Abstract
Background
Therapeutic hypothermia (TH) is regarded as the most efficacious therapy for neonatal hypoxic encephalopathy. However, limitations in previous systematic reviews and the publication of new data necessitate updating the evidence. We conducted this up-to-date systematic review to evaluate the effects of TH in neonatal encephalopathy on clinical outcomes.
Methods
In this systematic review and meta-analysis, we searched Medline, Cochrane Library, Embase, LIVIVO, Web of Science, Scopus, CINAHL, major trial registries, and grey literature (from inception to October 31, 2021), for randomized controlled trials (RCT) comparing TH vs normothermia in neonatal encephalopathy. We included RCTs enrolling neonates (gestation ≥35 weeks) with perinatal asphyxia and encephalopathy, who received either TH (temperature ≤34°C) initiated within 6 hours of birth for ≥48 hours, vs no cooling. We excluded non-RCTs, those with delayed cooling, or cooling to >34°C. Two authors independently appraised risk-of-bias and extracted data on mortality and neurologic disability at four time points: neonatal (from randomization to discharge/death), infancy (18-24 months), childhood (5-10 years), and long-term (>10 years). Other outcomes included seizures, EEG abnormalities, and MRI findings. Summary data from published RCTs were pooled through fixed-effect meta-analysis.
Results
We identified 36 863 citations and included 39 publications representing 29 RCTs with 2926 participants. Thirteen studies each had low, moderate, and high risk-of-bias. The pooled risk ratios (95% confidence interval, CI) were as follows: neonatal mortality: 0.87 (95% CI = 0.75, 1.00), n = 2434, I2 = 38%; mortality at 18-24 months: 0.88 (95% CI = 0.78, 1.01), n = 2042, I2 = 51%; mortality at 5-10 years: 0.81 (95% CI = 0.62, 1.04), n = 515, I2 = 59%; disability at 18-24 months: 0.62 (95% CI = 0.52, 0.75), n = 1440, I2 = 26%; disability at 5-10 years: 0.68 (95% CI = 0.52, 0.90), n = 442, I2 = 3%; mortality or disability at 18-24 months: 0.78 (95% CI = 0.72, 0.86), n = 1914, I2 = 54%; cerebral palsy at 18-24 months: 0.63 (95% CI = 0.50, 0.78), n = 1136, I2 = 39%; and childhood cerebral palsy: 0.63 (95% CI = 0.46, 0.85), n = 449, I2 = 0%. Some outcomes showed significant differences by study-setting; the risk ratio (95% CI) for mortality at 18-24 months was 0.79 (95% CI = 0.66,0.93), n = 1212, I2 = 7% in high-income countries, 0.67 (95% CI = 0.41, 1.09), n = 276, I2 = 0% in upper-middle-income countries, and 1.18 (95% CI = 0.94, 1.47), n = 554, I2 = 75% in lower-middle-income countries. The corresponding pooled risk ratios for ‘mortality or disability at 18-24 months’ were 0.77 (95% CI = 0.69, 0.86), n = 1089, I2 = 0%; 0.56 (95% CI = 0.41, 0.78), n = 276, I2 = 30%; and 0.92 (95% CI = 0.77, 1.09), n = 549, I2 = 86% respectively. Trials with low risk of bias showed risk ratio of 0.97 (95% CI = 0.80, 1.16, n = 1475, I2 = 62%) for neonatal mortality, whereas trials with higher risk of bias showed 0.71 (95% CI = 0.55, 0.91), n = 959, I2 = 0%. Likewise, risk ratio for mortality at 18-24 months was 0.96 (95% CI = 0.83, 1.13), n = 1336, I2 = 58% among low risk-of-bias trials, but 0.72 (95% CI = 0.56, 0.92), n = 706, I2 = 0%, among higher risk of bias trials.
Conclusions
Therapeutic hypothermia for neonatal encephalopathy reduces neurologic disability and cerebral palsy, but its effect on neonatal, infantile and childhood mortality is uncertain. The setting where it is implemented affects the outcomes. Low(er) quality trials overestimated the potential benefit of TH.
Neonatal hypoxic ischemic encephalopathy is a significant cause of mortality and morbidity. It is also associated with adverse outcomes such as cerebral palsy, cognitive dysfunction, epilepsy, and others, well beyond the neonatal period. These have a cascading impact on the community and society through increased health care utilization, need for special services, economic burden, and diminished workforce productivity. Several interventions have been explored to manage neonatal encephalopathy (NE). Among these, therapeutic hypothermia (TH) is ranked highest, with several studies and systematic reviews [1,2] reporting reduction in mortality and adverse neurological and/or neurodevelopmental outcomes during infancy [3,4]. TH involves controlled cooling of the body (or at least of the head) during the first 2-4 days of life, followed by a gradual rewarming to a euthermic state [1,5]. Currently, it is implemented globally, including in many low-resource health care settings [6-8], although the International Liaison Committee on Resuscitation advised its use only in institutions with adequate monitoring and intensive care facilities [9].
A recent multi-country HELIX trial reported that TH was associated with an alarming increase in both immediate and late mortality, prompting the authors to emphatically recommend its immediate discontinuation in resource-constrained settings [10]. This created considerable consternation, especially in some developing countries, with arguments about the trial methods, generalizability, and other issues [11-17]. However, critical appraisal of the trial confirmed its validity [18], despite some plausible explanations for the stark differences in key outcomes [19]. Additionally, a systematic review restricted to trials from developing countries reported limited benefit of TH in such settings [20].
These developments necessitate a detailed review of the available evidence. The Cochrane review published in 2013 is outdated, and also contained some data analysis errors, such as combining short-term and long-term outcomes in the same meta-analysis [1]. A more recent review, updated as of mid-2020, contained several errors such as duplication of data from some trials, presenting data from non-existent trials, missing relevant trials, combining short-term and long-term mortality together, and expressing relative risk with negative integers [2]. Therefore, we conducted an up-to-date systematic review of randomized controlled trials (RCTs) to evaluate the effects of therapeutic hypothermia (Intervention), vs normothermia or no hypothermia (Comparison), in neonates with hypoxic encephalopathy (Population), on mortality and neurological and/or neurodevelopmental features (Outcomes). The question of this review was: What are the effects of therapeutic hypothermia in newborns with hypoxic encephalopathy?
METHODS
This review was registered in PROSPERO (Registration number CRD42021279682, dated 20 October 2021) [21] and conducted in accordance with the Cochrane Handbook for systematic reviews [22]. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Protocols (PRISMA-P) 2020 statement [23].
Criteria for considering studies for this review
Types of studies: We included RCTs comparing the use of therapeutic hypothermia vs normothermia, or no hypothermia. We excluded non-randomized trials, cohort studies, trials with historic controls, case series, trials in animals, in vitro experiments, and ex vivo human studies.
Types of participants: We included RCTs enrolling newborn infants with a gestational age ≥35 weeks, having evidence of perinatal asphyxia and encephalopathy. Perinatal asphyxia was defined by one or more of the following: a) Apgar score ≤5 at 5 minutes of life; b) need for ongoing resuscitation or respiratory support at 10 minutes; or c) cord blood/arterial blood pH<7.1, or base deficit ≥12 within one hour of birth. Evidence of encephalopathy was based on Sarnat staging system or any other recognized staging/classification system.
Types of intervention: We included RCTs delivering TH (whole-body cooling [WBC] or selective head cooling [SHC]) by any device/equipment, initiated within 6 hours of birth, with documented reduction in core temperature (to ≤34°C in case of WBC) or middle ear temperature (to ≤34°C in case of SHC). We excluded trials where TH was initiated later than six hours after birth (in all or the majority of infants), or cooling was conducted without documentation of core temperature (as specified above), or was done for <48 hours.
Types of comparison: The comparator was normothermia, or no therapeutic cooling, or no intervention. We excluded studies without a comparison group, those in which the comparison group had received any cooling for any duration, or a historic comparison group.
Types of outcome measures: We considered the following outcomes: mortality, neurological impairment or disability (defined by any standard criteria), the composite outcome of mortality or disability, and cerebral palsy. We assessed these at four time points after randomization: a) Neonatal, ie, from randomization to discharge or death during the initial hospitalization; b) Infancy, ie, at the age of 18-24 months, c) Childhood, ie, at the age of 5-10 years, and d) Long-term, ie, beyond the age of 10 years. Other outcomes were seizures, electroencephalogram (aEEG) abnormalities, MRI findings suggesting neuronal damage during the initial hospitalization, duration of hospitalization, and quality of life. For this analysis, the primary outcome was listed as “mortality or neurological disability” at ≥18 months of age [21].
Information sources: Two authors independently searched the following databases: Medline, Embase, Cochrane Library, LIVIVO, Web of Science, Scopus, and CINAHL. We searched the following clinical trial registries: World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, and Clinical Trials Registry – India. We also hand-searched reference lists of included trials, as well as previous (narrative and systematic) reviews. In addition, we conducted a grey literature search using OpenGrey (www.opengrey.eu/), ProQuest, and Google Scholar. Each database was searched from its date of inception to October 31, 2021, without restrictions based on language or geography.
Search strategy: We used combinations of MeSH terms and synonyms of the following keywords, and their variations: neonate, newborn, perinatal, infant, hypothermia, therapeutic hypothermia, cool, cooling, therapeutic cooling, asphyxia, hypoxia, hypoxic-ischemic, encephalopathy, neonatal encephalopathy. The searches were pilot-tested before finalizing the strategy. The search strategy in representative databases is summarized in Table S1 in the Online Supplementary Document.
Selection of studies: Two review authors independently screened citation titles, followed by the abstracts of short-listed citations, followed by full-text of potentially eligible studies (and those without abstracts). Thereafter, two authors independently examined the full text versions of short-listed studies, to confirm eligibility for inclusion, and recorded reasons for exclusion of ineligible studies. Disagreements were discussed and resolved by consensus. After eliminating duplicate publications, a final list of studies was prepared. A PRISMA flow diagram was created, summarizing the search results and process of including studies.
Translation of languages other than English: Non-English publication abstracts were translated using open-source software; if eligible, the full text was translated as well.
Data extraction: Two review authors independently extracted the following information from the included studies.
Trial characteristics: design, study duration, setting, date of publication.
Participant characteristics: inclusion criteria, exclusion criteria, gestational age, birth weight, definition of perinatal asphyxia, definition and severity of encephalopathy, sample size.
Intervention characteristics: WBC or SHC, method of cooling, temperature targeted, method of determining target temperature, cooling duration, cooling cessation criteria.
Comparison characteristics: Temperature targeted, method of determining target temperature, and standard of care.
Outcomes: Data on the outcomes listed above were extracted along with notes/remarks.
Dealing with missing data: We attempted to contact the corresponding authors of studies with missing or unclear data.
Data synthesis and statistical analysis: We presented data on baseline characteristics with descriptive statistics. We pooled data on the outcomes of interest and performed meta-analysis, using Cochrane Review Manager version 5.4 [24]. For dichotomous outcomes, we calculated risk ratios (RR) with 95% confidence interval (CI) using the fixed-effect model. For continuous outcomes, we calculated the weighted mean difference with 95% CI (fixed-effect model). We opted for the fixed-effect model, as the alternative (random effects-model) tends to assign disproportionately greater weight to studies with smaller sample sizes. However, wherever the heterogeneity statistic exceeded 50%, we re-examined the pooled effect with the random effects model also. For data that could not be pooled by meta-analysis, we provided a description, summarizing the key results.
Assessment of methodological quality of included studies: Two authors independently assessed methodological quality, using version 2 of the Cochrane Risk-of-Bias (RoB) tool [25]. We assessed RoB for each reported outcome of each trial, and the overall RoB of each trial.
Assessment of heterogeneity: We assessed heterogeneity among trials by visual inspection of the forest plots, and the Higgins-Thompson I2 method. We interpreted heterogeneity as outlined in the Cochrane Handbook: 75%-100% = considerable heterogeneity, 50%-90% = may represent substantial heterogeneity, 30%-60% = may represent moderate heterogeneity, and 0%-40% = might not be important [22]. Where I2 exceeded 50%, we tried to identify explanations.
Subgroup analysis: We conducted a subgroup analysis based on the following criteria: a) Study setting (defined by the World Bank Classification of the country where the trial was conducted): high-income country (HIC), upper middle-income country (UMIC), lower middle-income country (LMIC), low-income country (LIC); and b) Type of cooling: WBC vs SHC. We planned subgroup analysis based on cooling method (formal devices vs informal methods), but there were insufficient studies.
Sensitivity analysis: We assessed the impact of low(er) quality studies, by excluding trials with moderate/high RoB.
RESULTS
We identified 36 863 citations, of which 85 citations were short-listed, and 39 publications [10,26-63] reporting 29 trials with 2926 participants [10,26-32,34-36,38,39,41-47,49,50,53,57-62] were included (Figure 1). Characteristics of the included studies are presented in Table 1, and their detailed description in Table S2 in the Online Supplementary Document. The reasons for excluding 46 studies [64-109] are presented in Table S3 in the Online Supplementary Document. Two authors independently categorized 13 studies each as having overall high, moderate, and low RoB (Table S4 in the Online Supplementary Document).
Figure 1.
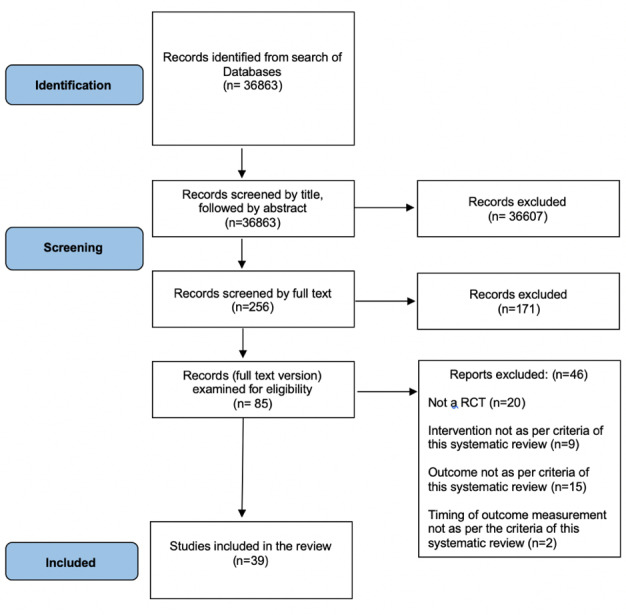
Flowchart highlighting screening and selection of studies.
Table 1.
Characteristics of the included studies
| No. |
Trials (n = 29) |
Publications (n = 39) |
Country |
Inclusion criteria |
Exclusion criteria |
Number randomized |
Type of cooling and method |
Site of temperature measurement |
Target temperature (°C) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
TH
|
Control
|
TH
|
Control
|
||||||||
| 1 |
Akisu |
Akisu 2003 [32] |
Turkey |
5 min AS<6; cord blood or arterial pH 7.1 or BD>10 mmol/l; encephalopathy (stupor, hypotonia, abnormal neonatal reflexes) |
Metabolic disorders, congenital malformations, chromosomal abnormalities, congenital infection, transitory drug depression |
11 |
10 |
SHC (cooling caps) |
EAC; rectal |
33.0-33.5 |
36.0-36.5 |
| 2 |
Battin |
Battin 2001 [34]*, Battin 2003 [63]† |
New Zealand |
GA≥37 wk; 5 min AS≤6 or cord/first arterial pH≤7.09; encephalopathy (lethargy/stupor, hypotonia, abnormal reflexes) |
Major congenital abnormalities |
25 |
15 |
SHC (cooling caps) |
rectal |
36.5-36 (n = 6); 35.9-35.5 (n = 6); 35.0 ± 0.5 (n = 6); 34.5 ± 0.5 (n = 7) |
37.0 ± 0.2 |
| 3 |
Bharadwaj |
Bharadwaj 2012 [35] |
India |
GA>37 wk with HIE; ABG pH≤7 or BE≥-12 meq within 1st h and also fulfilling any two of, AS≤6 at 10 min; evidence of fetal distress; assisted ventilation for at least 10 min after birth; evidence of any organ dysfunction; and history of acute perinatal event with evidence of encephalopathy |
Age >6 h, major congenital anomalies, if the infant did not establish spontaneous respiration by 20 min after birth, out-born babies |
65 |
65 |
WBC (gel packs) |
rectal |
33.0-34.0 |
36.5 |
| 4 |
Bhat |
Bhat 2006 [36] |
India |
NA |
NA |
20 |
15 |
WBC (not mentioned) |
rectal, skin |
33.5 |
NA |
| 5 |
Catherine |
Catherine 2020 [38] |
India |
pH≤7 or BD≤12 meq in cord blood; AS≤6 at 10 min, any clinical evidence of fetal distress, requiring assisted ventilation for at least 10 min after delivery, and any evidence of one or more organ dysfunction |
Age >6 h, out-born babies, major congenital abnormalities, no spontaneous respiratory efforts by 20 min following delivery |
78 |
84 |
WBC (phase changing material) |
rectal |
33.5 ± 0.5 |
36.5 |
| 6 |
Chen |
Chen 2018 [39] |
China |
NA |
NA |
20 |
20 |
SHC (not mentioned) |
rectal |
34.5-35.0 |
NA |
| 7 |
CoolCap Trial |
Gluckman 2005 [29] |
USA |
AS≤5 at 10 min after birth; need for resuscitation at 10 min after birth; or severe acidosis (pH<7.00 or BD≥16 mmol/l in cord, arterial or venous sample within 60min of birth) |
Age >5.5 h, prophylactic high-dose anticonvulsants, major congenital abnormalities, head trauma causing major intracranial hemorrhage, severe growth restriction, BW<1800 g, infants judged critically ill, unavailability of essential equipment, planned concurrent participation in other experimental treatments |
116 |
118 |
SHC (cooling caps) |
rectal |
34.0-35.0 |
36.8-37.2 |
| 8 |
Eicher |
Eicher 2005 [41] |
USA |
GA≥35 wk, BW 2000 g, hypoxic-ischemic insult, with one clinical sign & two neurologic findings of hypoxia-ischemia, cord pH 7.0 or BD 13, initial infant pH 7.1, AS 5 at 10 min, continued resuscitation after 5 min, fetal bradycardia with HR 80/min lasting 15 min, or postnatal hypoxic ischemic event with oxygen desaturation 70% or arterial oxygen tension 35 mm Hg for 20 min with evidence of ischemia |
Clinical sepsis, maternal chorioamnionitis, weight or head circumference <10th percentile for gestation age, or congenital abnormalities |
32 |
33 |
WBC (cooling blanket) |
rectal |
33.0 ± 0.5 |
37.0 ± 0.5 |
| 9 |
El Shimi |
El Shimi 2014 [57] |
Egypt |
pH≤7.0 or BD≥16 mmol/l in cord or any blood during 1st h after birth. If pH 7.01-7.15, BD 10.0-15.9 mmol/l, or blood gas unavailable, additional criteria viz. acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, hemorrhage, or cardiorespiratory arrest) and either 10 min AS≤5 or assisted ventilation initiated at birth and continued for >10 min |
Major congenital anomalies, severe growth restriction (BW 1800g), presence of an infectious cause, suspected inborn error of metabolism, age >6 h |
10 |
10 |
WBC (cool packs) |
rectal |
33.0-34.0 |
NA |
| 10 |
HELIX trial |
Thayyil 2021 [10] |
India, Sri Lanka, Bangladesh |
GA≥37 wk, BW≥1kg, need for resuscitation at 5 min of age or AS<6 at 5 min of age (for babies born in hospital), or both, or absence of crying by 5 min of age (for babies born at home); and evidence of moderate or severe encephalopathy between 1-6 h of age |
No heart rate at 10 min of age despite adequate resuscitation, with major life-threatening congenital malformations |
202 |
206 |
WBC (servo-controlled cooling device) |
rectal |
33.5 ± 0.10 |
36.7 ± 0.06 |
| 11 |
ICE trial |
Jacobs 2011 [26], Cheong 2012 [40] |
Australia, New Zealand, Canada, USA |
GA≥35 wk, age <6 h, moderate or severe encephalopathy and indicators of peripartum hypoxia-ischemia (≥2 of the following, AS≤5 at 10 min, continued need for mechanical ventilation at 10 min, and/or metabolic acidosis (cord, arterial or venous pH<7.00; or BD≥12 within 60 min of birth) |
Age >6 h, BW<2 kg, major congenital abnormalities, bleeding, >80% inspired oxygen, imminent death, TH before assessment |
110 |
111 |
WBC (gel packs) |
rectal |
33.0-34.0 |
37.0 |
| 12 |
Inder |
Inder 2004 [43] |
Australia |
GA≥35 with intrapartum hypoxia-ischemia comprising at least two of, AS≤5 at 10 min; ongoing resuscitation at 10 min; and metabolic acidosis (cord pH≤7 or BD≥12 mmol/l or more within 60 min of life) combined with clinical evidence of encephalopathy |
NA |
13 |
14 |
WBC (cool packs) |
rectal |
33.0-34.0 |
36.8-37.3 |
| 13 |
Jose |
Jose 2018 [44] |
India |
Moderate or severe encephalopathy within 6 h after birth after an acute perinatal event, with acidosis or resuscitation |
NA |
77 |
79 |
WBC (not mentioned) |
NA |
33.0 |
NA |
| 14 |
Joy |
Joy 2012 [45] |
India |
GA≥37 wk with cord or peripheral blood pH≤7 or BD≥12 mEq within 1 h with evidence of encephalopathy and with any two of, AS at 10 min ≤5; assisted ventilation for at least ≥10 min after birth; evidence of any organ dysfunction; history of acute perinatal event (intrapartum fetal distress, cord prolapse, placental abruption, uterine rupture, maternal trauma, or cardiac arrest) |
Age >6 h, major congenital abnormalities; no spontaneous respiration by 20 min, out-born babies |
58 |
58 |
WBC (gel packs) |
rectal |
33.0-34.0 |
36.5 |
| 15 |
Li |
Li 2009 [46] |
China |
GA≥37 wk; BW>2500 g, admitted to NICU within 10 h$$ history of asphyxia (AS at 5 min ≤5 with ABG pH<7.1 or BD>16 mmol/l within 1h birth); clinical evidence of encephalopathy |
Major congenital abnormalities; head trauma; skull fracture; enrollment >10 h after birth |
46 |
47 |
WBC (cooling mattress) |
rectal |
33.0-34.5 |
37.0 |
| 16 |
Lin |
Lin 2006 [47] |
China |
GA≥37 wk; AS at 5 min <6 with postnatal ABG pH<7.1 or BD>15 mEq/l; signs of postpartum encephalopathy (decreased muscle tone, lethargy, coma, or seizures within 6 h after birth) |
Major congenital abnormalities; prolonged hypoxemia due to severe persistent fetal circulation |
32 |
30 |
SHC (cooling caps) |
rectal; NP |
34.5 ± 0.5 (rectal); 34.4 ± 0.5 (NP) |
37.1 ± 0.5 (rectal); 36.8 ± 0.5 (NP) |
| 17 |
neo.nEURO.network trial |
Simbruner 2010 [58] |
Germany |
AS 5, or continued need for resuscitation at 10 min after birth, cord or any arterial pH 7.00 within 1 h after birth, BD 16 mmol/l, encephalopathy (lethargy, stupor, or coma and one of the following, hypotonia, abnormal reflexes, absent or weak suck), clinical seizures and abnormal standard EEG or aEEG findings |
Age >5.5 h, high-dose anticonvulsant therapy, BW 1800 g or GA 36 wk, head circumference <3rd percentile, major congenital malformations with poor developmental prognosis, Imperforate anus gross hemorrhage, infant “in extremis” |
64 |
65 |
WBC (cooling blanket) |
rectal |
33.0-34.0 |
36.5-37.5 |
| 18 |
NEST study |
Field 2013 [42] |
UK |
Standard criteria for ECMO eligibility based on the clinical decision of the local ECMO team |
Congenital diaphragmatic hernia, post-cardiac surgery, any therapeutic cooling before randomization |
56 |
55 |
WBC (heat exchanger in the ECMO circuit) |
rectal |
34.0 |
37.0 |
| 19 |
NICHD trial |
Shankaran 2005 [27],Shankaran 2008 [54], Shankaran 2012 [55], Shankaran 2012a [56], |
USA |
Cord or any blood pH≤7.0 or BD≥16 mmol/l during 1st h after birth. If pH 7.01-7.15, BD 10.0-15.9 mmol/l, or blood gas unavailable, additional criteria applied viz. acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, hemorrhage, or cardiorespiratory arrest) and either 10 min AS≤5 or assisted ventilation initiated at birth and continued for at least 10 min |
Age >6 h, major congenital abnormality, severe growth restriction (BW≤1800 g), refusal of consent by a parent or attending neonatologist; moribund infants |
102 |
106 |
WBC (cooling blanket) |
esophageal |
33.5 |
36.0-37.0 |
| 20 |
Rakesh |
Rakesh 2017 [49] |
India |
GA≥37 wk, cord or arterial blood pH≤7 or BD≥12 meq within 1st h, encephalopathy (Sarnat and Sarnat staging) |
Age >6 h; major congenital abnormalities, absent spontaneous respiratory efforts by 20min after birth; out-born babies |
60 |
60 |
WBC (phase) changing material) |
rectal |
33.0-34.0 |
NA |
| 21 |
Robertson |
Robertson 2008 [50] |
Uganda |
GA≥37 wk, need for resuscitation, and/or AS<6 at 5 min plus abnormal neurological assessment (>5 on Thompson score) from 30 min to 3 h after birth |
Apnea or cyanosis, absent cardiac output for >10 min after birth, BW<2 kg |
21 |
15 |
WBC (cooling mattress) |
rectal |
33.0-34.0 |
36.5 |
| 22 |
Shankaran |
Shankaran 2002 [53] |
USA |
Cord or any blood pH within 1st h 7.0 or BD 16 mEq/l. If blood gas unavailable or pH at 1h 7.01-7.15 or BD 10.0-15.9 mEq/l, additional history of acute perinatal event and either AS = 5 at 10 min or continued need for ventilation initiated at birth and continued for at least 10 min |
Age >6 h, chromosomal abnormality, major congenital anomaly, severe growth restriction (BW 1800 g), infant unlikely to survive, and parent or attending neonatologist refusal of consent |
9 |
10 |
WBC (cooling blanket) |
oesophageal |
34.5 |
36.5 |
| 23 |
Sun |
Sun 2012 [59] |
China |
AS<3 at 1 min and <5 at 5 min; pH<7; BD≤16 mmol/l; HIE |
Major congenital abnormalities, infection on admission, severe anemia, other encephalopathy |
23 |
28 |
SHC (cooling caps) |
rectal |
34.5-35.0 |
36-37.5 |
| 24 |
Tanigasalam |
Tanigasalam 2015 [60] |
India |
Encephalopathy, pH 7 or BD 12meq in cord blood and fulfilling any two of, AS≤5 at 10 min of life, evidence of fetal distress, assisted ventilation for at least 10 min after birth, evidence of any organ dysfunction |
Age >6 h, out-born babies, major congenital abnormalities, no spontaneous respiratory efforts by 20 min after birth or history of maternal renal failure |
60 |
60 |
WBC (gel packs) |
rectal |
33.0-34.0 |
36.5 |
| 25 |
Thayyil |
Thayyil 2013 [28] |
India |
Age ≤6 h, NE with Thompsons encephalopathy score >5 |
NA |
17 |
16 |
WBC (phase changing material) |
rectal |
33.5 |
36.4 |
| 26 |
THIN study |
Aker 2019 [31] |
India |
GA≥36 wk, BW>1800 g, age <5 h, perinatal asphyxia (umbilical cord or 1st h pH<7.0 or BD≥12), 5 min AS≤5, or need of PPV≥10 min at birth |
Major congenital anomalies or imminent death anticipated |
25 |
25 |
WBC (phase changing material) |
rectal |
33.5 ± 0.5 |
37.0 ± 0.5 |
| 27 |
TOBY trial |
Azzopardi 2009 [30], Azzopardi 2014 [33], Campbell 2018 [37], Perrone 2010 [48], Roka 2011 [51], Rutherford 2010 [52] |
UK |
GA≥36 wk, at 10 min after birth, either AS≤5 or continued need for resuscitation or, within 60 min after birth, acidosis (umbilical-cord, arterial, or capillary pH<7.00 or BD≥16mmol/l); moderate-to-severe encephalopathy (lethargy, stupor, or coma) and either hypotonia, abnormal reflexes, absent or weak suck, or clinical seizures; abnormal background activity for ≥30 min or seizures (on aEEG) |
Age >6 h, major congenital abnormalities that required surgery or were suggestive of chromosomal anomaly or syndromes that involve brain dysgenesis |
163 |
162 |
WBC (cooling blanket) |
rectal |
33.0-34.0 |
37.0 ± 0.2 |
| 28 |
Yang |
Yang 2020 [61] |
China |
Age <6 h; GA 37 wk and BW 2500 g; 1 min AS 3 and 5 min AS 5 |
Convulsions caused by electrolyte disorder, intracranial hemorrhage, brain injury caused by intrauterine infection, genetic and metabolic diseases, other congenital diseases; congenital malformation or congenital metabolic abnormality; suspicion of prenatal and intrapartum infection |
62 |
30 |
SHC (cooling caps) |
NA |
28.0-30.0 (head skin temp); 34.5 ± 0.5 (body surface skin); 35.5 ± 0.5 (anal temp) |
NA |
| 29 | Zhou | Zhou 2010 [62] | China | Age <6 h, GA 37 wk, BW 2500 g, with clinical evidence of perinatal hypoxia-ischemia or diagnosis of encephalopathy. AS≤3 at 1 min and ≤5 at 5 min; cord blood pH<7.0 or BD≤16 mmol/l; and need for resuscitation or ventilation at 5 min of age | Major congenital abnormalities; infection; other encephalopathy, severe anemia | 138 | 118 | SHC (cooling caps) | NP; rectal | 34.0 ± 0.2 (NP); 34.5-35.0 (rectal) | 36.0-37.5 (rectal) |
EAC – external auditory canal, ECMO – extracorporeal membrane oxygenation, HELIX – hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries, HIE – hypoxic ischemic encephalopathy, HT – hypothermia, ICE – The Infant Cooling Evaluation, NA – not reported, NE – neonatal encephalopathy, NEST – neonatal ECMO Study of Temperature, NICHD – National Institute of Child Health and Human Development, NP – nasopharyngeal, SHC – selective head cooling, WBC – Whole body cooling, THIN – Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy in India, TOBY – Total Body Hypothermia for Neonatal Encephalopathy Trial, wk – weeks
*In this trial, TH group had 4 subgroups with cooling to 36.5-36°C (n = 6): 35.9-35.5°C (n = 6): 35 ± 0.5°C (n = 6): 34.5 ± 0.5°C (n = 7). Only those with cooling to 34.5 ± 0.5°C were eligible for inclusion in this systematic review. The median time of initiation of intervention was within 4 hours of birth in the TH group; and 4.5 hours after birth in the control group.
†This study is the same as the Battin 2001 trial, however in this study data for TH group included participants cooled to temperature 35.0 ± 0.5°C (n = 6); or 34.5 ± 0.5°C (n = 7). Although, the latter conformed to the inclusion criteria of this review, outcome data could not be extracted separately for this group. Therefore, data from this study was unusable for meta-analysis.
Two of the 29 RCTs were multi-country trials [10,26]. Nine trials were conducted in India [28,31,35,36,38,44,45,49,60], six in China [39,46,47,59,61,62], four in the USA [26,29,41,53], two in the UK [30,42], and one each in Australia [43], Egypt [57], Germany [58], New Zealand [34], Turkey [32], and Uganda [50]. The sample sizes in the 29 trials ranged from 19 [53] to 408 [10]; with median (IQR) 93 (40, 158). Only 14 trials [10,26,27,29,30, 35,38,42,44,45,49,58,60,62] enrolled >100 participants each.
Nine RCTs [10,26,30,35,43,45,46,57,58] enrolled infants with moderate or severe encephalopathy, whereas another nine trials [27,29,41,44,47,49,59,60,62] also included some infants with mild encephalopathy. The proportion of such infants ranged from 0.2% to 23.3%. Eleven trials [28,31,32,34,36,38,39,42,50,53,61] did not describe the severity of encephalopathy. One study [48] presented data from a sub-group of participants reported in another study [30], hence data were extracted from the main publication.
Mortality
Twenty-two studies with 2434 participants reported neonatal mortality during the initial hospitalization. The pooled RR was 0.87, (95% CI = 0.75, 1.00), I2 = 38% (Figure 2). The absolute risk difference was -0.03 (95% CI = -0.06, 0.00), I2 = 47%. One trial [58] reported mortality only during the intervention period, but not the entire hospitalization, hence its data was not pooled. Among the 22 trials, 21 showed an uncertain effect; only the HELIX trial [10] showed increased mortality. Excluding its data yielded a pooled RR of 0.74 (95% CI = 0.62, 0.87), I2 = 0%.
Figure 2.
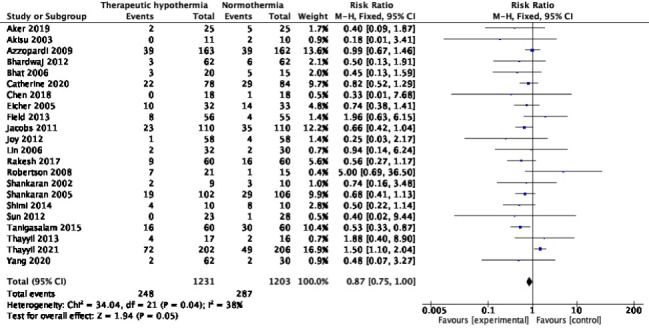
Meta-analysis of data on neonatal mortality (during the initial hospitalization).
Eleven trials with 2042 participants reported mortality at 18-24 months [10,26,27,29,30,34,38,42,46,58,62]; pooled RR (95% CI) was 0.88 (95% CI = 0.78, 1.01), I2 = 51% (Figure 3). The absolute risk difference was -0.04 (95% CI = -0.08, 0.00), I2 = 74%. Only one trial [27] showed statistically significant reduction, with a RR of 0.65 (95% CI = 0.43, 0.97); nine [26,29,30,34,38,42,46,58,62] showed statistically insignificant differences, and the HELIX trial [10] reported increased mortality, with an RR of 1.35 (95% CI = 1.04, 1.76). Excluding HELIX trial data [10] yielded a pooled RR of 0.77 (95% CI = 0.66, 0.90), I2 = 0%. Two trials [58,62] had data missing for >10% enrolled participants. In the Simbruner 2010 [58] trial, 17.2% and 10.8% in the intervention and comparison arms had missing data. In the Zhou 2010 [62] trial, the respective proportions were 27.5% and 20.3%. Exclusion of these two trials did not remarkably change the pooled effect; RR was 0.93 (95% CI = 0.81, 1.07), I2 = 53%.
Figure 3.
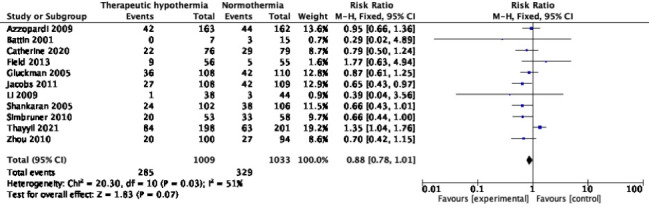
Meta-analysis of data on mortality at the age of 18-24 months.
Only two studies [33,56] with 515 survivors reported mortality during childhood. Although one trial [56] reported statistically significant reduction, pooled RR (95% CI) was 0.81 (95% CI = 0.62, 1.04), I2 = 59% (Figure 4). Random-effects model yielded RR 0.79 (95% CI = 0.53, 1.18). The absolute risk difference was -0.07 (95% CI = -0.15, 0.01), I2 = 67%. No trial reported data on children older than ten years.
Figure 4.
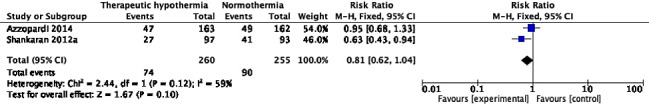
Meta-analysis of data on mortality between 5-10 years of age.
Unfavourable neurological and/or neurodevelopmental outcomes (ie, disability)
Eleven trials [10,27,29,30,34,38,39,42,46,54,62] with 1440 participants reported this outcome at 18-24 months of age. Among these, nine trials used the Bayley Scales of Infant Development (second or third edition) [10,27,29,30,34,39,42,46,54], and one each used the Gesell Child Development Age Scale and the Gross Motor Function Classification System (GMFCS) [62], and Developmental Assessment Scale for Indian Infants [38]. Although only three trials [38,39,62] showed statistically significant reduction, whereas the other eight were inconclusive, pooled RR (95% CI) was 0.62 (95% CI = 0.52, 0.75), I2 = 26% (Figure 5). The absolute risk difference was -0.11 (95% CI = -0.15, -0.07), I2 = 46%. Four trials [27,29,46,54] had missing data in >10% survivors in at least one of the trial arms. Additionally, two trials had >10% difference in inter-group attrition. In the Jacobs 2011 trial [27], data were missing in 3.6% and 14.5% of survivors in the intervention and comparison groups. The respective proportions in the Li 2009 trial [46] were 17.8% and 6.8%.
Figure 5.
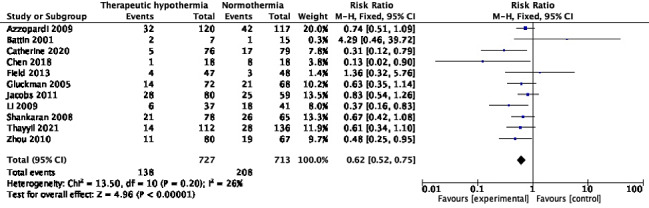
Meta-analysis of data on participants with neurologic disability at the age of 18-24 months.
Three publications [33,44,56] presented the proportion with neurological disability during childhood, among 442 survivors; pooled RR was 0.68 (95% CI = 0.52, 0.90), I2 = 3% (Figure 6). The absolute risk difference was -0.12 (95% CI = -0.21, -0.04), I2 = 0%. The denominators in two of these [33,56] were less than the number of survivors, suggesting missing data. In the third publication [44], the originally randomized number was unavailable. There were no studies reporting the outcome at 10 years of age.
Figure 6.
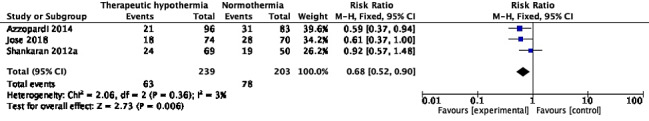
Meta-analysis of data on participants with neurologic disability between 5-10 years of age.
Mortality or disability
Ten trials with 1914 participants reported the composite outcome of death or disability at 18-24 months of age [10,26,27,29,30,34,38,46,58,62]. Pooled RR (95% CI) was 0.78 (95% CI = 0.72, 0.86), I2 = 54% (Figure 7). Random-effects model yielded a RR of 0.75 (95% CI = 0.66, 0.87). The absolute risk difference was -0.12 (95% CI = -0.17, -0.08), I2 = 59%. Unlike when the two outcomes were analysed separately, TH showed statistically significant improvement in the composite outcome in six of ten trials [26,27,38,46,58,62], and none including the HELIX trial [10] showed increased risk. Three trials [47,58,62] had data missing in >10% of participants in at least one arm. Excluding these trials yielded RR 0.84 (95% CI = 0.76, 0.92), I2 = 43%. In addition, the difference in attrition between the trial arms was >10% in the Li 2009 trial [46]. In the Zhou 2010 trial [62], the proportions with missing data were 27.5% in the intervention arm and 20.3% in the comparison arm. Exclusion of these trials did not significant alter the pooled effect; RR was 0.60 (95% CI = 0.46, 0.78), I2 = 41%.
Figure 7.
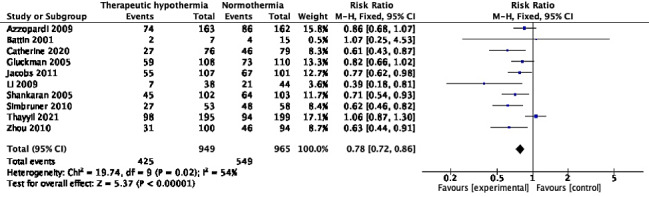
Meta-analysis of data on participants with death or neurologic disability at the age of 18-24 months.
Cerebral palsy
Eight trials (1136 participants) reported the proportion of infants with cerebral palsy (CP) at 18-24 months of age [10,26,27,30,42,46,58,62]. Although only four [10,30,58,62] independently showed statistically significant reduction, the pooled RR was 0.63 (95% CI = 0.50, 0.78), I2 = 39% (Figure 8). Two trials [46,62] had data missing from >10% survivors in at least one arm, but their exclusion did not change the pooled effect; the RR was 0.68 (95% CI = 0.54, 0.86), I2 = 43%. The absolute risk difference across the 8 trials was -0.10 (95% CI = -0.15, -0.06), I2 = 55%.
Figure 8.
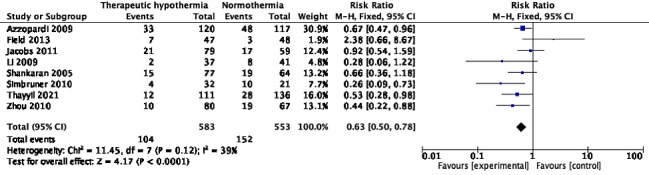
Meta-analysis of data on participants with cerebral palsy at the age of 18-24 months.
Three studies [33,44,56] (449 survivors) reported the proportions with cerebral palsy during childhood; pooled RR was 0.63 (95% CI = 0.46, 0.85), I2 = 0% (Figure 9). The denominators in two [33,56] of these were less than the number of survivors, suggesting missing data. In the third publication [44], the number originally randomized was unavailable. The absolute risk difference across the 3 studies was -0.13 (95% CI = -0.21, -0.04), I2 = 0%. No studies reported cerebral palsy at 10 years of age.
Figure 9.
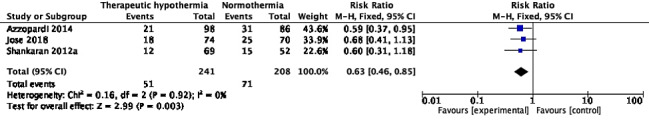
Meta-analysis of data on participants with cerebral palsy between 5-10 years of age.
Other outcomes
Seizures
Ten trials [10,26,28,29,31,32,41,43,50,53] with 1094 participants reported neonatal seizures. The pooled RR was 1.02 (95% CI = 0.95, 1.09), I2 = 17% (Figure 10). The absolute risk difference was 0.01 (95% CI = -0.03, 0.06), I2 = 42%
Figure 10.
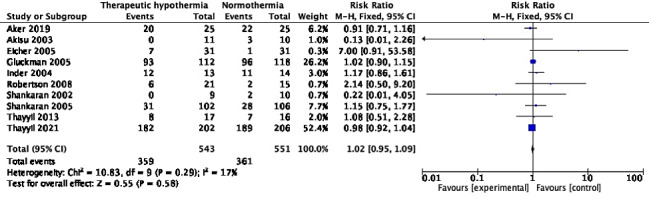
Meta-analysis of data on participants with neonatal seizures (during the initial hospitalization).
Only four trials (710 participants) reported the proportion of infants with seizures at 18-24 months, ie, infantile epilepsy as a sequel to neonatal encephalopathy [10,29,30,42]. The pooled RR was 0.87 (95% CI = 0.55, 1.37), I2 = 36% (Figure 11). The absolute risk difference was -0.01 (95% CI = -0.06, 0.03), I2 = 60%. One trial [29] had data missing from >10% survivors, however its exclusion did not change the pooled effect: 0.84 (95% CI = 0.48, 1.48), I2 = 56%.
Figure 11.
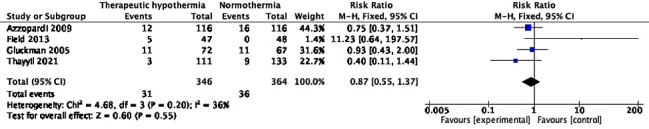
Meta-analysis of data on participants with seizures at the age of 18-24 months (ie, infantile epilepsy).
Only one [56] publication with 117 children presented data on seizures during childhood (ie, childhood epilepsy); there was no statistically significant impact, and RR was 0.65 (95% CI = 0.25, 1.68). The absolute risk difference was -0.06 (95% CI = -0.18, 0.07), N = 1, n = 117.
Length of hospital stay
Nine trials reported length of hospital stay during the initial hospitalization; five [26,32,35,53,58] yielded a pooled mean difference (95% CI) of -0.82 days (95% CI = -1.65, 0.02). The other four presented data as median (IQR) [10,30,38,42]. Although their hospitalization durations varied widely, they were comparable in both arms.
EEG abnormalities
Only two publications [32,51] with 45 participants reported the proportion with EEG abnormalities during the initial hospitalization. One trial [32] performed EEG, 4-10 days after birth, whereas the other performed aEEG during the first 72 hours and calculated the proportion with persisting abnormalities. Pooled RR (95% CI) was 0.34 (95% CI = 0.14, 0.83), I2 = 22% (Figure 12). The absolute risk difference was -0.36 (95% CI = -0.62, -0.10), I2 = 0%.
Figure 12.
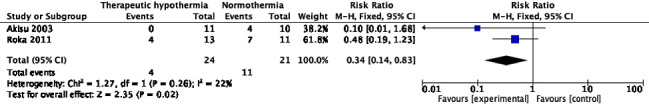
Meta-analysis of data on participants with EEG abnormalities during the neonatal period.
Abnormalities on MRI
Eight trials reported MRI abnormalities during the initial hospitalization [10,31,40,43,51-53,55]. The timing of MRI varied as follows: during 7-14 days after birth [10], on the 5th day after birth [31], within the first 10 days of birth [40], during the first 7 days of life [43], between days 5-14 of life [51], within the first 4 weeks of birth [52], and by 44 weeks of post-menstrual age [53,55]. The pooled RR for number of infants with “any MRI abnormality” was 0.68 (95% CI = 0.56, 0.83), I2 = 50%, 6 trials, 377 participants (Figure 13). Random-effects model yield RR of 0.73 (95% CI = 0.54, 0.98). The absolute risk difference was -0.19 (95% CI = -0.29, -0.10), I2 = 28%. Three trials [31,51,55] showed a lower proportion, whereas the others [43,52,53] reported uncertain effect. MRI abnormalities in the basal ganglia region, or thalamic injury were reported in five trials [10,40,43,52,55] (680 participants); pooled RR was 0.82 (95% CI = 0.68, 0.98), I2 = 37% (Figure 14). The absolute risk difference was -0.08 (95% CI = -0.14, -0.01), I2 = 64%. Two of these trials [52,55] showed statistically significant reduction. Four trials [10,40,52,55] with 659 participants reported those with lesions in the posterior limb of the internal capsule (PLIC). Although only one [55] showed statistically significant reduction with TH, pooled RR was 0.66 (95% CI = 0.52, 0.84), I2 = 0% (Figure 15). The absolute risk difference was -0.11 (95% CI = -0.18, -0.05), I2 = 0%. White matter injury was reported in various ways in five trials [10,40,43,52,55] (686 participants). Although a statistically significant reduction was seen in only two trials [40,52], pooled RR was 0.88 (95% CI = 0.78, 0.98), I2 = 76% (Figure 16). Random-effects model yielded RR 0.76 (95% CI = 0.54, 1.09). The absolute risk difference was -0.07 (95% CI = -0.13, -0.01), I2 = 62%.
Figure 13.
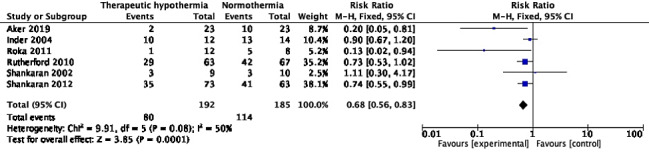
Meta-analysis of data on participants with ‘any MRI lesions’ during the neonatal period.
Figure 14.
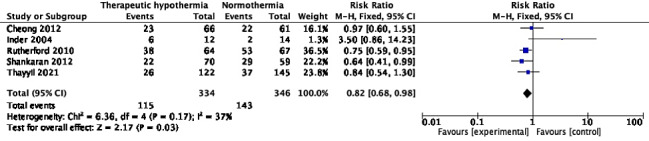
Meta-analysis of data on participants with basal ganglia lesions or thalamic injury on MRI, during the neonatal period.
Figure 15.
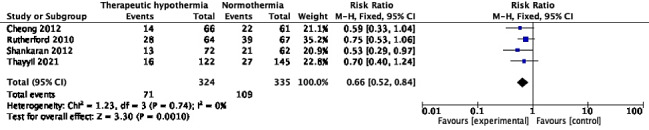
Meta-analysis of data on participants with PLIC lesions on MRI during the neonatal period.
Figure 16.
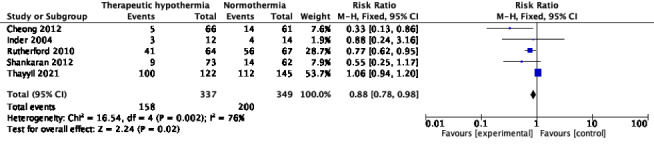
Meta-analysis of data on participants with white matter injury on MRI during the neonatal period.
Quality of life
A single trial [37] presented information on quality of life during childhood using various scoring systems. The proportion with Health Utilities Index (HUI3) score was not different in the two arms, RR was 0.76 (95% CI = 0.55, 1.04); and the mean difference of scores was also similar; 0.09 (95% CI = -0.06, 0.23).
Subgroup analysis
We examined the outcomes by study setting (Table 2). Neonatal mortality and neonatal seizures did not show statistically significant inter-group differences, in any of the four types of countries/settings. TH significantly reduced mortality at 18-24 months in HIC but did not show statistically significant differences in UMIC or LMIC. Similarly, the composite outcome of death or disability at 18-24 months was significantly lowered in HIC and UMIC, but not LMIC. However, neurological disability and cerebral palsy at 18-24 months showed statistically significant reduction across settings.
Table 2.
Analysis of outcomes by country/setting of the trials*
| Outcome | Overall | HIC | UMIC | LMIC | LIC |
|---|---|---|---|---|---|
|
Neonatal mortality
|
0.87 (0.75, 1.00), N = 22, n = 2434, I2 = 38% |
0.82 (0.65, 1.03), N = 6, n = 948, I2= 0%. |
0.47 (0.16, 1.32), N = 5, n = 262, I2 = 0% |
0.89 (0.74, 1.09), N = 10, n = 1188, I2 = 62% |
5.00 (0.69, 36.50), N = 1, n = 36 |
|
Mortality at 18-24 mo
|
0.88 (0.78, 1.01), N = 11, n = 2042, I2 = 51% |
0.79 (0.66, 0.93), N = 7, n = 1212, I2 = 7% |
0.67 (0.41, 1.09), N = 2, n = 276, I2 = 0% |
1.18 (0.94, 1.47), N = 2, n = 554, I2 = 75% |
No trial |
|
Mortality at 5-10 y of age
|
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
No trial |
No trial |
No trial |
|
Neurological disability at 18-24 mo
|
0.62 (0.52, 0.75), N = 11, n = 1440, I2 = 26% |
0.76 (0.61, 0.95), N = 6, n = 782, I2 = 0% |
0.38 (0.23, 0.62), N = 3, n = 261, I2 = 0% |
0.49 (0.30, 0.80), N = 2, n = 403, I2 = 32% |
No trial |
|
Neurological disability at 5-10 y of age
|
0.68 (0.52, 0.90), N = 3, n = 442, I2 = 3%. |
0.72 (0.51, 1.00), N = 2, n = 298, I2 = 42% |
No trial |
0.61 (0.37, 1.00), N = 1, n = 144 |
No trial |
|
Mortality or disability at 18-24 mo
|
0.78 (0.72, 0.86), N = 10, n = 1914, I2 = 54% |
0.77 (0.69, 0.86), N = 6, n = 1089, I2 = 0% |
0.56 (0.41, 0.78), N = 2, n = 276, I2 = 30% |
0.92 (0.77, 1.09), N = 2, n = 549, I2 = 86% |
No trial |
|
Cerebral palsy at 18-24 mo
|
0.63 (0.50, 0.78), N = 8, n = 1136, I2 = 39% |
0.72 (0.56, 0.92), N = 5, n = 664, I2 = 51% |
0.40 (0.21, 0.75), N = 2, n = 225, I2 = 0% |
0.53 (0.28, 0.98), N = 1, n = 247 |
No trial |
|
Cerebral palsy at 5-10 y of age
|
0.63 (0.46, 0.85), N = 3, n = 449, I2 = 0% |
0.60 (0.41, 0.88), N = 2, n = 305, I2 = 0% |
No trial |
0.68 (0.41, 1.13), N = 1, n = 144 |
No trial |
|
Neonatal seizures
|
1.02 (0.95, 1.09), N = 10, n = 1094, I2 = 17% |
1.09 (0.95, 1.24), N = 5, n = 546, I2 = 31% |
0.13 (0.01, 2.26), N = 1, n = 21 |
0.98 (0.92, 1.04), N = 3, n = 491, I2 = 0% |
2.14 (0.50, 9.20), N = 1, n = 36 |
|
Seizures at 18-24 mo (infantile epilepsy)
|
0.87 (0.55, 1.37), N = 4, n = 710, I2 = 36% |
1.01 (0.62, 1.65), N = 3, n = 466, I2 = 42% |
No trial |
0.40 (0.11, 1.44), N = 1, n = 244 |
No trial |
| Seizures at 5-10 y of age (childhood epilepsy) | 0.65 (0.25, 1.68), N = 1, n = 117 | 0.65 (0.25, 1.68), N = 1, n = 117 | No trial | No trial | No trial |
HIC – high-income countries, UMIC – upper middle-income countries, LMIC – lower middle-income countries, LIC – low-income countries, mo – months, y – years
*All data are presented as risk ratios (RR) with 95% confidence interval. ‘N’ represents the number of trials, and ‘n’ represents the number of participants.
Subgroup analysis by type of cooling (Table 3) showed statistically insignificant inter-group differences between WBC and SHC, for mortality (neonatal and at 18-24 months) and seizures at any age. Other outcomes at 18-24 months, namely neurological disability, composite of mortality or disability, and cerebral palsy, were all improved with TH, irrespective of whether the whole body or only the head was cooled.
Table 3.
Analysis of outcomes by type of cooling*
| Outcome | Overall | Whole-body cooling | Selective head cooling |
|---|---|---|---|
|
Neonatal mortality
|
0.87 (0.75, 1.00), N = 22, n = 2434, I2 = 38% |
0.88 (0.76, 1.02), N = 17, n = 2172, I2 = 50% |
0.47 (0.16, 1.32), N = 5, n = 262, I2 = 0% |
|
Mortality at 18-24 mo
|
0.88 (0.78, 1.01), N = 11, n = 2042, I2 = 51% |
0.91 (0.79, 1.06), N = 8, n = 1608, I2 = 62% |
0.79 (0.59, 1.05), N = 3, n = 434, I2 = 0% |
|
Mortality at 5-10 y of age
|
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
No trial |
|
Neurological disability at 18-24 mo
|
0.62 (0.52, 0.75), N = 11, n = 1440, I2 = 26% |
0.65 (0.53, 0.80), N = 7, n = 1095, I2 = 15% |
0.54 (0.36, 0.81), N = 4, n = 345, I2 = 48% |
|
Neurological disability at 5-10 y of age
|
0.68 (0.52, 0.90), N = 3, n = 442, I2 = 3% |
0.68 (0.52, 0.90), N = 3, n = 442, I2 = 3% |
No trial |
|
Mortality or disability at 18-24 mo
|
0.78 (0.72, 0.86), N = 10, n = 1914, I2 = 54% |
0.79 (0.72, 0.88), N = 7, n = 1480, I2 = 67% |
0.75 (0.63, 0.91), N = 3, n = 434, I2 = 0% |
|
Cerebral palsy at 18-24 mo
|
0.63 (0.50, 0.78), N = 8, n = 1136, I2 = 39% |
0.66 (0.52, 0.83), N = 7, n = 989, I2 = 41% |
0.44 (0.22, 0.88), N = 1, n = 147 |
|
Cerebral palsy at 5-10 y of age
|
0.63 (0.46, 0.85), N = 3, n = 449, I2 = 0% |
0.63 (0.46, 0.85), N = 3, n = 449, I2 = 0% |
No trial |
|
Neonatal seizures
|
1.02 (0.95, 1.09), N = 10, n = 1094, I2 = 17% |
1.03 (0.95, 1.11), N = 8, n = 843, I2 = 23% |
0.99 (0.87, 1.12), N = 2, n = 251, I2 = 55% |
|
Seizures at 18-24 mo (infantile epilepsy)
|
0.87 (0.55, 1.37), N = 4, n = 710, I2 = 36% |
0.84 (0.48, 1.48), N = 3, n = 571, I2 = 56% |
0.93 (0.43, 2.00), N = 1, n = 139 |
| Seizures at 5-10 y of age (childhood epilepsy) | 0.65 (0.25, 1.68), N = 1, n = 117 | 0.65 (0.25, 1.68), N = 1, n = 117 | No trial |
mo – months, y – years
*All data are presented as risk ratios (RR) with 95% CI. ‘N’ represents the number of trials, and ‘n’ represents the number of participants.
Sensitivity analysis
Sensitivity analysis excluding trials with moderate/high RoB (from the analysis) did not change the overall result for major clinical outcomes, although the magnitude of effect diminished for some outcomes (Table 4). However, the exclusion changed three statistically significant differences in MRI outcomes to statistically insignificant differences (Table 4). Examination of pooled risk ratios among trials with low RoB against those with moderate or high RoB showed that TH reduced neonatal mortality and mortality at 18-24 months in trials with moderate/high RoB, but not in trials with low RoB (Table 4). However neurological disability, cerebral palsy, and the composite outcome of disability or mortality at 18-24 months showed benefit with TH in both types of trials, although the magnitude was less in low RoB trials.
Table 4.
Analysis of outcomes by risk of bias within the trials*
| Outcome | Overall | Trials with low risk of bias | Trials with moderate or high risk of bias |
|---|---|---|---|
|
Neonatal mortality
|
0.87 (0.75, 1.00), N = 22, n = 2434, I2 = 38% |
0.97 (0.80, 1.16), N = 7, n = 1475, I2 = 62% |
0.71 (0.55, 0.91), N = 15, n = 959, I2 = 0% |
|
Mortality at 18-24 mo
|
0.88 (0.78, 1.01), N = 11, n = 2042, I2 = 51% |
0.96 (0.83, 1.13), N = 6, n = 1336, I2 = 58% |
0.72 (0.56, 0.92), N = 5, n = 706, I2 = 0% |
|
Mortality at 5-10 y of age
|
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
No trial |
0.81 (0.62, 1.04), N = 2, n = 515, I2 = 59% |
|
Neurological disability at 18-24 mo
|
0.62 (0.52, 0.75), N = 11, n = 1440, I2 = 26% |
0.68 (0.54, 0.85), N = 6, n = 941, I2 = 24% |
0.52 (0.38, 0.73), N = 5, n = 499, I2 = 28% |
|
Neurological disability at 5-10 y of age
|
0.68 (0.52, 0.90), N = 3, n = 442, I2 = 3% |
No trial |
0.68 (0.52, 0.90), N = 3, n = 442, I2 = 3% |
|
Mortality or disability at 18-24 mo
|
0.78 (0.72, 0.86), N = 10, n = 1914, I2 = 54% |
0.86 (0.77, 0.95), N = 6, n = 1322, I2 = 43% |
0.63 (0.53, 0.75), N = 4, n = 592, I2 = 0% |
|
Cerebral palsy at 18-24 mo
|
0.63 (0.50, 0.78), N = 8, n = 1136, I2 = 39%. |
0.68 (0.52, 0.90), N = 3, n = 622, I2 = 0% |
0.55 (0.38, 0.79), N = 5, n = 514, I2 = 53% |
|
Cerebral palsy at 5-10 y of age
|
0.63 (0.46, 0.85), N = 3, n = 449, I2 = 0% |
No trial |
0.63 (0.46, 0.85), N = 3, n = 449, I2= 0% |
|
Neonatal seizures
|
1.02 (0.95, 1.09), N = 10, n = 1094, I2 = 17% |
0.99 (0.94, 1.05), N = 2, n = 638, I2 = 0% |
1.11 (0.89, 1.38), N = 8, n = 456, I2 = 30% |
|
Seizures at 18-24 mo (infantile epilepsy)
|
0.87 (0.55, 1.37), N = 4, n = 710, I2 = 36% |
0.73 (0.45, 1.17). N = 3, n = 615, I2 = 0% |
11.23 (0.64, 197.57), N = 1, n = 95 |
| Seizures at 5-10 y of age (childhood epilepsy) | 0.65 (0.25, 1.68), N = 1, n = 117 | No trial | 0.65 (0.25, 1.68), N = 1, n = 117 |
mo – months, y – years
*All data are presented as risk ratios (RR) with 95% CI. ‘N’ represents the number of trials, and ‘n’ represents the number of participants.
DISCUSSION
This up-to-date systematic review showed that therapeutic hypothermia implemented for neonatal encephalopathy, did not result in statistically significant reductions in mortality during the neonatal period, infancy or later childhood. However, it reduced neurologic disability and cerebral palsy in infancy and childhood, resulting in reduction in the composite outcome of mortality or disability, despite absence of conclusive benefit on mortality alone. EEG abnormalities and multiple MRI outcomes were better in neonates who received TH. However, there was no statistically significant impact on seizures during the neonatal period, infantile epilepsy, or childhood epilepsy.
While the type of cooling (ie, WBC or SHC) did not affect the results, the setting where TH was implemented was relevant. TH reduced mortality at 18-24 months in high income countries, but not in other settings. While neonatal mortality and seizures were not reduced in any setting, disability and cerebral palsy in infancy were reduced in all settings.
More important, reduction in mortality reported in previous systematic reviews [1,2] was influenced by trials with higher risk of bias.
Thus, this systematic review uncovered several novel findings that contradict previous reviews [1,2]. This is partly because of the availability of new trials, notably the HELIX trial [10], but also due to methodological errors in the previous reviews. The Cochrane review combined immediate and later mortality in the same meta-analysis [1]. The later review failed to include some eligible trials, duplicated data from some trials, presented data from non-existent trials, combined immediate and later mortality, and even expressed relative risk with negative integers [2].
The HELIX trial [10] reported increased mortality (neonatal and infancy) with TH, in stark contrast to previous trials. This RCT was one of the best conducted trials with multiple methodological refinements, strict definitions, largest sample size, extremely low attrition rate, and low risk of bias. Extensive critical appraisal did not identify any major limitations [18], although some concerns were raised about the inclusion of out-born infants, slightly delayed initiation of cooling (though within the accepted limit of 6 hours), and possibly diverse causes of hypoxic encephalopathy in low-resource settings [19].
This systematic review had several strengths notably exhaustive literature search across published and grey literature, inclusion of the largest cohort of trials to date, searching and data extraction in duplicate, careful extraction of data meeting the review criteria (rather than including data reported by trials), and undertaking multiple subgroup and sensitivity analyses. There were no deviations from the protocol [21]. In fact, several additional outcomes were also presented. This fosters high confidence in the review findings.
We acknowledge several limitations in our review. We could not search Chinese language databases, or conference proceedings. We could not obtain individual participant data, or missing data for intention-to-treat analyses. In the protocol, we mentioned that randomized controlled trials would be included, but did not specify how quasi or pseudo randomized studies, would be handled. Analysis of the randomization method identified that 18 trials used an appropriate method of randomization, 1 trial used a quasi-randomization method, and 10 trials had an unclear method. Thus, the included trials had some quasi/pseudo randomized studies. The impact of this is evident from the differences in some outcomes among trials with low vs higher RoB.
The effect of therapeutic hypothermia may also be influenced by several factors such as the proportion of outborn neonates in studies, proportion with severe encephalopathy, method of cooling (servo vs non-servo), and severity of asphyxia. For example, 4 trials excluded outborn neonates, 14 trials included them (but only 8 of them reported the proportion of outborn babies), and 11 trials did not provide any information, Similarly, 15 studies reported data of participants with only severe or moderate neonatal encephalopathy, 9 studies included those with mild encephalopathy also, but the proportion was <25% of the total, and 15 studies did not report details of severity. Among these 15, data on Apgar score and/or cord blood parameters suggested severe disease in some (Table S2 in the Online Supplementary Document). Thirteen studies did not report any data on Apgar scores or cord blood parameters, whereas 26 studies reported either or both (Table S2 in the Online Supplementary Document). In the absence of individual patient data, it is not possible to account for these factors.
Before initiating this review, we listed the primary outcome as mortality or neurologic disability at the age of 18-24 months, in alignment with previous systematic reviews [1,2], and major trials [1,2,10,26,29,30]. Although the composite outcome provides useful information, we believe that it is skewed by the beneficial effects of TH on neurologic outcomes, masking the lack of statistically significant impact on mortality.
Adverse effects of therapeutic hypothermia were reported in various ways, and at various time points, in several trials. Although these are very important to consider, for making informed decisions (at the practice as well as policy levels), in this systematic review, we focused on evidence of efficacy, and did not examine adverse events.
We expected attrition in trials would bias the results in favor of the intervention, but did not observe this for most outcomes.
Finally, is more research required on therapeutic hypothermia for neonatal encephalopathy? Some experts would argue that more trials should be conducted until an optimal information size is achieved following which, further research can be discontinued. This would be very expensive in terms of time and resources. Instead, we suggest that research in local health care systems in resource-constrained settings, could focus on resolving issues such as which neonates are most likely to benefit from TH, predictors of failure, and of course primary prevention.
CONCLUSIONS
This up-to-date systematic review of randomized controlled trials confirmed that therapeutic hypothermia implemented for neonatal encephalopathy reduces neurologic disability and cerebral palsy in diverse settings. However, it has an unclear effect on neonatal, infantile, and childhood mortality. It also does not impact neonatal seizures, or epilepsy during infancy and childhood. The previously reported reduction in mortality was associated with trials of lower methodological quality, but not substantiated by trials with high(er) quality.
Additional material
Acknowledgements: None.
Funding: None.
Footnotes
Authorship contributions: JLM conceptualized the study, prepared the systematic review instruments, undertook formal literature search, screened studies for eligibility, extracted and verified data, conducted formal analysis, supervised, and validated the other authors’ work, wrote, revised, and finalized the manuscript. NK and JMD independently undertook formal literature search, screened studies for eligibility, extracted and verified data, participated in data analysis, and edited the manuscript. All authors approved the final version of the manuscript, and are accountable for all aspects of the work.
Competing interests: The authors completed the ICMJE Unified Competing Interest Form (available upon request from the corresponding author) and declare no conflicts of interest.
REFERENCES
- 1.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG.Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate BB, Bimerew M, Gebremichael B, Mengesha KA, Kassaw M, Gebremeskel T, et al. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: A systematic review and meta-analysis of randomized control trials. PLoS One. 2021;16:e0247229. 10.1371/journal.pone.0247229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CYZ, Chakranon P, Lee SWH.Comparative Efficacy and Safety of Neuroprotective Therapies for Neonates With Hypoxic Ischemic Encephalopathy: A Network Meta-Analysis. Front Pharmacol. 2019;10:1221. 10.3389/fphar.2019.01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd E, Salam RA, Middleton P, Han S, Makrides M, McIntyre S, et al. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews. Cochrane Database Syst Rev. 2018;6:CD012409. 10.1002/14651858.CD012409.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Leeuwen GM, Hand JW, Lagendijk JJ, Azzopardi DV, Edwards AD.Numerical modeling of temperature distributions within the neonatal head. Pediatr Res. 2000;48:351-6. 10.1203/00006450-200009000-00015 [DOI] [PubMed] [Google Scholar]
- 6.Perlman JM, Davis P, Wyllie J, Kattwinkel J.Therapeutic hypothermia following intrapartum hypoxia-ischemia. An advisory statement from the Neonatal Task Force of the International Liaison Committee on Resuscitation. Resuscitation. 2010;81:1459-61. 10.1016/j.resuscitation.2010.07.006 [DOI] [Google Scholar]
- 7.Azzopardi D, Strohm B, Linsell L, Hobson A, Juszczak E, Kurinczuk JJ, et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK–analysis of national data. PLoS One. 2012;7:e38504. 10.1371/journal.pone.0038504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 Suppl 1):S204-41. 10.1161/CIR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 9.Wyckoff MH, Wyllie J, Aziz K, de Almeida MF, Fabres J, Fawke J, et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142(16_suppl_1):S185-221. [DOI] [PubMed] [Google Scholar]
- 10.Thayyil S, Pant S, Montaldo P, Shukla D, Oliveira V, Ivain P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9:e1273-85. 10.1016/S2214-109X(21)00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aneja S, Sharma S.Hypoxic ischaemic encephalopathy in low resource settings-time to stop cooling? Lancet Glob Health. 2021;9:e1187-8. 10.1016/S2214-109X(21)00343-0 [DOI] [PubMed] [Google Scholar]
- 12.Krishnan V, Kumar V, Shankaran S, Thayyil S.Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial. Indian J Pediatr. 2021; Epub ahead of print. 10.1007/s12098-021-03861-y [DOI] [PubMed] [Google Scholar]
- 13.Jayaraj D, Rajendran SP.Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence. Indian J Pediatr. 2022;89:305. 10.1007/s12098-021-03962-8 [DOI] [PubMed] [Google Scholar]
- 14.Thomas N, Støen R, Aker K, Martinez-Biarge M, Nakken I, Håberg AK, et al. Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence. Indian J Pediatr. 2022;89:299-300. 10.1007/s12098-021-03967-3 [DOI] [PubMed] [Google Scholar]
- 15.Amboiram P, Balakrishnan U.Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence. Indian J Pediatr. 2022;89:293-4. 10.1007/s12098-021-03964-6 [DOI] [PubMed] [Google Scholar]
- 16.Plakkal N, Murki S.Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence. Indian J Pediatr. 2022;89:306. 10.1007/s12098-021-03966-4 [DOI] [PubMed] [Google Scholar]
- 17.Serane TV, Toshniwal PU.Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence. Indian J Pediatr. 2022;89:295-6. 10.1007/s12098-021-03961-9 [DOI] [PubMed] [Google Scholar]
- 18.Mathew JL.Randomized Controlled Trial Evaluating Hypothermia for Neonatal Encephalopathy in Low- and Middle-Income Countries: Evidence-based Medicine Viewpoint. Indian Pediatr. 2021;58:978-84. 10.1007/s13312-021-2335-y [DOI] [PubMed] [Google Scholar]
- 19.Rao PNS.Randomized Controlled Trial Evaluating Hypothermia for Neonatal Encephalopathy in Low- and Middle-Income Countries: Neonatologist’s Viewpoint. Indian Pediatr. 2021;58:984-5. [PubMed] [Google Scholar]
- 20.Pauliah SS, Shankaran S, Wade A, Cady EB, Thayyil S.Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: a systematic review and meta-analysis. PLoS One. 2013;8:e58834. 10.1371/journal.pone.0058834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew JL, Kaur N, Dsouza JM. Systematic review evaluating therapeutic hypothermia for newborns with hypoxic ischaemic encephalopathy. PROSPERO 2021 CRD42021279682. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021279682. Accessed: 11 November 2021.
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available: https://training.cochrane.org/handbook. Accessed: 22 October 2021.
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RevMan for non-Cochrane reviews. Available: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews. Accessed:N 11 November 2021.
- 25.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 26.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Infant Cooling Evaluation Collaboration Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692-700. 10.1001/archpediatrics.2011.43 [DOI] [PubMed] [Google Scholar]
- 27.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574-84. 10.1056/NEJMcps050929 [DOI] [PubMed] [Google Scholar]
- 28.Thayyil S, Shankaran S, Wade A, Cowan FM, Ayer M, Satheesan K, et al. Whole-body cooling in neonatal encephalopathy using phase changing material. Arch Dis Child Fetal Neonatal Ed. 2013;98:F280-1. 10.1136/archdischild-2013-303840 [DOI] [PubMed] [Google Scholar]
- 29.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663-70. 10.1016/S0140-6736(05)17946-X [DOI] [PubMed] [Google Scholar]
- 30.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349-58. 10.1056/NEJMoa0900854 [DOI] [PubMed] [Google Scholar]
- 31.Aker K, Støen R, Eikenes L, Martinez-Biarge M, Nakken I, Håberg AK, et al. Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy in India (THIN study): a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;105:405-11. 10.1136/archdischild-2019-317311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akisu M, Huseyinov A, Yalaz M, Cetin H, Kultursay N.Selective head cooling with hypothermia suppresses the generation of platelet-activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:45-50. 10.1016/S0952-3278(03)00055-3 [DOI] [PubMed] [Google Scholar]
- 33.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140-9. 10.1056/NEJMoa1315788 [DOI] [PubMed] [Google Scholar]
- 34.Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ.Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107:480-4. 10.1542/peds.107.3.480 [DOI] [PubMed] [Google Scholar]
- 35.Bharadwaj SK, Bhat BV.Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource-limited settings: a randomized controlled trial. J Trop Pediatr. 2012;58:382-8. 10.1093/tropej/fms005 [DOI] [PubMed] [Google Scholar]
- 36.Bhat MA.Re: Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F464. 10.1136/fnn.2006.097915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell H, Eddama O, Azzopardi D, Edwards AD, Strohm B, Rivero-Arias O.Hypothermia for perinatal asphyxia: trial-based quality of life at 6-7 years. Arch Dis Child. 2018;103:654-9. 10.1136/archdischild-2017-313733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catherine RC, Ballambattu VB, Adhisivam B, Bharadwaj SK, Palanivel C.Effect of Therapeutic Hypothermia on the Outcome in Term Neonates with Hypoxic Ischemic Encephalopathy-A Randomized Controlled Trial. J Trop Pediatr. 2021;67:fmaa073. 10.1093/tropej/fmaa073 [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Peng W, Zhang Z, Zhao Q, Zhou Y, Chen L, et al. Efficacy and safety of selective brain hypothermia therapy on neonatal hypoxic-ischemic encephalopathy. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:1046-50. [DOI] [PubMed] [Google Scholar]
- 40.Cheong JL, Coleman L, Hunt RW, Lee KJ, Doyle LW, Inder TE, et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch Pediatr Adolesc Med. 2012;166:634-40. 10.1001/archpediatrics.2012.284 [DOI] [PubMed] [Google Scholar]
- 41.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005;32:18-24. 10.1016/j.pediatrneurol.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 42.Field D, Juszczak E, Linsell L, Azzopardi D, Cowan F, Marlow N, et al. Neonatal ECMO study of temperature (NEST): a randomized controlled trial. Pediatrics. 2013;132:e1247-56. 10.1542/peds.2013-1754 [DOI] [PubMed] [Google Scholar]
- 43.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835-7. 10.1016/j.jpeds.2004.07.034 [DOI] [PubMed] [Google Scholar]
- 44.Jose S.K MI. Effect of hypothermia for perinatal asphyxia on childhood outcomes. Int J Contemp Pediatrics. 2017;5:86-91. 10.18203/2349-3291.ijcp20175489 [DOI] [Google Scholar]
- 45.Joy R, Pournami F, Bethou A, Bhat VB, Bobby Z.Effect of therapeutic hypothermia on oxidative stress and outcome in term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2013;59:17-22. 10.1093/tropej/fms036 [DOI] [PubMed] [Google Scholar]
- 46.Li T, Xu F, Cheng X, Guo X, Ji L, Zhang Z, et al. Systemic hypothermia induced within 10 hours after birth improved neurological outcome in newborns with hypoxic-ischemic encephalopathy. Hosp Pract (1995). 2009;37:147-52. [DOI] [PubMed] [Google Scholar]
- 47.Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY.Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J Perinatol. 2006;26:180-4. 10.1038/sj.jp.7211412 [DOI] [PubMed] [Google Scholar]
- 48.Perrone S, Szabó M, Bellieni CV, Longini M, Bangó M, Kelen D, et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr Neurol. 2010;43:236-40. 10.1016/j.pediatrneurol.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 49.Rakesh K, Vishnu Bhat B, Adhisivam B, Ajith P.Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia - a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31:2418-23. 10.1080/14767058.2017.1344633 [DOI] [PubMed] [Google Scholar]
- 50.Robertson NJ, Nakakeeto M, Hagmann C, Cowan FM, Acolet D, Iwata O, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372:801-3. 10.1016/S0140-6736(08)61329-X [DOI] [PubMed] [Google Scholar]
- 51.Roka A, Kelen D, Halasz J, Beko G, Azzopardi D, Szabo M.Serum S100B and neuron-specific enolase levels in normothermic and hypothermic infants after perinatal asphyxia. Acta Paediatr. 2012;101:319-23. 10.1111/j.1651-2227.2011.02480.x [DOI] [PubMed] [Google Scholar]
- 52.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39-45. 10.1016/S1474-4422(09)70295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shankaran S, Laptook A, Wright LL, Ehrenkranz RA, Donovan EF, Fanaroff AA, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110:377-85. 10.1542/peds.110.2.377 [DOI] [PubMed] [Google Scholar]
- 54.Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791-8. 10.1542/peds.2008-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085-92. 10.1056/NEJMoa1112066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Shimi MS, Awad HA, Hassanein SM, Gad GI, Imam SS, Shaaban HA, et al. Single dose recombinant erythropoietin versus moderate hypothermia for neonatal hypoxic ischemic encephalopathy in low resource settings. J Matern Fetal Neonatal Med. 2014;27:1295-300. 10.3109/14767058.2013.855894 [DOI] [PubMed] [Google Scholar]
- 58.Simbruner G, Mittal RA, Rohlmann F, Muche R.neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771-8. 10.1542/peds.2009-2441 [DOI] [PubMed] [Google Scholar]
- 59.Sun J, Li J, Cheng G, Sha B, Zhou W.Effects of hypothermia on NSE and S-100 protein levels in CSF in neonates following hypoxic/ischaemic brain damage. Acta Paediatr. 2012;101:e316-20. 10.1111/j.1651-2227.2012.02679.x [DOI] [PubMed] [Google Scholar]
- 60.Tanigasalam V, Bhat V, Adhisivam B, Sridhar MG.Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia?–a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29:2545-8. [DOI] [PubMed] [Google Scholar]
- 61.Yang T, Li S.Efficacy of different treatment times of mild cerebral hypothermia on oxidative factors and neuroprotective effects in neonatal patients with moderate/severe hypoxic-ischemic encephalopathy. J Int Med Res. 2020;48:300060520943770. 10.1177/0300060520943770 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367-72. 10.1016/j.jpeds.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 63.Battin MR, Penrice J, Gunn TR, Gunn AJ.Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111:244-51. 10.1542/peds.111.2.244 [DOI] [PubMed] [Google Scholar]
- 64.Akula VP, Joe P, Thusu K, Davis AS, Tamaresis JS, Kim S, et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J Pediatr. 2015;166:856-61.e1. 10.1016/j.jpeds.2014.12.061 [DOI] [PubMed] [Google Scholar]
- 65.Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD.Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000;106:684-94. 10.1542/peds.106.4.684 [DOI] [PubMed] [Google Scholar]
- 66.Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, et al. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. 10.1186/1471-2431-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azzopardi D, TOBY study group Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F80-2. 10.1136/archdischild-2013-303710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basu SK, Salemi JL, Gunn AJ, Kaiser JR.CoolCap Study Group. Hyperglycaemia in infants with hypoxic-ischaemic encephalopathy is associated with improved outcomes after therapeutic hypothermia: a post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed. 2017;102:F299-306. 10.1136/archdischild-2016-311385 [DOI] [PubMed] [Google Scholar]
- 69.Bonifacio SL, Glass HC, Vanderpluym J, Agrawal AT, Xu D, Barkovich AJ, et al. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158:360-5. 10.1016/j.jpeds.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonifacio SL, Saporta A, Glass HC, Lee P, Glidden DV, Ferriero DM, et al. Therapeutic hypothermia for neonatal encephalopathy results in improved microstructure and metabolism in the deep gray nuclei. AJNR Am J Neuroradiol. 2012;33:2050-5. 10.3174/ajnr.A3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catherine RC, Bhat BV, Adhisivam B, Bharadwaj SK, Vinayagam V, Chinnakali P.Neuronal Biomarkers in Predicting Neurodevelopmental Outcome in Term Babies with Perinatal Asphyxia. Indian J Pediatr. 2020;87:787-92. 10.1007/s12098-020-03283-2 [DOI] [PubMed] [Google Scholar]
- 72.Debillon T, Daoud P, Durand P, Cantagrel S, Jouvet P, Saizou C, et al. Whole-body cooling after perinatal asphyxia: a pilot study in term neonates. Dev Med Child Neurol. 2003;45:17-23. 10.1111/j.1469-8749.2003.tb00854.x [DOI] [PubMed] [Google Scholar]
- 73.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11-7. 10.1016/j.pediatrneurol.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 74.Filippi L, Fiorini P, Daniotti M, Catarzi S, Savelli S, Fonda C, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI). BMC Pediatr. 2012;12:144. 10.1186/1471-2431-12-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filippi L, Fiorini P, Catarzi S, Berti E, Padrini L, Landucci E, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): a feasibility study. J Matern Fetal Neonatal Med. 2018;31:973-80. 10.1080/14767058.2017.1304536 [DOI] [PubMed] [Google Scholar]
- 76.Gane BD, Bhat V, Rao R, Nandhakumar S, Harichandrakumar KT, Adhisivam B.Effect of therapeutic hypothermia on DNA damage and neurodevelopmental outcome among term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2014;60:134-40. 10.1093/tropej/fmt098 [DOI] [PubMed] [Google Scholar]
- 77.Groenendaal F, Casaer A, Dijkman KP, Gavilanes AW, de Haan TR, ter Horst HJ, et al. Introduction of hypothermia for neonates with perinatal asphyxia in the Netherlands and Flanders. Neonatology. 2013;104:15-21. 10.1159/000348823 [DOI] [PubMed] [Google Scholar]
- 78.Guillet R, Edwards AD, Thoresen M, Ferriero DM, Gluckman PD, Whitelaw A, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71:205-9. 10.1038/pr.2011.30 [DOI] [PubMed] [Google Scholar]
- 79.Gunn AJ, Gluckman PD, Gunn TR.Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885-92. 10.1542/peds.102.4.885 [DOI] [PubMed] [Google Scholar]
- 80.Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55-8. 10.1016/j.jpeds.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 81.Horn AR, Woods DL, Thompson C, Eis I, Kroon M.Selective cerebral hypothermia for post-hypoxic neuroprotection in neonates using a solid ice cap. S Afr Med J. 2006;96:976-81. [PubMed] [Google Scholar]
- 82.Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2012;32:1888-96. 10.1038/jcbfm.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322-8. 10.1177/0883073810380915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laptook AR, Shankaran S, Ambalavanan N, Carlo WA, McDonald SA, Higgins RD, et al. Outcome of term infants using apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619-26. 10.1542/peds.2009-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laptook AR, Kilbride H, Shepherd E, McDonald SA, Shankaran S, Truog W, et al. Temperature control during therapeutic hypothermia for newborn encephalopathy using different Blanketrol devices. Ther Hypothermia Temp Manag. 2014;4:193-200. 10.1089/ther.2014.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, et al. Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:1550-60. 10.1001/jama.2017.14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maoulainine FMR, Elbaz M, Elfaiq S, Boufrioua G, Elalouani FZ, Barkane M, et al. Therapeutic Hypothermia in Asphyxiated Neonates: Experience from Neonatal Intensive Care Unit of University Hospital of Marrakech. Int J Pediatr. 2017;2017:3674140. 10.1155/2017/3674140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Massaro AN, Jeromin A, Kadom N, Vezina G, Hayes RL, Wang KK, et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr Crit Care Med. 2013;14:310-7. 10.1097/PCC.0b013e3182720642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Natarajan G, Shankaran S, Laptook AR, Pappas A, Bann CM, McDonald SA, et al. Apgar scores at 10 min and outcomes at 6-7 years following hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2013;98:F473-9. 10.1136/archdischild-2013-303692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pappas A, Shankaran S, McDonald SA, Vohr BR, Hintz SR, Ehrenkranz RA, et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics. 2015;135:e624-34. 10.1542/peds.2014-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parikh NA, Lasky RE, Garza CN, Bonfante-Mejia E, Shankaran S, Tyson JE.Volumetric and anatomical MRI for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. J Perinatol. 2009;29:143-9. 10.1038/jp.2008.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Persianinov LS, Rasstrigin NN, Dizna SN.(Controlled craniocerebral hypothermia in the overall treatment of posthypoxic states in the newborn). Akush Ginekol (Mosk). 1978;9:40-5. [PubMed] [Google Scholar]
- 93.Nuñez-Ramiro A, Benavente-Fernández I, Valverde E, Cordeiro M, Blanco D, Boix H, et al. Topiramate plus Cooling for Hypoxic-Ischemic Encephalopathy: A Randomized, Controlled, Multicenter, Double-Blinded Trial. Neonatology. 2019;116:76-84. 10.1159/000499084 [DOI] [PubMed] [Google Scholar]
- 94.Rivero-Arias O, Eddama O, Azzopardi D, Edwards AD, Strohm B, Campbell H.Hypothermia for perinatal asphyxia: trial-based resource use and costs at 6-7 years. Arch Dis Child Fetal Neonatal Ed. 2019;104:F285-92. 10.1136/archdischild-2017-314685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robertson NJ, Hagmann CF, Acolet D, Allen E, Nyombi N, Elbourne D, et al. Pilot randomized trial of therapeutic hypothermia with serial cranial ultrasound and 18-22 month follow-up for neonatal encephalopathy in a low resource hospital setting in Uganda: study protocol. Trials. 2011;12:138. 10.1186/1745-6215-12-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogers EE, Bonifacio SL, Glass HC, Juul SE, Chang T, Mayock DE, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014;51:657-62. 10.1016/j.pediatrneurol.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Róka A, Bodrogi E, Szabó M, Machay T.A hypoxiás-ischaemiás encephalopathia kezelése mérsékelt, teljestest-hypothermia alkalmazásával érett újszülöttekben–biztonságossági vizsgálat Magyarországon [Whole body hypothermia for the treatment of hypoxic-ischaemic encephalopathy in term infants–a safety study in Hungary]. Orv Hetil. 2007;148:993-8. [DOI] [PubMed] [Google Scholar]
- 98.Selway LD.State of the science: hypoxic ischemic encephalopathy and hypothermic intervention for neonates. Adv Neonatal Care. 2010;10:60-6. 10.1097/ANC.0b013e3181d54b30 [DOI] [PubMed] [Google Scholar]
- 99.Shankaran S, Laptook AR, McDonald SA, Higgins RD, Tyson JE, Ehrenkranz RA, et al. Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatr Crit Care Med. 2012;13:53-9. 10.1097/PCC.0b013e31821926bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629-39. 10.1001/jama.2014.16058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shankaran S, Laptook AR, McDonald SA, Hintz SR, Barnes PD, Das A, et al. Acute Perinatal Sentinel Events, Neonatal Brain Injury Pattern, and Outcome of Infants Undergoing a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2017;180:275-8.e2. 10.1016/j.jpeds.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:57-67. 10.1001/jama.2017.7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thayyil S, Oliveira V, Lally PJ, Swamy R, Bassett P, Chandrasekaran M, et al. Hypothermia for encephalopathy in low and middle-income countries (HELIX): study protocol for a randomised controlled trial. Trials. 2017;18:432. 10.1186/s13063-017-2165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walsh BH, Boylan GB, Dempsey EM, Murray DM.Association of nucleated red blood cells and severity of encephalopathy in normothermic and hypothermic infants. Acta Paediatr. 2013;102:e64-7. 10.1111/apa.12086 [DOI] [PubMed] [Google Scholar]
- 105.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, Wang A, Cook N, Donnelly M, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724-8. 10.1177/0883073810390036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912-21. 10.1542/peds.2006-2839 [DOI] [PubMed] [Google Scholar]
- 107.Zhou WH, Shao XM, Zhang XD, Chen C, Huang GY.Effects of hypothermia on cardiac function in neonates with asphyxia. Zhonghua Er Ke Za Zhi. 2003;41:460-2. [PubMed] [Google Scholar]
- 108.Zonnenberg IA, Koopman C, van Schie PE, Vermeulen RJ, Groenendaal F, van Weissenbruch MM.Comparison of psychomotor outcome in patients with perinatal asphyxia with versus without therapeutic hypothermia at 4 years using the Ages and Stages Questionnaire screening tool. Eur J Paediatr Neurol. 2016;20:545-8. 10.1016/j.ejpn.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 109.Zupan V.Systemic hypothermia in infants with hypoxic-ischaemic encephalopathy: a French pilot study. Dev Med Child Neurol Suppl. 2001;86:32. 10.1111/j.1469-8749.2001.tb04147.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


