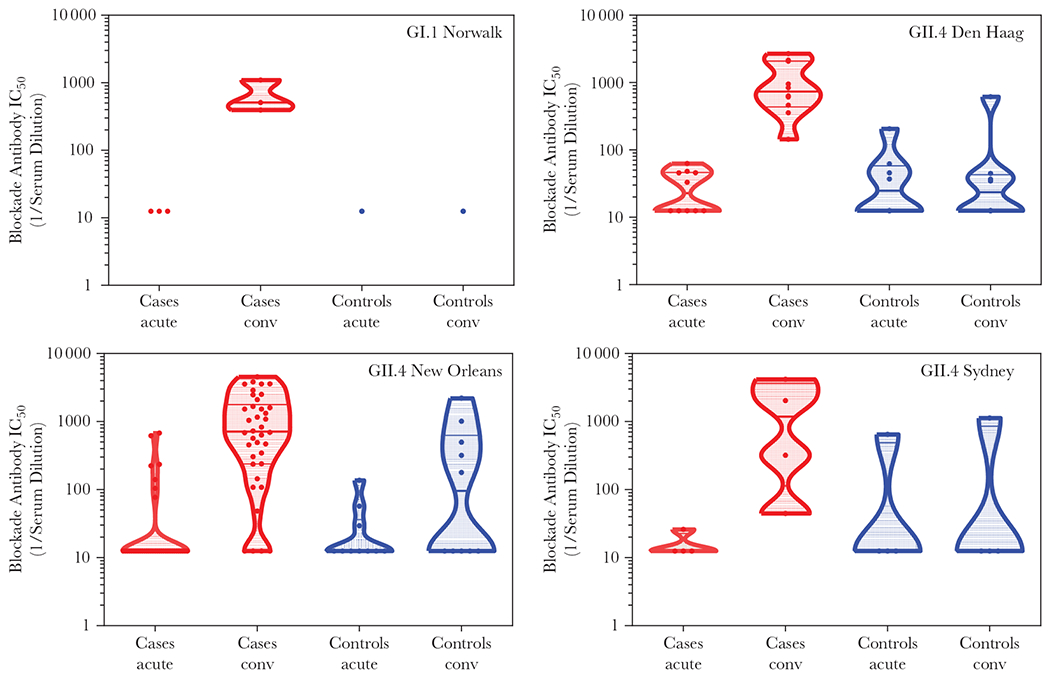
Norovirus strain-specific blockade antibody. Violin plots represent the 25%–75% percentile of reactivity for each norovirus genotype in samples collected from cases (red) and exposed controls (blue) during outbreaks. Outbreak genotypes are listed in the upper right corner of each graph. A hypothetical value of 12.5 (half of the lowest tested dilution) was assigned to negative samples. Abbreviations: IC50, half maximal inhibitory concentration; conv, convalescent. See online version for color figure.
The GMFRs for IgG, IgA, and blockade antibodies in cases without virus shedding (n = 10) were 3.2 (95% CI, .9–11.3), 4.1 (95% CI, .8–20.3), and 5.3 (95% CI, 1.5–19.3), respectively (Supplementary Table 7). Seroconversion for IgG, IgA, and blockade antibodies was detected in 5 of 10 cases, suggesting that although norovirus was not detected, these 5 individuals had been infected. The GMFR for IgG, IgA, and blockade antibodies in asymptomatic shedders (n = 2) were 7.26 (95% CI, 1.4–37.8), 4.3 (95% CI, 3.6–5.1), and 5.4 (95% CI, .7–40.3), respectively, with both asymptomatic shedders showing ≥4-fold rises for each immunological marker. Taking together the data from norovirus-specific salivary IgA response, systemic IgA, IgG, and blockade antibodies, confirmed norovirus infection in 5 (50%) of 10 participants with symptoms of AGE but without virus shedding and 2 (100%) of 2 asymptomatic shedders (Figure 5).
Figure 5.
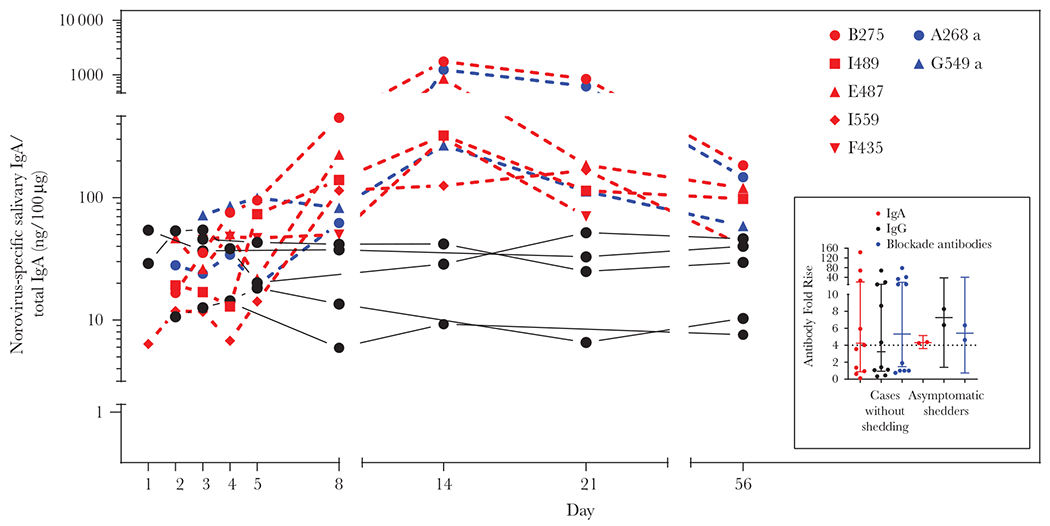
Norovirus-specific salivary immunoglobulin A (IgA) and systemic IgA/immunoglobulin G (IgG) fold rise in cases with negative stool samples or asymptomatic shedders. Norovirus GII.4 variant-specific salivary IgA antibody levels for each time point are presented for 10 cases (red and black lines) with negative acute stool sample and 2 controls (blue lines). On the side, fold rise on norovirus strain-specific IgG, IgA and blockade antibody for the 12 participants. See online version for color figure.
Correlation Between Salivary IgA, Serum IgA Antibody Levels, and Blockade Antibodies
We found a strong correlation between systemic IgA and IgG (r = 0.6082; P < .0001), systemic IgA and blockade antibodies (r = 0.6611; P < .0001), and systemic IgG and blockade antibodies (r = 0.7009; P < .0001) in convalescent but not in acute sera (Figure 6A and 6B). Correlations between convalescent serum IgA titers and salivary IgA levels consistently increased from day 5, with correlation factors equal to 0.6689, 0.7552, and 0.7569 at days 8, 14, and 21, respectively (all P < .0001). Blockade antibodies and IgA levels in saliva were strongly correlated on days 14, 21, and 56 (r = 0.7517, r =0.7910, r =5799, respectively; all P < .0001) (Figure 6C and 6D). No correlation was observed between viral load, shedding, disease duration, or disease severity and immune response.
Figure 6.
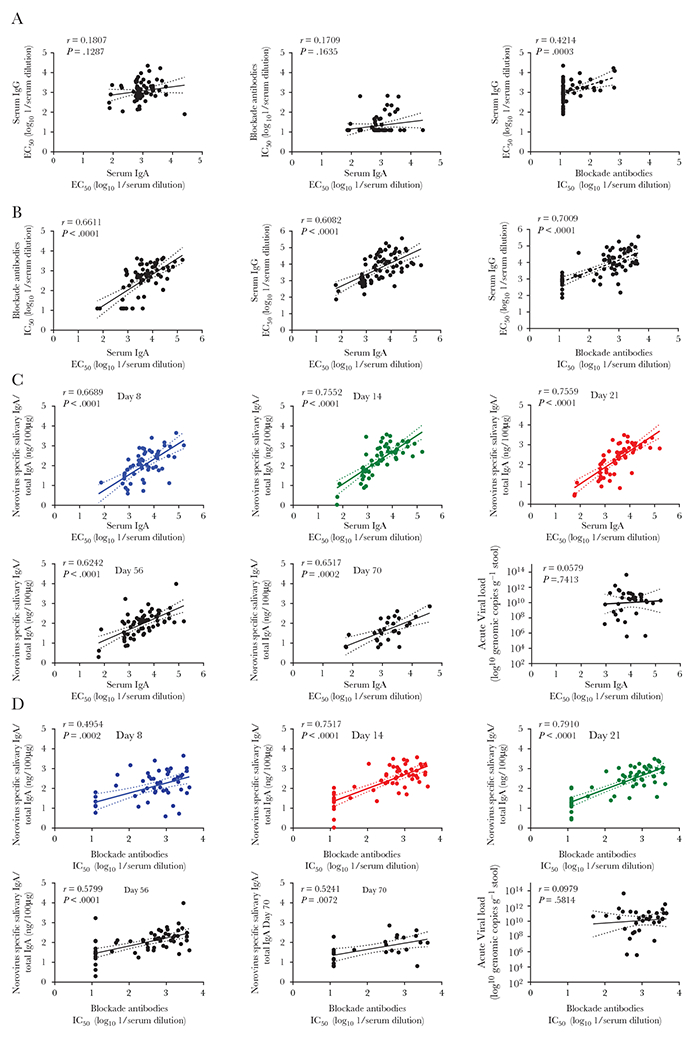
Correlation between systemic and mucosal immune response. A and B, Correlation between systemic IgA, IgG, and blockade antibody responses from cases and control. Scatterplots of norovirus-specific serum IgA vs IgG, blockade antibodies vs serum IgA, and serum IgG vs blockade antibodies from cases (n = 54) and controls (n = 23): (A) acute serum; (B) convalescent serum. C and D, Correlation between systemic and mucosal immune responses. Scatterplots of (C) convalescent serum IgA and (D) convalescent blockade antibodies vs normalized norovirus-specific salivary IgA levels (at day 8, 14, 21, 56, and 70) or viral load. Linear regression best-fit line (fitted lines) with 95% confidence bands (dotted lines). Spearman correlation coefficients and associated P value were calculated. Abbreviations: EC50, half maximal effective concentration; IC50, half maximal inhibitory concentration; IgA, immunoglobulin A; IgG, immunoglobulin G. See online version for color figure.
DISCUSSION
Salivary IgA levels remained elevated above baseline for almost 3 months after infection with norovirus. Similar to other studies [16, 21, 28], IgA titers began to increase at day 5, peaked at day 14, followed by a gradual decrease until day 84. The pattern of salivary IgA was similar for symptomatic and asymptomatic individuals. During the 2009–2010 season, norovirus baseline salivary IgA levels were similar among residents and health care workers within an institution, but significantly different among different institutions, most likely reflecting differences in exposure history. In the 2010–2011 season, however, baseline salivary IgA levels were low and similar among all institutions, which may be explained by lack of exposure to the GII.4 New Orleans strain that had emerged in the prior season (2009–2010) or that no infections had occurred during the 3 months prior to baseline saliva collection.
We confirmed that in a highly exposed LTCF population, recent human norovirus infections can be readily assessed by the detection of IgA in saliva. Similarly, the presence of salivary IgA can be used to discriminate infected from uninfected individuals in well-controlled challenge studies [19, 28]. Moreover, ROC analysis showed good discriminatory power of the salivary IgA test at day 14. Retrospective assessment of infection based on salivary IgA levels on day 14 using the cutoff derived from the ROC curves correctly assigned 92% of the participants (with norovirus-negative or uncollected stool specimens) as infected or noninfected. The salivary IgA assay sensitivity and specificity was based on the known genotype of each outbreak. Previous studies have shown no cross-reactivity of salivary IgA between different genogroups [29] and high specificity for norovirus genotype-specific salivary IgG [30]. Because our study was not designed to evaluate salivary IgA cross-reactivity between genogroups/genotypes, further studies will be required to evaluate the assay when the outbreak strain is unknown.
Systemic IgA and IgG antibody responses correlated well with the blockade antibody responses. Among norovirus cases, 80%, 78%, and 87% showed seroconversion for IgA, IgG, and blockade antibodies, respectively, while among controls, seroconversion rates were 22% for IgA, 18% for IgG, and 22% for blockade antibodies. All controls who seroconverted were asymptomatic shedders. We previously reported that the highest viral loads correlated with the recent emergence of new GII.4 variants [24]. However, fluctuation of salivary IgA levels, IgG, IgA, or blockade antibody rises did not differ by norovirus genotype during outbreaks.
Salivary antibodies can be of systemic origin, and therefore may reflect levels in serum, or they can be produced locally in mucosal tissues. Recent studies in healthy adults have reported significant correlation between serum and salivary IgA, suggesting that salivary IgA levels depend on the systemic response [19, 21]. In contrast, mucosal production of antibodies is characterized by a dimeric IgA that binds to a secretory component. Tamminen et al showed that salivary IgA was associated with secretory component, suggesting that most of the IgA was produced locally [20]. We found an increase in correlation between convalescent serum IgA and norovirus-specific salivary IgA, suggesting that salivary IgA levels depend on the systemic response. Correlations between blockade antibodies and IgA levels in saliva were strong between days 8 and 21 and moderate between days 21 and 70, similar to a recent report on salivary IgA response to an experimental norovirus vaccine [21].
Our study has several limitations. First, the data were limited to a small number of LTCFs, with populations that are generally older and more medically frail and therefore the results cannot be directly extrapolated to the general population. Second, individuals were not prescreened for norovirus antibodies, the presence of which could affect the immune response to new infections. Third, collection of saliva samples did not always start on day 0 and not all participants provided all samples. Serum samples during the acute stage of disease were collected during the first week but not on the same day after onset for all cases. These samples were only tested for antibodies with VLPs matching the genotype for each outbreak. Finally, although individual variations in salivary IgA titers were corrected by normalizing to total salivary IgA, protein loss could have occurred during sampling.
In summary, we described fluctuations in norovirus-specific salivary IgA titers through the course of 10 norovirus outbreaks in LTCFs. Salivary IgA titers were elevated, starting 8 days after infection, and could serve as a screening method for vaccine response, especially when collection of blood is challenging or infeasible. A single salivary sample could be used to identify acute infection in a suspected outbreak or to monitor population salivary IgA. Further studies are needed to determine whether blockade antibodies are present in saliva [20], and whether they are produced locally in mucosal tissue or as part of the systemic response. These data will help to further elucidate potential correlates of protection, which are critically needed to assess efficacy of future norovirus vaccines.
Supplementary Material
Acknowledgments.
We are grateful to Matthew Jaqua, John Oh, Rachele Cruz, Sandra Martin, and Keenan Williamson of the Oregon Health Authority (OHA) and Catherine Yen (CDC) for coordinating enrollment and data/sample collection. We thank LaDonna Grenz, Marjorie Yungclass, Laura Tsaknaridis, and Vanda Makris at OHA for technical assistance. We also thank all residents, health care workers, and authorities at all the long-term care facilities for their participation in this study.
Financial support.
This work was supported by the National Institute of Food and Agriculture, United States Department of Agriculture (grant number 2011-68003-30395); and a grant to the CDC Foundation from Takeda Pharmaceuticals.
Footnotes
Presented in part: 6th International Calicivirus Conference, Savannah, GA, 9–13 October 2016.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke RM, Shih SM, Yen C, et al. Burden of severe norovirus disease in Taiwan, 2003–2013. Clin Infect Dis 2018; 67:1373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol 2009; 44:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Kirk MD, Pires SM, Black RE, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 2015; 12:e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon JL, Barclay L, Collins NR, et al. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses. J Clin Microbiol 2017; 55:2208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Beek J, de Graaf M, Al-Hello H, et al. ; NoroNet. Molecular surveillance of norovirus, 2005-16: an epidemiological analysis of data collected from the NoroNet network. Lancet Infect Dis 2018; 18:545–53. [DOI] [PubMed] [Google Scholar]
- 7.Ramani S, Estes MK, Atmar RL. Norovirus vaccine development. In: Svensson L, Desselberger U, Estes MK, Greenberg HB, eds. Viral gastroenteritis. Philadelphia, PA: Elsevier, 2016. [Google Scholar]
- 8.Blazevic V, Malm M, Salminen M, et al. Multiple consecutive norovirus infections in the first 2 years of life. Eur J Pediatr 2015; 174:1679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazevic V, Malm M, Vesikari T. Induction of homologous and cross-reactive GII.4-specific blocking antibodies in children after GII.4 New Orleans norovirus infection. J Med Virol 2015; 87:1656–61. [DOI] [PubMed] [Google Scholar]
- 10.Parra GI, Green KY. Sequential gastroenteritis episodes caused by 2 norovirus genotypes. Emerg Infect Dis 2014; 20:1016–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein DI, Atmar RL, Lyon GM, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindesmith LC, Ferris MT, Mullan CW, et al. Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial. PLoS Med 2015; 12:e1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindesmith LC, McDaniel JR, Changela A, et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity 2019; 50:1530–1541.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon JL, Lopman BA, Payne DC, Vinjé J. Birth cohort studies assessing norovirus infection and immunity in young children: a review. Clin Infect Dis 2019; 69:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 2005; 79:2900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindesmith LC, Donaldson E, Leon J, et al. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 2010; 84:1800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14:1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramani S, Neill FH, Opekun AR, et al. Mucosal and cellular immune responses to Norwalk virus. J Infect Dis 2015; 212:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamminen K, Malm M, Vesikari T, Blazevic V. Norovirus-specific mucosal antibodies correlate to systemic antibodies and block norovirus virus-like particles binding to histo-blood group antigens. Clin Immunol 2018; 197:110–7. [DOI] [PubMed] [Google Scholar]
- 21.Atmar RL, Cramer JP, Baehner F, Han C, Borkowski A, Mendelman PM. An exploratory study of the salivary immunoglobulin A responses to 1 dose of a norovirus virus-like particle candidate vaccine in healthy adults. J Infect Dis 2019; 219:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah MP, Wikswo ME, Barclay L, et al. Near real-time surveillance of U.S. norovirus outbreaks by the norovirus sentinel testing and tracking network - United States, August 2009–July 2015. MMWR Morb Mortal Wkly Rep 2017; 66:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis 2011; 52:466–74. [DOI] [PubMed] [Google Scholar]
- 24.Costantini VP, Cooper EM, Hardaker HL, et al. Epidemiologic, virologic, and host genetic factors of norovirus outbreaks in long-term care facilities. Clin Infect Dis 2016; 62:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall AJ, Vinje J, Lopman B, et al. Update norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep 2011; 60:1–18. [PubMed] [Google Scholar]
- 26.Lindesmith LC, Brewer-Jensen PD, Mallory ML, et al. Antigenic characterization of a novel recombinant GII.P16-GII.4 Sydney norovirus strain with minor sequence variation leading to antibody escape. J Infect Dis 2018; 217:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Choo S, Everard J, Jennings R, Finn A. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect Immun 2000; 68:2692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moe CL, Sair A, Lindesmith L, Estes MK, Jaykus LA. Diagnosis of Norwalk virus infection by indirect enzyme immunoassay detection of salivary antibodies to recombinant Norwalk virus antigen. Clin Diagn Lab Immunol 2004; 11:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin SM, Chen IM, Fout GS, Wade TJ, Egorov AI. Development of a multiplex microsphere immunoassay for the quantitation of salivary antibody responses to selected waterborne pathogens. J Immunol Methods 2011; 364:83–93. [DOI] [PubMed] [Google Scholar]
- 30.Pisanic N, Ballard SB, Colquechagua FD, et al. Minimally invasive saliva testing to monitor norovirus infection in community settings. J Infect Dis 2019; 219:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


