Abstract
Objectives:
Dispersion in cognitive test performance within a single testing session is proposed as an early marker of poor brain health. Existing research, however, has not investigated factors that may explain individual differences in cognitive dispersion. We investigate the extent to which the Big Five personality traits are associated with cognitive dispersion in older adulthood.
Method:
To promote transparency and reliability, we applied preregistration and conceptual replication via coordinated analysis. Drawing data from seven longitudinal studies of aging (Ntotal = 33,581; Mage range = 56.4–71.2), cognitive dispersion scores were derived from cognitive test results. Independent linear regression models were fit in each study to examine personality traits as predictors of dispersion scores, adjusting for mean cognitive performance and sociodemographics (age, sex, education). Results from individual studies were synthesized using random effects meta-analyses.
Results:
Synthesized results revealed that openness was positively associated with cognitive dispersion, 0.028, 95% CI [0.003, 0.054]. There was minimal evidence for associations between cognitive dispersion and the other personality traits in independent analyses or meta-analyses. Mean cognitive scores were negatively associated with cognitive dispersion across the majority of studies, while sociodemographic variables were not consistently associated with cognitive dispersion.
Conclusion:
Higher levels of openness were associated with greater cognitive dispersion across seven independent samples, indicating that individuals higher in openness had more dispersion across cognitive tests. Further research is needed to investigate mechanisms that may help to explain the link between openness and cognitive dispersion, as well as to identify additional individual factors, beyond personality traits, that may be associated with cognitive dispersion.
Keywords: intravisit cognitive variability, personality, older adults, coordinated analysis
Cognitive aging research has traditionally focused on the study of individual differences in cognitive function; specifically, this literature emphasizes investigation of mean population differences or within-person changes in performance over time in one or more cognitive domains. The concept of cognitive dispersion, instead, refers to the degree of relative within-person variation in performance across cognitive tasks assessing various cognitive domains at the same testing occasion (Hilborn et al., 2009; Hultsch et al., 2002). Specifically, computation of cognitive dispersion captures an individual’s relative strengths and weaknesses across cognitive tests within a neuropsychological test battery, which may provide a more sensitive index of cognitive ability compared to composite scores based on central tendency. Indeed, existing research suggests that a more uniform cognitive profile represents better cognitive health (Christensen et al., 1999; Hilborn et al., 2009), which is consistent with neuroimaging research indicating that higher white matter integrity is associated with less intraindividual variability across a neuropsychological battery (Halliday et al., 2019). A deeper understanding of the extent to which individual factors, such as personality traits, predict cognitive dispersion may contribute to further understanding of the dynamics between personality traits and cognition, and how personality is involved in the cognitive aging process.
An important body of literature suggests that cognitive dispersion may be an early marker of poor brain health, dementia, and mortality. That is, while some cognitive dispersion is normal, a high degree may represent inefficient cognitive processing, beyond performance on any individual neuropsychological test (Holtzer et al., 2008; Malek-Ahmadi et al., 2018). In support of this idea, various publications have reported an association between cognitive dispersion and neuropathology in cortical (Bielak et al., 2010; Bunce et al., 2013; Das et al., 2014; Fjell et al., 2011) and neocortical (Bangen et al., 2019) areas, as well as with increased levels of amyloid beta (Duchek et al., 2009) and neurofibrillary tangles (NFT) in healthy individuals and in individuals with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) dementia (Malek-Ahmadi et al., 2017). Furthermore, higher baseline cognitive dispersion scores predict progression to MCI and dementia (Holtzer et al., 2008; Roalf et al., 2016; Tales et al., 2012) and may be similarly or independently sensitive to early pathological change compared with APOEe4 carrier status, as well as measures of hippocampal atrophy and cerebral spinal fluid (Anderson et al., 2016; Gleason et al., 2018).
This accumulated evidence has led to the postulation that cognitive dispersion may be a valuable index to identify individuals at increased risk of poor brain health for selection into trials and interventions aiming to delay the onset or reduce the risk of cognitive deterioration. Importantly, estimation of within-person cognitive dispersion based on neuropsychological procedures is simple and cost-effective for clinicians (Holtzer et al., 2008). In particular, the adoption of these indices would repurpose familiar and currently available neuropsychological tools to potentially optimize their sensitivity and specificity for dementia detection (Gleason et al., 2018; Holtzer et al., 2008; Watermeyer et al., 2020), thereby relieving clinical and research groups from the pressures surrounding the implementation of novel assessment protocols, such as expertise acquisition through staff training. This may be particularly pertinent to areas of the country or world where resources for such activities are limited (e.g., developing nations). A deeper understanding of the extent to which individual factors, such as personality traits, predict cognitive dispersion may further assist clinicians. That is, while neuropsychologists may consider dispersion to some extent when making a clinical diagnosis, the understanding that inconsistent cognitive profiles are characteristic of individuals high or low in a particular personality trait may better equip medical practitioners to evaluate normative patterns of cognitive variability across individual tests within a neuropsychological battery. For instance, knowledge that individuals higher in neuroticism are more likely to be characterized by dispersion across cognitive tests may help clinicians to better understand what constitutes normal versus abnormal cognitive dispersion in their patients, potentially providing incremental, but important, information for diagnostic screening or present impairment.
A growing body of research has examined the role of cognitive dispersion as a risk factor for dementia biomarkers and other adverse outcomes, but few studies have focused on factors that may explain individual differences in cognitive dispersion. Personality traits offer a practical option for assessing individuals’ tendencies to think, feel, react, and behave in a relatively consistent manner across the lifespan (Costa & McCrae, 1992; McCrae & Costa, 2004). While systematic reviews, meta-analyses, and coordinated analyses based on extensive reports and samples indicate that personality traits have important implications for cognitive decline (Graham et al., 2021; Luchetti et al., 2016), cognitive complaints (Aschwanden et al., 2020), and risk of dementia (Aschwanden et al., 2021), personality may also have implications for cognitive dispersion in older adulthood. For instance, conscientiousness, characterized by competence, dutifulness, and self-discipline (Costa et al., 1991), is positively associated with subjective self-regulation (Reed et al., 2020), while neuroticism, characterized by anxiety, depressive symptoms, and emotional instability (Eysenck & Eysenck, 1985), is associated with error-prone performance and impulsivity on measures of executive functioning (Crow, 2019). As the ability to self-regulate and control impulses are likely important for consistent performance across tests within a neuropsychological battery, cognitive dispersion may be characteristic of individuals low in conscientiousness and high in neuroticism.
Additionally, personality traits are related to the experience and perception of stress. High neuroticism contributes to cumulative susceptibility of psychological distress, as well as the associated damaging effects of consistent hypothalamic–pituitary–adrenal axis activation (Sapolsky, 1996). Likewise, extraversion, which is characterized by sociability, liveliness, and activity (Eysenck & Eysenck, 1985), is positively associated with the subjective experience of stress in some studies (Swickert et al., 2002). Individuals high in neuroticism and extraversion may thus demonstrate more variability in performance on a neuropsychological test battery due to test anxiety and emotional instability. Further, as outlined by Curtis et al. (2015), people high in extraversion may perform better on cognitive tasks due to assertiveness, faster responding, and lower general arousal (Chamorro-Premuzic & Furnham, 2004), though individuals high in extraversion may also be more easily distracted and have a lower patience for repetition (Gold & Arbuckle, 1990). Similarly, low conscientiousness and high neuroticism are associated with unhealthy diurnal cortisol patterns, reflecting poor biological coping mechanisms in the face of stress (Montoliu et al., 2020), which may be exacerbated by cognitive testing in older adulthood. Finally, high openness to experience is characterized by desire for and depth of varied emotional experience, as well as cognitive flexibility and intellectual engagement (Costa & McCrae, 2008; McCrae & Sutin, 2007). Research indicates that MCI (Traykov et al., 2007) and the prodromal stages of vascular cognitive impairment (Garrett et al., 2004) are characterized by poor cognitive flexibility, and that individuals with poor cognitive flexibility are more likely to convert from MCI to dementia (Tatsuoka et al., 2013). As such, openness to experience may contribute to homogeneity in performance across cognitive tests (i.e., less dispersed cognitive performance), as individuals high in openness may approach a neuropsychological battery with cognitive flexibility and receptiveness to cognitive engagement.
Although personality traits are associated with individual differences in cognitive functioning (e.g., Crowe et al., 2006; Graham et al., 2021; Luchetti et al., 2016), to our knowledge, no research has examined the extent to which personality traits predict cognitive dispersion, and only one study has investigated the role of personality in cognitive inconsistency (Munoz et al., 2020). Cognitive dispersion is related to, but distinct from, the concept of cognitive inconsistency, which refers to within-person inconsistencies or fluctuations in performance at repeated attempts at the same task within the same testing occasion (Hultsch et al., 2002). Specifically, Munoz et al. (2020) evaluated the role of neuroticism and negative affect in explaining within-person variability across reaction time trials administered 60 times to each participant at the same testing occasion. Munoz et al. (2020) posited that individuals high in neuroticism would have erratic responses in reaction time tasks due to poorer flexibility in emotional and cognitive processes. Consistent with their hypothesis, findings revealed that higher neuroticism was associated with greater variability on repeated reaction time tasks beyond mean reaction time, indicating that neuroticism may be important in the identification and intervention of cognitive dysfunction in older adults.
Munoz et al. (2020) focused on only one personality trait (neuroticism) and variability on repeated administration of only one cognitive functioning test, yet, as postulated above, other personality traits may contribute to variability in performance on cognitive tasks. Previous work indicates that cognitive dispersion is positively associated with cognitive inconsistency, which is expected if variability across various cognitive tests and repeated reaction time tests reflects relatively stable mechanisms (e.g., neurodegeneration) as opposed to dynamic or fluid influences (e.g., pain, fatigue; Hilborn et al., 2009; Hultsch et al., 2002). Given that clinicians aim to make inferences regarding neurological integrity based on performance across multiple cognitive tests, existing literature, and particularly Munoz et al.’s (2020) findings, justify further exploration of the association between individual differences in personality traits and cognitive dispersion, an index that integrates various cognitive tests and more closely reflects neuropsychological practice.
In the present study, we aim to extend this initial investigation of neuroticism as a predictor of cognitive variability by evaluating the association of all Big Five personality traits and cognitive dispersion across several cognitive measures, drawing data from seven studies of older adults. In an effort to contribute to cumulative science, generate evidence for replicability and generalizability of our research question, and protect against Type I and Type II errors, we employ a coordinated data analytical approach (Hofer & Piccinin, 2009). Coordinated analysis is a form of integrative data analysis in which variables are coded consistently across multiple data sets, which are then analyzed independently using the same analytical technique. This approach facilitates the comparison of differences in results based on study-level characteristics (e.g., baseline age of sample, number of cognitive tests), as well as identification of potential patterns of associations across studies. Further, coordinated analysis generates evidence for the replicability and generalizability of a given set of questions. Our preregistered hypotheses are based on existing literature examining the associations between personality traits and cognitive functioning (Aschwanden et al., 2020, 2021; Baker & Bichsel, 2006; Boyle et al., 2010; Curtis et al., 2015; Duberstein et al., 2011; Soubelet & Salthouse, 2011; Stephan et al., 2021). Specifically, we expect that neuroticism and extraversion will be associated with greater cognitive dispersion; that openness and conscientiousness will be associated with less dispersion; and that agreeableness will not be associated with dispersion.
Method
Data
Cross-sectional data were drawn from seven international studies of older adults, described briefly below. The measurement occasion in which the Big Five personality traits were first assessed was used in the current analyses. For this project, eligibility criteria required that participants did not have a dementia diagnosis, had data for at least one personality trait, and had cognitive performance data for at least three cognitive tests. All participants provided informed consent, and ethical approval for each study was granted by governing research committees. Data are available to other researchers by data request via Maelstrom (https://www.maelstrom-research.org/). Detailed analytic methods and hypotheses are available via the project preregistration on the Open Science Framework (https://osf.io/wrnjq/).
Cognition and Aging in the USA
The Cognition and Aging in the USA (CogUSA) study is a longitudinal study of 1,514 adults over the age of 51 living in the United States (McArdle et al., 2015). Data collection took place in three waves between 2007 and 2009. Personality traits were first assessed with the Big Five Inventory (BFI; John & Srivastava, 1999) in the second wave, which was selected for analysis. Participants were administered auditory working memory (WM), word recall, number series, picture vocabulary, block design, and the stop/go switch tasks to assess cognition.
English Longitudinal Study of Aging
The English Longitudinal Study of Aging (ELSA) is a longitudinal study of more than 12,000 English adults over the age of 50 who responded to the Health Survey for England (Steptoe et al., 2013). Data collection began in 2002 with additional measurement waves every 2 years. Personality traits were first assessed in Wave 5, which was selected for analysis, using the Midlife Developmental Inventory (MIDI; Lachman & Weaver, 1997). Participants were administered word recall, letter cancelation, and verbal fluency tasks to assess cognition.
Health and Retirement Study
The Health and Retirement Study (HRS) is a nationally representative longitudinal panel study of more than 20,000 adults in the United States who were surveyed every 2 years starting in 1992 (Sonnega et al., 2014). Personality traits were first assessed in 2006 and 2008, which were selected for analysis, using the MIDI (Lachman & Weaver, 1997). Participants were administered word recall, numeracy, and backward counting tasks to assess cognition.
Long Beach Longitudinal Study
The Long Beach Longitudinal Study (LBLS) is a longitudinal study of 2,125 adults aged 28–84 living in California (Zelinski et al., 2010). Data collection began in 1978, with an additional six waves added between 1981 and 2008, and two additional cohorts added in 1994 and 2000. Personality traits were first assessed in 1994, which was selected for analysis, using the The Revised NEO Personality Inventory; NEO-PI-R (Costa & McCrae, 2008). Participants were administered computation span, word recognition, letter series, and verbal fluency tasks to assess cognition.
Midlife in the United States Study
The Midlife in the United States Study (MIDUS) is a nationally representative longitudinal study of 7,108 adults aged 28–74 (Brim et al., 2004). Data collection began in 1994, with additional waves collected in 2004 and 2013. The Big Five personality traits were assessed at all waves, using the MIDI (Lachman & Weaver, 1997); therefore, we selected the variables collected in 1994 for analysis. Participants were administered digit span, word recall, number series, verbal fluency, and stop/go switch tasks to assess cognition.
Swedish Adoption/Twin Study of Aging
The Swedish Adoption/Twin Study of Aging (SATSA) is a longitudinal study of 2,019 adults aged 26–93 years. Data collection began in 1984, with additional measurement waves occurring every 3 years (Pedersen et al., 1991). Personality traits were first assessed in 1984, which was selected for analysis, using the NEO-PI (Costa & McCrae, 1985) and Eysenck Personality Questionnaire; EPQ (Eysenck, 1977). Participants were administered digit span, Thurstone’s picture test, Wechsler Adult Intelligence Scale (WAIS) information test, and digit symbol tasks to assess cognition.
Wisconsin Longitudinal Study
The Wisconsin Longitudinal Study (WLS) is a longitudinal study of 22,334 Wisconsin residents who graduated from high school in 1957 and their siblings (Herd et al., 2014; Sewell et al., 2003). Personality traits were first assessed in 1992–1994, which was selected for analysis, using the shortened BFI (John et al., 1991). Participants were administered digit ordering, delayed word recall, number series, and category fluency tasks to assess cognition.
Statistical Approach
Cognitive dispersion scores were derived in each of the studies following an updated formulation of a previously published index of dispersion (Hultsch et al., 2002). The method applies a z-transformation to the raw scores of each test using parameters from the distribution of the entire sample, and then, the application of the formula:
where Tik is the k-th test (transformed) for participant i, K is the number of tests, and Si is participant i’s mean of the transformed scores. Then, linear regression analyses were used to test the association of dispersion scores with each of the personality traits in univariate models and in conditional models adjusted for mean cognitive performance, education, sex, and age. All variables were z-scored to facilitate interpretation of the results. Education was measured in years in CogUSA, HRS, LBLS, SATSA, and WLS. Education was assessed using ordinal scales in MIDUS (via a 12-point scale) and ELSA (via a 7-point scale), in which higher values represent higher educational attainment. The mean for MIDUS was 7.3 (SD = 2.5), which indicates 3 or more years of college, but no completed degree. The mean for ELSA was 4.01 (2.27), which indicates National Vocational Qualification Level 2 (Grades A–C on First Diploma in the U.K.). For more information regarding the education variables in MIDUS and ELSA, see (https://www.icpsr.umich.edu/web/NACDA/studies/04652/datasets/0001/variables/B1PB1?archive=nacda and http://doc.ukdataservice.ac.uk/doc/5050/mrdoc/pdf/5050_harmonized_elsa_e.pdf, respectively). As the focus of these analyses was not education, and to promote coordination and facilitate interpretation across studies, all education variables were standardized. Sex was coded as a binary variable (males = 0; females = 1).
Meta-Analyses
Estimates of the associations between dispersion scores and personality traits from each of the studies were meta-analyzed with random effects using the metafor package (Viechtbauer, 2010) in R. The resulting partial correlation coefficients provide an indication of the overall, synthesized association in terms of direction and significance. While this process may obscure meaningful differences between studies to some extent, meta-analysis minimizes Type I and Type II errors. Further, the random effects approach explicitly addresses between-study variability due to nonidentical study characteristics (i.e., no assumption of one true effect size underlying the included studies; instead there may be different true effects for each population; Hedges & Vevea, 1998). We used the I2 index (Higgins et al., 2003) to evaluate relative heterogeneity across samples (i.e., the proportion of true variability of the effect relative to the total variability in observed effects) and τ2 as a measure of between-study variance. In particular, I2 is recommended as a criterion for a decision whether subgroup analysis or moderator analysis is indicated (Borenstein et al., 2009), while τ2 is used to assign weight to the studies within a meta-analysis under the random effects model. We preregistered meta-analytic between-study moderator analyses examining the extent to which age and number of cognitive tests included in the computation of cognitive dispersion scores accounted for the association between personality traits and cognitive tests in meta-analytic models indicating substantial heterogeneity.
Transparency and Openness
Following open science recommended practices, we preregistered our analytical approach and specific hypotheses (osf.io/wrnjq/). Within the preregistration document, we also report eligibility criteria for participants, inference criteria, all study variables, and links for each of the study data sets. All analyses were conducted in R, and all analysis code and information regarding research materials are available on the open science webpage.
Results
Across studies, baseline characteristics, personality traits, and dispersion scores are reported in Table 1, and ethnic characteristics are reported in Table 2. Table 3 lists the cognitive tests administered and used to compute cognitive dispersion indices, while Table 4 reports the mean cognitive test scores and computed cognitive dispersion scores across studies. The proportion of men and women differed across studies (χ2 = −73.79, p < .001). Similarly, there were differences in age, analysis of variance (ANOVA), F(6, 16894) = 158.5, p < .01, and education, ANOVA, F(5, 19578) = 9167.9, p < .01, across studies.
Table 1.
Baseline Characteristics and Personality Traits Across Studies
| Study | N | Female | Male | Age | Education | Neuroticism | Openness | Conscientiousness | Extraversion | Agreeableness |
|---|---|---|---|---|---|---|---|---|---|---|
| CogUSA | 1,207 | 647 (56%) | 533 (44%) | 64.09 (10.65) | 14.19 (2.35) | 37.27 (20.57) | 68.05 (17.78) | 77.44 (15.25) | 59.08 (20.45) | 81.77 (13.41) |
| ELSA | 8,771 | 4,907 (56%) | 3,864 (44%) | 66.68 (10.04) | 4.01 (2.27) | 2.59 (0.47) | 2.12 (0.56) | 1.60 (0.51) | 1.85 (0.56) | 1.49 (0.48) |
| HRS | 14,863 | 8,862 (60%) | 6,001 (40%) | 67.22 (11.30) | 12.84 (3.03) | 2.03 (0.62) | 2.91 (0.58) | 3.36 (0.50) | 3.17 (0.58) | 3.51 (0.50) |
| LBLS | 590 | 307 (52%) | 283 (48%) | 69.45 (13.92) | 13.81 (3.01) | 79.43 (20.78) | 105.79 (17.65) | 121.27 (17.64) | 104.27 (18.32) | 125.09 (15.92) |
| MIDUS | 3,671 | 2029 (55%) | 1,642 (45%) | 55.43 (12.45) | 7.3 (2.5) | 2.07 (0.63) | 2.90 (0.54) | 3.46 (0.45) | 3.11 (0.57) | 3.45 (0.50) |
| SATSA | 625 | 385 (62%) | 240 (38%) | 65.53 (8.39) | NA | 2.82 (2.32) | 2.99 (0.43) | 3.84 (0.48) | 4.75 (2.26) | 3.92 (0.44) |
| WLS | 3,855 | 2087 (54%) | 1,768 (46%) | 71.27 (0.95) | 13.6 (2.3) | 14.82 (4.71) | 20.25 (4.90) | 27.96 (4.75) | 22.22 (5.49) | 28.28 (4.82) |
Note. Values are (sub)sample size and %, or means and standard deviations; CogUSA = The Cognition and Aging in the USA; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LBLS = Long Beach Longitudinal Study; MIDUS = Midlife in the United States; SATSA = Swedish Adoption Twin Study of Aging; WLS = Wisconsin Longitudinal Study; Age and education in years, except for education in ELSA and MIDUS, which were measured according to ordinal scales variables ranging 1–7 and 1–12, respectively, in which higher values indicate high educational attainment.
Table 2.
Ethnicity Across Studies
| Study | White | Black | Other | N | Analytic sample (N) |
|---|---|---|---|---|---|
| CogUSA | 1,081 (90.08%) | 66 (5.50%) | 53 (4.42%) | 1,200 | 1,207 |
| ELSA | 8,373 (97.47%) | 58 (0.68%) | 159 (1.85%) | 8,590 | 8,771 |
| HRS | 8,903 (83.33%) | 1,278 (11.96%) | 503 (4.71%) | 10,684 | 14,863 |
| LBLS | 510 (90.11%) | 10 (1.77%) | 46 (8.13%) | 566 | 590 |
| MIDUS | 3,114 (95.20%) | 82 (2.51%) | 75 (2.29%) | 3,271 | 3,373 |
| SATSA | 625 (100%) | 0 (0%) | 0 (0%) | 625 | 625 |
| WLS | 6,713 (98.71%) | 13 (0.19%) | 75 (1.10%) | 6,801 | 3,461 |
Note. The table reports (sub)samples and percentages for each ethnicity category across studies. Reported distributions are based on the ethnicity data available from the analytic sample in all cases, except for WLS and LBLS, in which the ethnicity distributions are based on the full samples in 2005 and 1994, respectively, as these data are not available publicly. CogUSA = The Cognition and Aging in the USA; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LBLS = Long Beach Longitudinal Study; MIDUS = Midlife in the United States; SATSA = Swedish Adoption Twin Study of Aging; WLS = Wisconsin Longitudinal Study. “Other” category across samples: ELSA includes Asian, Asian British, and any other group. HRS includes American Indian, Alaskan Native, Asian, and Pacific Islander. LBLS includes American Indian, Alaskan native, Asian or Pacific Islander, and Hispanic. MIDUS includes Native American, Aleutian Islander, Asian or Pacific Islander, and Other. WLS includes Black, Indian (American) or Alaskan Native, Asian, Pacific Islander (none reported), Hispanic/Latino, and Other.
Table 3.
Cognitive Tasks Administered Aligned According to Cognitive Domains Across Studies
| Cognitive tests | |||||||
|---|---|---|---|---|---|---|---|
| Study | Working memory | Declarative memory | Fluid intelligence | Crystalized intelligence | Visuospatial reasoning | Speed | Verbal fluency |
| CogUSA | Auditory WM | Word recall | Number series | Picture vocabulary | Block design | Stop/go switch | |
| ELSA | Word recall | Numeracy | Verbal fluency | ||||
| HRS | Word recall | Numeracy | Backward counting | ||||
| LBLS | Computation span | Word recognition | Letter series | Figure rotation | Verbal fluency | ||
| MIDUS | Digit span | Word recall | Number series | Stop/Go switch | Verbal fluency | ||
| SATSA | Digit span | Thurstone picture | TUFF battery | WAIS information | Block design | Digit symbol | |
| WLS | Digit ordering | Delayed word recall | Number series | Category fluency | |||
Note. CogUSA = The Cognition and Aging in the USA; WM = working memory; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LBLS = Long Beach Longitudinal Study; MIDUS = Midlife in the United States; SATSA = Swedish Adoption Twin Study of Aging; TUFF = Test battery for investigating functional disorders; WAIS = Wechsler Adult Intelligence Scale; WLS = Wisconsin Longitudinal Study.
Table 4.
Average Cognitive Test Scores and Computed Dispersion Scores Across Studies
| Cognitive tests, aligned according to cognitive domains | ||||||||
|---|---|---|---|---|---|---|---|---|
| Working memory | Declarative memory | Fluid intelligence | Crystalized intelligence | Visuospatial reasoning | Speed | Verbal fluency | Computed cognitive dispersion score | |
| Study | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| CogUSA | 516.73 (22.36) | 11.11 (3.20) | 55.53 (10.79) | 55.55 (11.10) | 51.25 (9.92) | 534.65 (28.25) | 0.70 (0.27) | |
| ELSA | 4.59 (2.16) | 4.26 (1.95) | 5.75 (2.25) | 0.75 (0.40) | ||||
| HRS | 9.75 (3.25) | 508.34 (40.34) | 1.87 (0.50) | 0.72 (0.54) | ||||
| LBLS | 17.77 (2.44) | 11.27 (6.75) | 18.29 (10.97) | 33.23 (12.53) | 0.71 (0.35) | |||
| MIDUS | 4.96 (1.53) | 4.36 (2.63) | 2.18 (1.53) | −1.10 (0.29) | 18.85 (6.17) | 0.77 (0.34) | ||
| SATSA | 9.68 (2.15) | 20.60 (4.62) | 30.91 (8.41) | 18.44 (7.70) | 38.38 (12.29) | 0.71 (0.27) | ||
| WLS | 5.63 (1.42) | 3.44 (1.79) | 8.39 (3.67) | 11.15 (4.17) | 0.82 (0.36) | |||
Note. All reported values are raw scores, except speed for MIDUS, which is transformed so that higher values indicate better cognitive performance (these values were standardized prior to analysis). CogUSA = The Cognition and Aging in the USA; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LBLS = Long Beach Longitudinal Study; MIDUS = Midlife in the United States; SATSA = Swedish Adoption Twin Study of Aging; WLS = Wisconsin Longitudinal Study. See study description in text and Table 3 for specific cognitive tasks administered aligned according to cognitive domains.
Dispersion Scores and Personality Traits
Standardized coefficients for personality traits and mean cognitive scores from the fully adjusted linear regression models are reported in Table 5, while the full model results, including p values and estimates for all covariates, are reported in Supplemental Table 1. Meta-analytic results for the association between dispersion scores and each personality trait are reported in the following subsections.
Table 5.
Personality Trait and Mean Cognitive Score Estimates From Multivariable Linear Regression Models Fitted to Cognitive Dispersion Scores in Each of the Seven International Studies Adjusted for Age, Sex, Education, Mean Cognitive Scores, and Personality Traits
| Study | CogUSA | ELSA | HRS | LBLS | MIDUS | SATSA | WLS |
|---|---|---|---|---|---|---|---|
| Variable | β (SE) | ||||||
| Cognitive dispersion | |||||||
| Neuroticism | −0.002 (0.007) | −0.001 (0.004) | 0.003 (0.004) | −0.008 (0.015) | −0.001 (0.006) | 0.002 (0.011) | 0.003 (0.006) |
| Mean Cog score | −0.143 (0.014)** | −0.011 (0.007) | −0.495 (0.006)** | −0.052 (0.028) | 0.015 (0.010) | −0.076 (0.016)** | −0.045 (0.010)** |
| Cognitive dispersion | |||||||
| Extraversion | 0.015 (0.007)* | −0.007 (0.004) | −0.002 (0.003) | −0.0001 (0.015) | 0.020 (0.006)** | 0.013 (0.011) | 0.013 (0.006)* |
| Mean Cog score | −0.143 (0.014)** | −0.014 (0.007)* | −0.496 (0.006)** | −0.050 (0.028) | 0.015 (0.010) | −0.076 (0.016)** | −0.045 (0.010)** |
| Cognitive dispersion | |||||||
| Openness | 0.012 (0.008) | −0.006 (0.004) | 0.014 (0.004)** | 0.006 (0.016) | 0.022 (0.006)** | −0.008 (0.012) | 0.014 (0.006)* |
| Mean Cog score | −0.146 (0.014)** | −0.015 (0.007)* | −0.497 (0.006)** | −0.052 (0.028) | 0.014 (0.010) | 0.014 (0.006)** | −0.05 (0.01)** |
| Cognitive dispersion | |||||||
| Conscientiousness | −0.002 (0.007) | −0.009 (0.004)* | 0.006 (0.004) | −0.005 (0.015) | 0.010 (0.006) | −0.004 (0.011) | 0.007 (0.006) |
| Mean Cog score | −0.142 (0.014)** | −0.014 (0.007)* | −0.497 (0.006)** | −0.050 (0.028) | 0.015 (0.010) | −0.067 (0.017)** | −0.046 (0.010)** |
| Cognitive dispersion | |||||||
| Agreeableness | 0.000 (0.007) | −0.008 (0.004) | 0.006 (0.004)* | −0.016 (0.015) | 0.013 (0.006)* | −0.001 (0.012) | 0.003 (0.006) |
| Mean Cog score | −0.142 (0.014)** | −0.012 (0.007) | −0.497 (0.006)** | −0.049 (0.028) | 0.015 (0.010) | −0.068 (0.017)** | −0.045 (0.010)** |
Note. Estimates are reported as standardized coefficients; CogUSA = The Cognition and Aging in the USA; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LBLS = Long Beach Longitudinal Study; MIDUS = Midlife in the United States; SATSA = Swedish Adoption Twin Study of Aging; WLS = Wisconsin Longitudinal Study; SE = standard error; mean Cog score = mean cognitive score. See Supplemental Table 1 for all estimates from the models (trait, mean cognitive score, age, sex, and education) and all p values.
p < .05.
p < .001.
Neuroticism
Neuroticism was not associated with dispersion in cognitive performance in univariate or in fully adjusted models. Although none of the estimates of the association of neuroticism with cognitive dispersion reached preregistered significance thresholds (5%), estimates of the association between neuroticism and cognitive dispersion were negative in four studies (CogUSA, ELSA, LBLS, and MIDUS) and positive in three studies (HRS, SATSA, and WLS). The pooled effect size from the random effects meta-analysis based on independent analysis of each study was estimated as 0.0017, SE = 0.0056, 95% CI [−0.0092, 0.0126]. Heterogeneity estimates indicated no between-study variance (τ2 = 0, SE = 0.0001) and I2 was calculated as 0%, which indicates relative homogeneity between studies. See Supplemental Figure 1 for a graphical representation of the meta-analytic results.
Extraversion
In CogUSA, MIDUS, and WLS, positive and statistically significant associations between extraversion and cognitive dispersion in fully adjusted models emerged, suggesting that extroverts have more dispersion in cognitive performance across cognitive tasks. In SATSA, the estimate of the association between extraversion and cognitive dispersion scores was also positive, though nonsignificant, whereas in ELSA, HRS, and LBLS, estimates were negative and nonsignificant (Supplemental Figure 2). The estimated pooled effect of the association of extraversion and cognitive dispersion was 0.0194, SE = 0.0147, 95% CI [−0.0131, 0.0246]. τ2 and I2 were 0.0003 (SE = 0.0003) and 54.67%, respectively.
Openness
The association between openness and cognitive dispersion reached conventional significance levels in HRS, MIDUS, and WLS (β = 0.014, SE = 0.004; β = 0.022, SE = 0.006; and β = 0.014, SE = 0.006, respectively) in fully adjusted models. These results indicate that individuals with higher levels of openness had more dispersion in cognitive performance. As these are standardized scores, we can interpret the results accordingly. For example, in MIDUS, individuals who are 1 SD higher in openness are, on average, 0.02 SDs higher in cognitive dispersion. The remainder of the estimates were not statistically significant: two were negative (ELSA and SATSA), while two were positive (LBLS and CogUSA; Figure 1). The estimated pooled effect was 0.0285, SE = 0.0129, 95% CI [0.0030, 0.0537], which was significant at a 10% level. τ2 and I2 were 0.0006 (SE = 0.006) and 74.58%, respectively.
Figure 1.
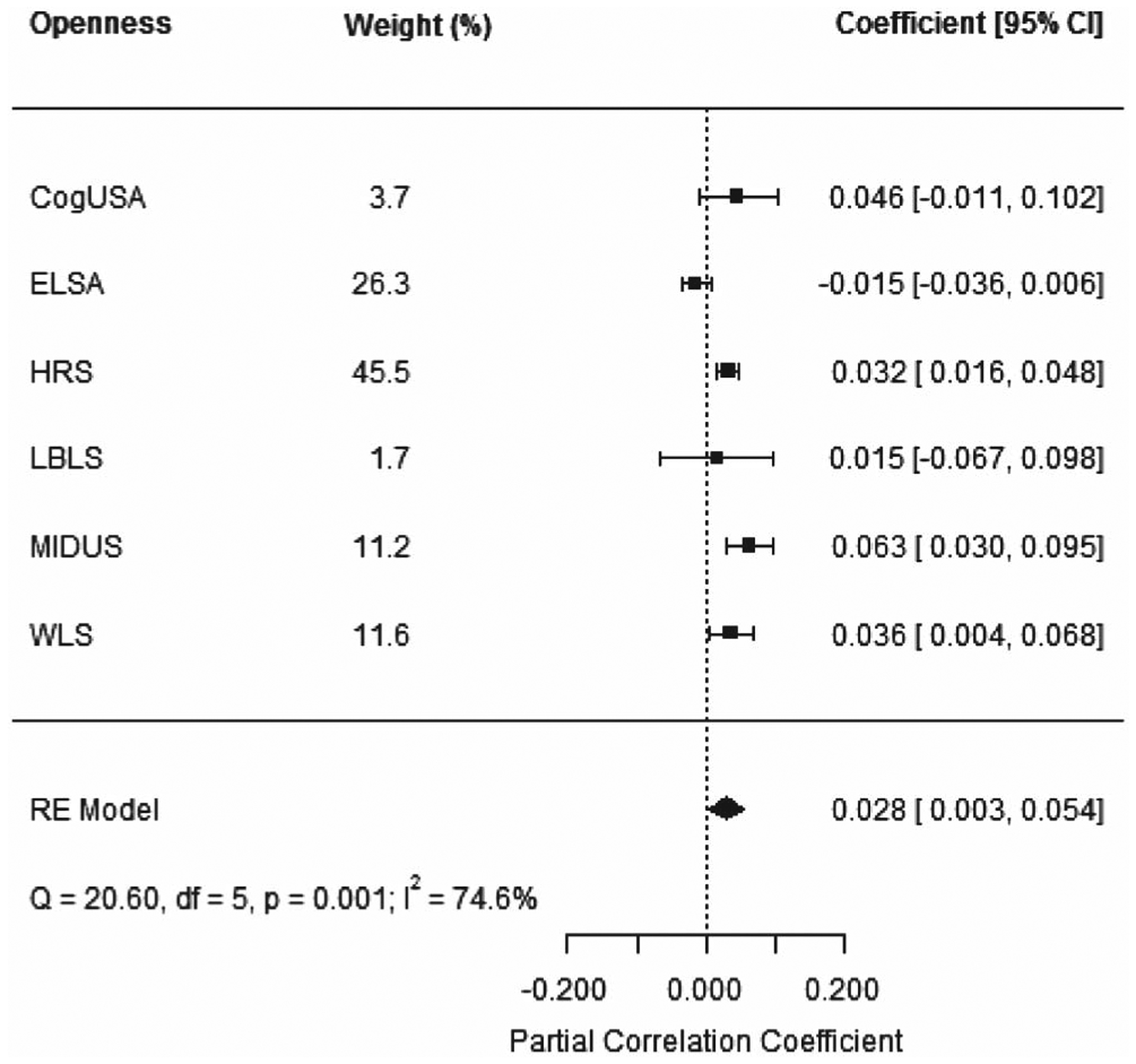
Meta-Analytic Results for the Partial Correlation Coefficient Between Openness and Cognitive Dispersion Across Studies
Conscientiousness
The association between conscientiousness and cognitive dispersion reached traditional significance levels only in ELSA, in which conscientiousness was negatively associated with dispersion scores (β = −0.009, SE = 0.004, p = .04). For CogUSA, LBLS, and SATSA, estimates were also negative, whereas in HRS, MIDUS, and WLS, estimates were positive, although none of these estimates reached statistical significance levels (Supplemental Figure 3). The estimated pooled effect of the association of conscientiousness and cognitive dispersion was 0.0058, SE = 0.0096, 95% CI [−0.0131, 0.0246]. τ2 and I2 were 0.0003 (SE = 0.0003) and 54.67%, respectively.
Agreeableness
In MIDUS and HRS, results revealed significant associations between agreeableness and cognitive dispersion; however, the estimate was positive in MIDUS (β = 0.013, SE = 0.006, p = .02) and negative in HRS (β = −0.006, SE = 0.004, p = .01). Although nonsignificant, in ELSA, LBLS, and SATSA, estimates were also negative, whereas in CogUSA and WLS, estimates were positive (Supplemental Figure 4). The pooled estimate of the association between agreeableness and cognitive dispersion was −0.004, SE = 0.0108, 95% CI [−0.0253, 0.0172]. τ2 and I2 were 0.0004 (SE = 0.0004) and 63.83%, respectively.
Sociodemographic Characteristics and Cognitive Dispersion
Mean cognitive performance was negatively associated with cognitive dispersion in all studies except two, which indicates that individuals with higher mean performance had less dispersed cognitive scores. Mean cognitive estimates were negative and nonsignificant in LBLS, and positive and nonsignificant in MIDUS.
A consistent pattern of results did not emerge across studies for the association between any of the sociodemographic characteristics and cognitive dispersion. Age at testing was negatively associated with cognitive dispersion in HRS and SATSA, suggesting that older adults had higher dispersion scores. Baseline age estimates were positive in MIDUS, and significant at p ≤ .05 for all personality traits except openness, indicating that younger adults had higher dispersion scores. Estimates for age at testing were nonsignificant across the other data sets, but positive in ELSA, negative in WLS and LBLS, and neutral in CogUSA. In MIDUS, the association between sex and cognitive dispersion was positive and significant, suggesting that female participants had higher dispersion scores than male participants. Across the remainder of studies, the association was also consistently positive, but nonsignificant. In HRS and LBLS, a significant association emerged between education and cognitive dispersion, suggesting that more educated individuals had higher dispersion scores compared to less educated individuals. In the other studies, the associations between education and dispersion were not significant and the estimated direction of the effects was inconsistent (see Supplemental Table 1). Overall, results suggest heterogeneity in the association between sociodemographic variables and cognitive dispersion.
Moderator Meta-Analyses
We preregistered study-level moderator tests for the models with substantial heterogeneity to examine if average baseline age (±65 years) or number of cognitive tests included in the computation of cognitive dispersion scores accounted for the association between personality traits and cognitive tests. After preparing the data, however, we realized that comparing the studies based on over or under 65 years old at baseline was not a meaningful comparison, as mean age substantially overlapped across studies, particularly given the deviation in mean age. That is, five of the seven studies were relatively homogeneous in terms of age, except for MIDUS, which included relatively young older adults (Mage = 56.4, SD = 12.3), and WLS, which included relatively old older adults (Mage = 71.2, SD = 0.9). Therefore, we deviated from the original preregistration and did not execute moderator analyses examining the impact of mean age. For heterogeneous models (all models except neuroticism), we executed moderator meta-analyses examining the impact of number of cognitive tests included in the computation of cognitive dispersion. Results revealed that the number of cognitive tests used in the computation of cognitive dispersion did not moderate the association between personality and cognitive dispersion.
Discussion
The present study examined five preregistered hypotheses focused on the relationships between personality traits and dispersion in cognitive performance applying a coordinated analysis approach to data from seven studies of older adults. We postulated that neuroticism and extraversion would be associated with more dispersion, whereas openness and conscientiousness would be associated with less dispersion, and no association would exist between agreeableness and dispersion. Results from the random effects meta-analyses showed that the only statistically significant pooled estimate was a positive association between openness and cognitive dispersion, which was inconsistent with our expectations based on existing literature. Overall, these findings suggest weak evidence in support of our predictions. Analyses based on individual studies, however, revealed some significant associations between individual differences in personality traits and cognitive dispersion. Specifically, consistent with our predictions, results revealed significant positive estimates between extraversion and cognitive dispersion in three out of seven associations, but the overall meta-analytic estimate was nonsignificant. Further, though conscientiousness and cognitive dispersion were negatively associated in one study, the remainder of results revealed nonsignificant associations in inconsistent directions. Finally, consistent with our expectation that no association would emerge between agreeableness and cognitive dispersion, only one study revealed a significant association, though these findings were similar to the inconsistency observed across the other traits. While findings based on individual study results may provide direction for future research, we focus our discussion on synthesized results based on the meta-analyses.
As mentioned, synthesized results revealed a positive association between openness and cognitive dispersion for individuals of the same age and gender, with the same education, and average cognitive performance, suggesting that individuals with higher openness scores have higher cognitive dispersion. The meta-analytic estimate in the opposite direction warrants careful interpretation, as we preregistered the hypothesis of a negative association between openness and cognitive dispersion. We predicted that the characteristics of individuals high in openness may contribute to a better ability to shift between cognitive tests (e.g., creativity, cognitive flexibility, and receptiveness to cognitive engagement). Moreover, existing literature indicates that higher openness is associated with better cognitive functioning (Curtis et al., 2015; Luchetti et al., 2016; Soubelet & Salthouse, 2011). Nevertheless, we cautiously consider these findings. It is possible that, rather than high openness leading to more flexibility between cognitive tasks, individuals higher in openness are more engaged by cognitive tasks that require more flexibility and creativity. For instance, given the tendency to be more imaginative and gravitate toward varied emotional experience, individuals high in openness may thrive on cognitive tasks that elicit more creativity (e.g., verbal fluency, word recognition) or perspective shifting (e.g., digit symbol, figure rotation), relative to cognitive assessment that requires memorization or WM (e.g., number series, computation span, backward counting).
We did not, however, consider the shape of cognitive dispersion profile within these analyses; as such, two individuals could have quite heterogeneous cognitive profiles despite identical cognitive dispersion scores. Future research examining the association of facets of openness with cognitive dispersion, as well as shape of cognitive dispersion profiles, may provide a more nuanced understanding of the mechanisms underlying these findings and opportunities for application within clinical settings. For instance, in the context of a substantial association between personality traits (or trait facets) and cognitive dispersion, clinicians may consider including a personality test to bolster their assessment of health and risk stratification. Within the current analyses, the strength of the association between openness and cognitive dispersion was small, though a variety of factors likely influence cognitive inconsistency, impairment, or decline in older adulthood; thus, any individual predictor is likely to demonstrate only a small-to-medium relationship. Future work could explore this association relative to more established risk factors for cognitive impairment, such as genetic, health, and lifestyle indicators, in order to guide clinical interpretations of patients’ cognitive dispersion metrics alongside personality parameters.
The meta-analytic models revealed heterogeneity levels that ranged from small (e.g., neuroticism, I2 = 0%) to substantial (openness, I2 = 74.58%). We executed meta-analytic between-study moderator analyses to examine if the number of cognitive tests used in the computation of cognitive dispersion moderated the association between personality traits and cognitive dispersion in models with substantial heterogeneity (all models except neuroticism). Results revealed that the number of cognitive tests used in the computation of cognitive dispersion did not moderate the association between personality and cognitive dispersion across any trait. We encourage follow-up investigation in this area, however, as I2 may be biased in meta-analyses based on a small number of independent effects (von Hippel, 2015).
Personality traits as predictors of individual differences in cognitive dispersion is an emerging area of inquiry, which limits our ability to compare our findings with existing reports. Munoz et al. (2020) found that neuroticism predicted greater reaction time variability (i.e., cognitive inconsistency) across ages, independent of mean response time and demographic covariates. Our results failed to expand upon these findings in our examination of the association between cognitive dispersion and neuroticism in the overall, synthesized results or in the independent analyses across seven samples. Interestingly, the estimates between neuroticism and cognitive dispersion were negative but not significant in four of the seven studies, suggesting that individuals with lower levels of neuroticism may have more dispersion in cognition than individuals with higher levels of neuroticism. These results are in partial contradiction with the results reported by Munoz et al. (2020), though our findings are not directly comparable as cognitive dispersion indices measure a different construct than reaction time variability. Yet, given previous evidence showing that individuals with higher levels of neuroticism are more error prone while completing cognitive tasks (Robinson et al., 2006), we encourage further investigation of potential explanations for these findings.
We found some evidence of a negative association between mean cognitive performance and cognitive dispersion, which indicates that individuals with higher average cognitive performance had less dispersion in performance across cognitive tasks. Across the majority of studies and models, sociodemographic variables were not significantly associated with cognitive dispersion; further, mixed results emerged regarding the direction of trends. Previous investigations of intraindividual variability in reaction time have generally shown increased variability in older participants and in participants with lower mean cognitive performance (Bielak et al., 2010). However, as previously mentioned, cognitive dispersion captures a distinct construct compared to cognitive inconsistency in reaction time.
It is worth noting that compared to the other studies, MIDUS had the most significant associations between personality traits and cognitive dispersion (openness, extraversion, and agreeableness were positively associated with cognitive dispersion). Although the average dispersion scores in MIDUS did not differ significantly from average dispersion in other studies, MIDUS was also the only study where mean cognitive performance was positively associated with cognitive dispersion, albeit a statistically nonsignificant association. Interestingly, the cognitive battery and individual cognitive tests available in MIDUS overlapped substantially with other studies; for instance, MIDUS administered identical cognitive tests (digit span, word recall, number series, Stop/Go switch task, and verbal fluency) compared to the other included studies. As average dispersion in MIDUS did not differ from other studies, and due to similarity in administration of cognitive tests compared to the other studies, differences in the MIDUS results may be due to other between-study differences in study characteristics. For instance, MIDUS is the youngest of the cohorts considered here (Mage = 56.4, SD = 12.3), and participants have the fewest years of education (Meduc = 7.28, SD = 2.54).
Importantly, age at testing was significantly and positively associated with dispersion scores in MIDUS, whereas age at testing was significantly and negatively associated with cognitive dispersion in HRS and SATSA, which both include relatively older participants. Given that participants in MIDUS were on average a decade younger than participants in HRS and SATSA, these results indicate that in a sample of relatively younger adults, the youngest adults tend to have higher dispersion scores, whereas in a sample of relatively older adults, the oldest adults have higher dispersion scores. Together, these results point to the possibility of a nonlinear, U-shaped relationship between cognitive dispersion and age; specifically, cognitive dispersion may be more substantial in young-old adults (better performance on some tests) and old-old adults (worse performance on some tests), while middle-old adults may tend to regress to their mean. These findings are consistent with research showing that age significantly impacts the association between cognitive dispersion and NFT (Malek-Ahmadi et al., 2017), such that cognitive dispersion is more strongly related to NFT for those dying at younger ages. Likewise, existing research suggests that educational attainment may influence cognitive dispersion in middle-old adults (~65 years old), but not in late-old adults (~80–90 years old), suggesting more efficient compensatory strategies in response to cognitive or neuronal senescence in younger age groups (De Felice & Holland, 2018; Watermeyer et al., 2020, 2021). We encourage researchers to extend our investigation to examine age-related compensatory strategies that may occur in response to cognitive senescence.
This investigation includes both strengths and limitations. The independent analyses conducted using seven large established studies of older adults substantiate the limited findings, while the synthesis of results further enhance the current research. In addition, we preregistered the project, including the hypotheses, on the Open Science Framework, which contributes to transparency of research. The only deviation from our original plan was not executing a meta-analysis examining age (over/under Mage = 65 years) as a between-study moderator, since mean age was clustered around 65 years in five of the studies included. Furthermore, utilizing a coordinated analysis approach, we maximized the use of all available data from cognitive tests in each of the studies, rather than coordinating at the lowest possible denominator. As such, there were between-study differences in the measures included in the derivation of cognitive dispersion. However, results remained relatively consistent across studies (i.e., limited evidence for an association between cognitive dispersion and individual differences in personality traits), despite differences and similarities in the individual tests included in the derivation of dispersion scores. Previous studies also vary in the measures included in the derivation of the scores, which suggests the index may be robust to these differences. Nevertheless, future research could consider the association between personality traits and the shape of cognitive dispersion profile, similarly to Peters et al. (2005).
The included studies also varied in several key characteristics, including the personality tests that were administered and the age at which personality was first assessed, which subsequently impacted the age at which we investigated the association between personality traits and cognitive dispersion. Another possible limitation is that we restricted our analyses to personality and cognitive dispersion, potentially neglecting other important variables, such as health (or disease) factors, which might contribute to inconsistency across tasks and level of cognitive performance, as well as correlate with personality domains (Strickhouser et al., 2017). Unfortunately, this was due in large part to inconsistencies in health assessments across the studies, which were selected on the basis of prioritizing the harmonization of personality measures and cognitive domains. Finally, the included studies are based on industrialized countries, the majority of participants are highly educated, and 83%–100% of participants identified as White across the seven studies (see Table 2). As such, our results may be limited to generalizing to predominately highly educated, industrialized, and White populations. Research based on personality surveys in 23 low- and middle-income countries (N = 94,751) suggests that assessment of the Big Five personality traits may not validly capture the intended personality traits outside of Western, educated, industrialized, rich, and democratic populations (Laajaj et al., 2019). Follow-up research focused on diverse samples that accounts for potential cross-cultural differences in interpretation of trait scales would benefit the existing literature.
To our knowledge, this investigation is the first to examine the relationship between personality traits and cognitive dispersion. It builds upon growing evidence that supports cognitive variability as a marker for cognitive and neurological dysfunction by attempting to delineate the influence of individual differences in personality traits on dispersion scores across several older age groups. Apart from age and education, there has been limited exploration of other variables that may influence cognitive variability. We encourage researchers to further examine the associations between personality traits and cognitive dispersion longitudinally and at different times during older adulthood, as it is possible that associations emerge as individuals’ cognitive functioning deteriorates and inconsistency in performance across tests becomes more substantial.
Supplementary Material
Key Points
Question:
Are personality traits associated with individual differences in cognitive dispersion (i.e., relative variation in performance across cognitive tasks)?
Findings:
Across seven independent samples of older adults, individuals higher in openness to experience had greater cognitive dispersion.
Importance:
The knowledge that inconsistent cognitive profiles may be characteristic of individuals high in openness, and that associations between other personality traits and cognitive dispersion are likely to be small or null, may better equip medical practitioners to evaluate healthy versus unhealthy cognitive functioning in older adulthood.
Next Steps:
Future research should investigate mechanisms that may help to explain the link between openness and cognitive dispersion, as well as additional individual factors that may contribute to cognitive dispersion.
Acknowledgments
Research reported in this publication was supported by the Alzheimer Society Research Program, Social Sciences and Humanities Research Council, and the National Institute on Aging of the National Institutes of Health under Award Numbers P01AG043362, R01-AG018436, and R01AG067622. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Tomiko Yoneda played lead role in project administration and writing of review and editing; supporting role in formal analysis, funding acquisition, and methodology; and equal role in visualization and writing of original draft. Alejandra Marroig played lead role in formal analysis and software and equal role in visualization. Eileen K. Graham played supporting role in conceptualization, funding acquisition, methodology, supervision, writing of original draft, and writing of review and editing and equal role in data curation. Emily C. Willroth played supporting role in methodology, writing of original draft, and writing of review and editing and equal role in data curation. Tamlyn Watermeyer played supporting role in writing of original draft and writing of review and editing. Emorie D. Beck played supporting role in writing of original draft and equal role in data curation. Elizabeth M. Zelinski played supporting role in supervision and equal role in investigation. Chandra A. Reynolds played supporting role in supervision and equal role in investigation. Nancy L. Pedersen played supporting role in supervision and equal role in investigation. Scott M. Hofer played supporting role in supervision and writing of original draft and equal role in funding acquisition. Daniel K. Mroczek played supporting role in conceptualization, methodology, supervision, and writing of original draft and equal role in funding acquisition. Graciela Muniz-Terrera played lead role in conceptualization, methodology, and supervision; supporting role in project administration and writing of review and editing; and equal role in funding acquisition and writing of original draft.
Footnotes
Supplemental materials: https://doi.org/10.1037/neu0000782.supp
The experiment materials are available at https://osf.io/5gxa3/.
The preregistered design is available at https://osf.io/h8jn5/.
References
- Anderson AA, Keren N, Lilja A, Godby KM, Gilbert SB, & Franke WD (2016). Utility of baroreflex sensitivity as a marker of stress. Journal of Cognitive Engineering and Decision Making, 10(2), 167–177. 10.1177/1555343416653887 [DOI] [Google Scholar]
- Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, & Terracciano A (2021). Is personality associated with dementia risk? A meta-analytic investigation. Ageing Research Reviews, 67, Article 101269. 10.1016/j.arr.2021.101269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden D, Sutin AR, Luchetti M, Allemand M, Stephan Y, & Terracciano A (2020). A systematic review and meta-analysis of the association between personality and cognitive failures/complaints. Social and Personality Psychology Compass, 14(11), Article e12565. 10.1111/spc3.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TJ, & Bichsel J (2006). Personality predictors of intelligence: Differences between young and cognitively healthy older adults. Personality and Individual Differences, 41(5), 861–871. 10.1016/j.paid.2006.02.017 [DOI] [Google Scholar]
- Bangen KJ, Weigand AJ, Thomas KR, Delano-Wood L, Clark LR, Eppig J, Werhane ML, Edmonds EC, & Bondi MW (2019). Cognitive dispersion is a sensitive marker for early neurodegenerative changes and functional decline in nondemented older adults. Neuropsychology, 33(5), 599–608. 10.1037/neu0000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak AAM, Hultsch DF, Strauss E, MacDonald SWS, & Hunter MA (2010). Intraindividual variability is related to cognitive change in older adults: Evidence for within-person coupling. Psychology and Aging, 25(3), 575–586. 10.1037/a0019503 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2009). Introduction to meta-analysis. Wiley. [Google Scholar]
- Boyle LL, Lyness JM, Duberstein PR, Karuza J, King DA, Messing S, & Tu X (2010). Trait neuroticism, depression, and cognitive function in older primary care patients. The American Journal of Geriatric Psychiatry, 18(4), 305–312. 10.1097/JGP.0b013e3181c2941b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, & Kessler RC (Eds.) (2004). How healthy are we? A national study of well-being at Midlife. John D. and Catherine T. MacArthur Foundation Series on Mental Health and Human Development: Studies in Successful Midlife Development. University of Chicago Press. https://search.proquest.com.ezproxy.library.uvic.ca/books/how-healthy-are-we-national-study-well-being-at/docview/56197354/se-2?accountid=14846 [Google Scholar]
- Bunce D, Bielak AAM, Cherbuin N, Batterham PJ, Wen W, Sachdev P, & Anstey KJ (2013). Utility of intraindividual reaction time variability to predict white matter hyperintensities: A potential assessment tool for clinical contexts? Journal of the International Neuropsychological Society, 19(9), 971–976. 10.1017/S1355617713000830 [DOI] [PubMed] [Google Scholar]
- Chamorro-Premuzic T, & Furnham A (2004). A possible model for understanding the personality—intelligence interface. British Journal of Psychology, 95(2), 249–264. 10.1348/000712604773952458 [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, & Jacomb P (1999). Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 6(3), 214–228. 10.1076/anec.6.3.214.779 [DOI] [Google Scholar]
- Costa PT Jr., & McCrae RR (1985). The NEO personality inventory manual. Psychological Assessment Resources. [Google Scholar]
- Costa PT Jr., & McCrae RR (1992). Four ways five factors are basic. Personality and Individual Differences, 13(6), 653–665. 10.1016/0191-8869(92)90236-I [DOI] [Google Scholar]
- Costa PT Jr., & McCrae RR (2008). The revised NEO Personality Inventory (NEO-PI-R). In Boyle GJ, Matthews G, & Saklofske DH (Eds.), The SAGE handbook of personality theory and assessment, Volume 2. Personality measurement and testing (pp. 179–198). SAGE Publications. 10.4135/9781849200479.n9 [DOI] [Google Scholar]
- Costa PT Jr., McCrae RR, & Dye DA (1991). Facet scales for agreeableness and conscientiousness: A revision of the NEO Personality Inventory. Personality and Individual Differences, 12(9), 887–898. 10.1016/0191-8869(91)90177-D [DOI] [Google Scholar]
- Crow AJD (2019). Associations between neuroticism and executive function outcomes: Response inhibition and sustained attention on a continuous performance test. Perceptual and Motor Skills, 126(4), 623–638. 10.1177/0031512519848221 [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, & Gatz M (2006). Personality and risk of cognitive impairment 25 years later. Psychology and Aging, 21(3), 573–580. 10.1037/0882-7974.21.3.573 [DOI] [PubMed] [Google Scholar]
- Curtis RG, Windsor TD, & Soubelet A (2015). The relationship between Big-5 personality traits and cognitive ability in older adults—A review. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 22(1), 42–71. 10.1080/13825585.2014.888392 [DOI] [PubMed] [Google Scholar]
- Das D, Tan X, Bielak AAM, Cherbuin N, Easteal S, & Anstey KJ (2014). Cognitive ability, intraindividual variability, and common genetic variants of catechol-O-methyltransferase and brain-derived neurotrophic factor: A longitudinal study in a population-based sample of older adults. Psychology and Aging, 29(2), 393–403. 10.1037/a0035702 [DOI] [PubMed] [Google Scholar]
- De Felice S, & Holland CA (2018). Intra-individual variability across fluid cognition can reveal qualitatively different cognitive styles of the aging brain. Frontiers in Psychology, 9, Article 1973. 10.3389/fpsyg.2018.01973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, & Franks P (2011). Personality and risk for Alzheimer’s disease in adults 72 years of age and older: A 6-year follow-up. Psychology and Aging, 26(2), 351–362. 10.1037/a0021377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse C-S, Holtzman DM, Fagan AM, & Goate AM (2009). The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology, 23(6), 746–758. 10.1037/a0016583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ (1977). Manual of the Eysenck personality questionnaire (junior and adult): H. J. Eysenck and Sybil Eysenck Hodder and Stoughton (1975). 47 pp., together with test blanks and scoring keys for junior and adult versions. Specimen set £1.80. Behaviour Research and Therapy, 15(4), 369–370. 10.1016/0005-7967(81)90058-9 [DOI] [Google Scholar]
- Eysenck HJ, & Eysenck MW (1985). Personality and individual differences: A natural science approach. Plenum Press. http://uvic.summon.serialssolutions.com/2.0.0/link/0/eLvHCXMwfV07C8IwED58LIKDT7Q-6OSmNC-TzmJxcFBwL01MwMXJ_4-XPrQUcUyGIzku-e6R7wLA6C7aNu4ErSxzzBhOtNEx4YabiEnlnJVWibz5fo1N9sm_NTsnlsSTP_UZKveCtaGNfojnc1zPlXFhYEH3isq8Yos4jJEN52UHnmoc1Vp_lgiTDKDjWQdDaNnnGDaXr4scYqgfPj60qbD60QTP9wSC5Hg7nLZeWlomYtJicXQK_cy_Xn--cpbbfQZhRhG1iXP6jmjJYqE0UQY141mtkVV0DqMfkoKfswvokViJIk2whK5DM7arYmPrXCVvocxwsA [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, & Walhovd KB (2011). Reduced white matter integrity is related to cognitive instability. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(49), 18060–18072. 10.1523/JNEUROSCI.4735-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett KD, Browndyke JN, Whelihan W, Paul RH, DiCarlo M, Moser DJ, Cohen RA, & Ott BR (2004). The neuropsychological profile of vascular cognitive impairment—no dementia: Comparisons to patients at risk for cerebrovascular disease and vascular dementia. Archives of Clinical Neuropsychology, 19(6), 745–757. 10.1016/j.acn.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Gleason CE, Norton D, Anderson ED, Wahoske M, Washington DT, Umucu E, Koscik RL, Maritza Dowling N, Johnson SC, Carlsson CM, Asthana S, & the Alzheimer’s Disease Neuroimaging Initiative. (2018). Cognitive variability predicts incident Alzheimer’s disease and mild cognitive impairment comparable to a cerebrospinal fluid biomarker. Journal of Alzheimer’s Disease, 61(1), 79–89. 10.3233/JAD-170498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DP, & Arbuckle TY (1990). 13 interactions between personality and cognition and their implications for theories of aging. In Lovelace EA (Ed.), Advances in psychology (Vol. 72, pp. 351–377). North-Holland. 10.1016/S0166-4115(08)60794-3 [DOI] [Google Scholar]
- Graham EK, James BD, Jackson KL, Willroth EC, Luo J, Beam CR, Pedersen NL, Reynolds CA, Katz M, Lipton RB, Boyle P, Wilson R, Bennett DA, & Mroczek DK (2021). A coordinated analysis of the associations among personality traits, cognitive decline, and dementia in older adulthood. Journal of Research in Personality, 92, Article 104100. 10.1016/j.jrp.2021.104100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DWR, Gawryluk JR, Garcia-Barrera MA, & MacDonald SWS (2019). White matter integrity is associated with intraindividual variability in neuropsychological test performance in healthy older adults. Frontiers in Human Neuroscience, 13, Article 352. 10.3389/fnhum.2019.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, & Vevea JL (1998). Fixed- and random-effects models in meta-analysis. Psychological Methods, 3(4), 486–504. 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- Herd P, Carr D, & Roan C (2014). Cohort profile: Wisconsin longitudinal study (WLS). International Journal of Epidemiology, 43(1), 34–41. 10.1093/ije/dys194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.), 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, & Hunter MA (2009). Intraindividual variability across cognitive domains: Investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology, 31(4), 412–424. 10.1080/13803390802232659 [DOI] [PubMed] [Google Scholar]
- Hofer SM, & Piccinin AM (2009). Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychological Methods, 14(2), 150–164. 10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, & Lipton RB (2008). Within-person across-neuropsychological test variability and incident dementia. Journal of the American Medical Association, 300(7), 823–830. 10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, & Dixon RA (2002). Variability in reaction time performance of younger and older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 57(2), P101–P115. 10.1093/geronb/57.2.P101 [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, & Kentle RL (1991). The Big Five inventory—Versions 4a and 54. University of California, Berkeley, Institute of Personality and Social Research. [Google Scholar]
- John OP, & Srivastava S (1999). The Big Five trait taxonomy: History, measurement, and theoretical perspectives. In Pervin L & John OP (Eds.), Handbook of personality: Theory and research (2nd ed., pp. 102–138). Guilford Press. [Google Scholar]
- Laajaj R, Macours K, Pinzon Hernandez DA, Arias O, Gosling SD, Potter J, Rubio-Codina M, & Vakis R (2019). Challenges to capture the big five personality traits in non-WEIRD populations. Science Advances, 5(7), Article eaaw5226. 10.1126/sciadv.aaw5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M, & Weaver S (1997). The Midlife Development Inventory (MIDI) personality scales: Scale construction and scoring (pp. 1–9). Brandeis University. [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, & Sutin AR (2016). Personality and cognitive decline in older adults: Data from a longitudinal sample and meta-analysis. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 71(4), 591–601. 10.1093/geronb/gbu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Lu S, Chan Y, Perez SE, Chen K, & Mufson EJ (2017). Cognitive domain dispersion association with Alzheimer’s disease pathology. Journal of Alzheimer’s Disease, 58(2), 575–583. 10.3233/JAD-161233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi M, O’Connor K, Schofield S, Coon DW, & Zamrini E (2018). Trajectory and variability characterization of the Montreal cognitive assessment in older adults. Aging Clinical and Experimental Research, 30(8), 993–998. 10.1007/s40520-017-0865-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J, Rodgers W, Willis R, & Inter-university Consortium for Political and Social Research. (2015). Cognition and Aging in the USA (CogUSA) 2007–2009 (ICPSR 36053). https://www.icpsr.umich.edu/web/NACDA/studies/36053/versions/V1
- McCrae RR, & Costa PT Jr. (2004). A contemplated revision of the NEO five-factor inventory. Personality and Individual Differences, 36(3), 587–596. 10.1016/S0191-8869(03)00118-1 [DOI] [Google Scholar]
- McCrae RR, & Sutin AR (2007). New Frontiers for the five-factor model: A preview of the literature. Social and Personality Psychology Compass, 1(1), 423–440. 10.1111/j.1751-9004.2007.00021.x [DOI] [Google Scholar]
- Montoliu T, Hidalgo V, & Salvador A (2020). Personality and hypothalamic-pituitary-adrenal axis in older men and women. Frontiers in Psychology, 11, Article 983. 10.3389/fpsyg.2020.00983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz E, Stawski RS, Sliwinski MJ, Smyth JM, & MacDonald SWS (2020). The ups and downs of cognitive function: Neuroticism and negative affect drive performance inconsistency. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 75(2), 263–273. 10.1093/geronb/gby032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, & DeFaire U (1991). The Swedish Adoption Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae, 40(1), 7–20. 10.1017/S0001566000006681 [DOI] [PubMed] [Google Scholar]
- Peters KR, Graf P, Hayden S, & Feldman H (2005). Neuropsychological subgroups of cognitively-impaired-not-demented (CIND) individuals: Delineation, reliability, and predictive validity. Journal of Clinical and Experimental Neuropsychology, 27(2), 164–188. 10.1080/13803390490515496 [DOI] [PubMed] [Google Scholar]
- Reed RG, Combs HL, & Segerstrom SC (2020). The structure of self-regulation and its psychological and physical health correlates in older adults. Collabra. Psychology, 6(1), Article 23. 10.1525/collabra.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Mechanic-Hamilton D, Wolk DA, Arnold SE, Moberg PJ, & the Alzheimer’s Disease Neuroimaging Initiative. (2016). Within-individual variability: An index for subtle change in neurocognition in mild cognitive impairment. Journal of Alzheimer’s Disease, 54(1), 325–335. 10.3233/JAD-160259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Wilkowski BM, & Meier BP (2006). Unstable in more ways than one: Reaction time variability and the Neuroticism/Distress relationship. Journal of Personality, 74(2), 311–344. 10.1111/j.1467-6494.2005.00377.x [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1996). Why stress is bad for your brain. Science, 273(5276), 749–750. 10.1126/science.273.5276.749 [DOI] [PubMed] [Google Scholar]
- Sewell WH, Hauser RM, Springer KW, & Hauser TS (2003). As we age: A review of the Wisconsin longitudinal study, 1957–2001. Research in Social Stratification and Mobility, 20, 3–111. 10.1016/S0276-5624(03)20001-9 [DOI] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, & Weir DR (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubelet A, & Salthouse TA (2011). Personality-cognition relations across adulthood. Developmental Psychology, 47(2), 303–310. 10.1037/a0021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2021). Memory and personality development in adulthood: Evidence from four longitudinal studies. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 76(1), 88–97. 10.1093/geronb/gbaa086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Breeze E, Banks J, & Nazroo J (2013). Cohort profile: The English longitudinal study of ageing. International Journal of Epidemiology, 42(6), 1640–1648. 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickhouser JE, Zell E, & Krizan Z (2017). Does personality predict health and well-being? A metasynthesis. Health Psychology, 36(8), 797–810. 10.1037/hea0000475 [DOI] [PubMed] [Google Scholar]
- Swickert RJ, Rosentreter CJ, Hittner JB, & Mushrush JE (2002). Extraversion, social support processes, and stress. Personality and Individual Differences, 32(5), 877–891. 10.1016/S0191-8869(01)00093-9 [DOI] [Google Scholar]
- Tales A, Leonards U, Bompas A, Snowden RJ, Philips M, Porter G, Haworth J, Wilcock G, & Bayer A (2012). Intra-individual reaction time variability in amnestic mild cognitive impairment: A precursor to dementia? Journal of Alzheimer’s Disease, 32(2), 457–466. 10.3233/JAD-2012-120505 [DOI] [PubMed] [Google Scholar]
- Tatsuoka C, Tseng H, Jaeger J, Varadi F, Smith MA, Yamada T, Smyth KA, Lerner AJ, & the Alzheimer’s Disease Neuroimaging Initiative. (2013). Modeling the heterogeneity in risk of progression to Alzheimer’s disease across cognitive profiles in mild cognitive impairment. Alzheimer’s Research & Therapy, 5(2), 14–14. 10.1186/alzrt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traykov L, Raoux N, Latour F, Gallo L, Hanon O, Baudic S, Bayle C, Wenisch E, Remy P, & Rigaud A-S (2007). Executive functions deficit in mild cognitive impairment. Cognitive and Behavioral Neurology, 20(4), 219–224. 10.1097/WNN.0b013e31815e6254 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- von Hippel PT (2015). The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Medical Research Methodology, 15(1), Article 35. 10.1186/s12874-015-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermeyer T, Goerdten J, Johansson B, & Muniz-Terrera G (2021). Cognitive dispersion and ApoEe4 genotype predict dementia diagnosis in 8-year follow-up of the oldest-old. Age and Ageing, 50(3), 868–874. 10.1093/ageing/afaa232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermeyer T, Marroig A, Ritchie CW, Ritchie K, Blennow K, & Muniz-Terrera G (2020). Cognitive dispersion is not associated with cerebrospinal fluid biomarkers of Alzheimer’s disease: Results from the European Prevention of Alzheimer’s Dementia (EPAD) v500.0 Cohort. Journal of Alzheimer’s Disease, 78(1), 185–194. 10.3233/JAD-200514 [DOI] [PubMed] [Google Scholar]
- Zelinski E, Kennison R, & Inter-university Consortium for Political and Social Research. (2010). Long beach longitudinal study (ICPSR 26561). https://www.icpsr.umich.edu/web/NACDA/studies/26561/versions/V2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


