Abstract
The efficacy of FK463, a new (1,3)-β-d-glucan synthase inhibitor, against azole-resistant Candida albicans strains has been studied. The MIC of FK463 was lower than those of azoles and amphotericin B against CDR1-expressing C26 and CaMDR-expressing C40 strains. All mice treated with FK463 (1 mg/kg) survived disseminated murine candidiasis. The fungal burden in the kidney after 6 days was markedly reduced after therapy with FK463 and amphotericin B sodium deoxycholate, and plasma (1,3)-β-d-glucan concentration was found to be lower in FK463-treated mice. In our study, FK463 was found to be a potent antifungal agent against disseminated infection with azole-resistant C. albicans.
In the past two decades, there has been increasing evidence that fungi cause life-threatening infections in hospitalized patients, Candida infection being the most notorious. Individuals with impaired immune system due to AIDS, cancer chemotherapy, or drugs designed to prevent rejection of transplanted organs are especially susceptible to invasive fungal infections including candidiasis (12). There are substantially fewer antifungal drugs than antibacterial agents. Azole derivatives inhibit sterol biosynthesis and are fungistatic and effective against many pathogenic fungi including Candida spp. with minimal side effects, but resistance to azoles is becoming an increasing problem as their use increases. In particular, they are not effective against several strains of Candida albicans isolated from oropharyngeal lesions in patients with AIDS (4, 13).
The echinocandins and the closely related pneumocandins act by specific and noncompetitive inhibition of the (1,3)-β-d-glucan synthase enzyme complex that forms glucan polymers, a major component of the cell wall of many pathogenic fungi. By virtue of their novel mechanism of action, antifungal spectrum and potency, apparently suitable disposition, and expected low toxicity, (1,3)-β-d-glucan synthase inhibitors hold great promise as valuable assets in the current armamentarium of agents for the treatment of Candida and Aspergillus infections (3, 9). FK463, a new agent, acts by specific and noncompetitive inhibition of the (1,3)-β-d-glucan synthase enzyme complex that forms (1,3)-β-d-glucan (S. Ueda, M. Tanaka, M. Ezaki, K. Sakamoto, S. Hashimoto, K. Ito, K. Nagao, T. Higaki, N. Oohata, M. Tsuboi, and M. Yamashita, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-145, 1998). The present study is aimed at evaluating the efficacy of FK463 in experimental murine disseminated candidiasis caused by azole-resistant strains of C. albicans.
The antifungal agents used in the present study were fluconazole (FLCZ) (Pfizer Central Research, Sandwich, United Kingdom), ketoconazole (KTZ), itraconazole (ITCZ) (Janssen Research Foundation, Beerse, Belgium), amphotericin B (AmB), Fungizone (AmB sodium deoxycholate; D-AmB) (Bristol-Myers Squibb, N.J.), and FK463 (Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan). The compounds were dissolved in dimethyl sulfoxide, and the final concentration of dimethyl sulfoxide was less than 1% of the total volume of medium. Two clinical isolates of C. albicans, C26 and C40, isolated from AIDS patients with oropharyngeal candidiasis and maintained at our laboratory were used. Strain C26 was azole resistant, overexpressing CDR1 mRNA, and strain C40 was azole resistant, overexpressing CaMDR mRNA (10).
The MICs of the antifungal agents were determined by the microdilution method modified from the macrodilution method of the National Committee for Clinical Laboratory Standards (7). The stock solution of the antifungal agents was diluted 100-fold with susceptibility testing culture medium, and a series of 10 twofold-diluted solutions were prepared. The solutions were pipetted in 100-μl volumes in rows of wells of flat-bottomed 96-well microdilution plates (Iwaki Glass, Inc., Funabashi City, Chiba, Japan). The final concentrations of FLCZ ranged from 128 to 0.25 μg/ml, concentrations of ITCZ and KTZ ranged from 8 to 0.0156 μg/ml, and FK463 and AmB ranged from 1 to 0.0018 μg/ml, in serial twofold dilutions. C. albicans cells from deep-frozen stock cultures were inoculated into Sabouraud dextrose agar (SDA) (BBL, Cockeysville, Md.) culture plates and were incubated at 37°C for 24 h. The inoculum size was adjusted to 103 CFU/ml. The plates were incubated at 37°C for 48 h in the presence of moisture. The MIC was defined as the lowest concentration of an antifungal that substantially inhibits growth of the organism as detected visually. The MIC of FK463 was read as the lowest drug concentration that prevented any discernible growth.
The experimental protocol was approved by the Ethics Review Committee for animal experimentation of Nagasaki University School of Medicine. The guidelines for animal experimentation of the Laboratory Animal Center for Biomedical Research at our institution were followed. Six-week-old, male BALB/c mice were purchased from Charles River, Inc. (Yokohama, Japan), and were housed in standard conditions. Animal inoculation was carried out as follows: yeast cell colonies were picked from overnight culture on SDA plates, and counting with a hemacytometer quantitated cell concentrations. The viable count was confirmed by serial 10-fold dilution and plating the inoculum on SDA plates. Each mouse was inoculated with 107 cells (C26 or C40) for survival and 106 cells for fungal burden and the assay of (1,3)-β-d-glucan in 100 μl of normal saline through lateral tail vein. Treatment started 2 h after inoculation. Different groups of mice were treated with FK463 or D-AmB intravenously once a day for 5 days. Dextrose (5%) was injected intravenously as a control. FK463 and D-AmB were dissolved in 5% dextrose.
Ten animals from each group were sacrificed on day 6, and kidneys were resected, a pair of kidneys was homogenized in sterile normal saline, and serially 10-fold-diluted homogenized tissue samples were cultured in SDA for assessment of fungal burden as cells per kidney of homogenates. Therapeutic efficacy monitoring by estimation of plasma (1,3)-β-d-glucan was done by Fungitec G test (Seikagaku Kogyo Co., Ltd., Tokyo, Japan) as has been described in our previous study (4). Briefly, a 5-μl plasma sample from each of five mice was pretreated with 20 μl of the test solution containing 0.15 M KOH, 0.3 M KCl, and 0.1% polybrene. The mixture was incubated for 10 min at 37°C. The pretreated sample was added to 100 μl of factor G dissolved in 0.1 M HEPES buffer (pH 7.6) and was then incubated at 37°C for 30 min. The optical density at 405 nm was measured by using the kinetic mode of a computerized well reader (SK601; Seikagaku Kogyo, Tokyo, Japan). Pachyman, (1,3)-β-d-glucan from Poria cocos was used as a standard. Duplicate assays were performed for each sample, and the average was recorded.
Each experiment was repeated twice to ascertain its reproducibility. Data were expressed as means ± standard deviations. Tests for differences in survival distributions were based on a generalized Wilcoxon test from survival rates calculated by the Kaplan-Meier method. The mean CFUs in kidney tissues were compared by Scheffe's multiple comparison test. The data obtained from Fungitec G test were compared by Student's t test.
The MICs against two strains of azole-resistant C. albicans were measured. In C26 strain, the MICs of FLCZ, ITCZ, KTZ, AmB, and FK463 were >128, >8, >8, 0.5, and 0.0156 μg/ml, respectively. The MICs of FLCZ, ITCZ, KTZ, AmB, and FK463 for C40 strain were 128, 0.5, >8, 0.25, and 0.0156 μg/ml, respectively.
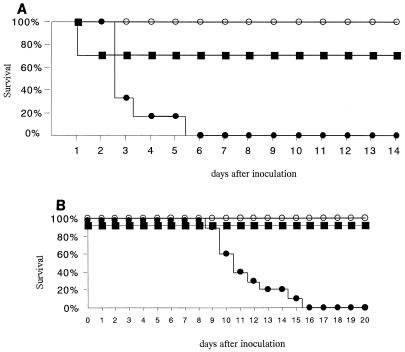
All the untreated control mice injected with 5% dextrose died within 6 days after inoculation of C26 strain. Following therapy with D-AmB (1 mg/kg), 70% of mice survived. All mice treated with FK463 (1 mg/kg) survived for more than 14 days after inoculation (Fig. 1A). After inoculation of C40, all the untreated mice died within 16 days. All mice survived following the therapy with D-AmB (1 mg/kg) and FK463 (1 mg/kg) (Fig. 1B). Table 1 indicates the numbers (log10 CFU/kidney) of yeast cells in mouse kidney 6 days after inoculation. The therapy with FK463 (1 mg/kg) or D-AmB (1 mg/kg) significantly inhibited the growth of both C26 and C40. The concentration of plasma (1,3)-β-d-glucan measured 6 days after inoculation are shown in Table 1. The concentration of (1,3)-β-d-glucan after inoculation of C26 was the lowest in the D-AmB (1 mg/kg)-treated mice. The concentration of (1,3)-β-d-glucan was also decreased following the therapy of FK463 (1 mg/kg). In C40-inoculated mice, the concentration of (1,3)-β-d-glucan was the lowest in mice treated with FK463.
FIG. 1.
Survival rate of mice with experimental disseminated candidiasis infected with CDR1-expressing (C26) (A) and CaMDR-expressing (C40) (B) azole-resistant C. albicans treated with 5% dextrose (filled circle), Fungizone (D-AmB) (1 mg/kg) (filled square), and FK463 (1 mg/kg) (open circle). Treatment started 2 h after inoculation. Different groups of mice were treated with FK463 or D-AmB intravenously for 5 days. Ten mice were included in each group (P < 0.05 for FK463 and D-AmB compared with 5% dextrose by generalized Wilcoxon test).
TABLE 1.
Numbers of yeasts in the kidneys and the concentration of plasma (1,3)-β-d-glucan in mice with disseminated candidiasis treated with FK463 and D-AmB
| Treatment | No. of yeasts (log10 CFU/kidney)a
|
Plasma concn of (1,3)-β-d-glucan (pg/ml)b
|
||
|---|---|---|---|---|
| C26 | C40 | C26 | C40 | |
| Control | 5.76 ± 0.31 | 5.15 ± 0.63 | 139.1 ± 117.9 | 51.1 ± 20.5 |
| FK463 | 0.33 ± 1.04‡ | 1.51 ± 2.28‡ | 35.6 ± 28.3 | 3.7 ± 7.6‡ |
| D-AmB | 0.33 ± 1.04‡ | 0.77 ± 0.02‡ | 18.6 ± 7.6† | 22.7 ± 11.9† |
The numbers of yeasts in the kidney were measured 6 days after inoculation and were treated with antifungal agents for 5 days. The control mice were injected with 5% dextrose intravenously. The values represent means standard deviations in five mice. ‡, P < 0.01 compared with control (Scheffe's multiple comparison test).
The concentration of (1,3)-β-d-glucan in plasma was measured 6 days after inoculation and was treated with antifungal agents for 5 days. The control mice were injected with 5% dextrose intravenously. The values represent means ± standard deviations in each five mice. †, P < 0.05; ‡, P < 0.01 compared with control (Student's t test).
The MICs of FK463 were the lowest against two strains of azole-resistant C. albicans used in this study. The multidrug efflux is commonly believed to be one of the mechanisms for the development of resistance to azole antifungal agents used against C. albicans (1, 11). Clear differences were noted in multidrug efflux mechanism encoded by the CDR and CaMDR genes (1). The CDR1-expressed azole-resistant strain C26 showed cross resistance to several azoles; however, CaMDR-expressed C40 was less resistant to ITCZ (5). FK463 may be effective against azole-resistant C. albicans strains depending on CDR and CaMDR genes, because FK463 was susceptible to both C26 and C40 strains in this study.
In our study, FK463 showed efficacy against murine disseminated candidiasis caused by infection with azole-resistant C. albicans strains. Treatment with FK463 led to 100% survival in therapy for murine candidiasis caused by both C26 and C40 strains. AmB was also potent against azole-resistant C. albicans infection; however, 30% of mice died on the first day, immediately after injection. As was suspected, these mice died due to the acute toxicity of D-AmB. However, further studies are needed to compare the safety of FK463 and to the safety of D-AmB. FK463 significantly reduced the numbers of yeast cells in the kidney, as was also observed in the D-AmB-treated mice. This data suggested that FK463 has a fungicidal efficacy for the therapy of the azole-resistant C. albicans infections. In this study, the efficacy of FK463 was evaluated in immunocompetent mice. However, the disseminated Candida infection often occurs in immunocompromised patients. The comparative study of the efficacy of FK463 and AmB for the therapy of disseminated Candida infection in immunocompromised mice is under consideration for the assessment of the effects of immunosuppression on the efficacy of FK463.
In order to reduce candidiasis-related morbidity and mortality, early diagnosis and definitive treatment are essential. The blood culture is often negative and takes several days, as does measurement of disseminated candidiasis. Methods for the serodiagnosis of deep mycosis, including detection of (1,3)-β-d-glucan, are helpful for the diagnosis of Candida infections (6). Monitoring the concentration of (1,3)-β-d-glucan in the plasma from the patients with Candida infections has been found to be useful for diagnosis, as has the evaluation of the efficacy of the antifungal treatment (8). In our murine model of disseminated Candida infection, the concentration of (1,3)-β-d-glucan in plasma from the untreated mice was elevated. The concentration of (1,3)-β-d-glucan was notably lower in the plasma following treatment with FK463. These results also suggested that FK463 could effectively inhibit the growth of the cells in mice, resulting in suppression of the production and release of (1,3)-β-d-glucan from the yeast cell wall.
In the present study, FK463 was found to be an effective antifungal agent against azole-resistant strains of C. albicans. The results also revealed FK463 might add to the armamentarium for the treatment of infection by azole-resistant C. albicans.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain M A, Miyazaki T, Mitsutake K, Kakeya H, Yamamoto Y, Yanagihara K, Kawamura S, Otsubo T, Hirakata Y, Tashiro T, Kohno S. Comparison between Wako-WB003 and Fungitec G tests for detection of (1,3)-β-d-glucan in systemic mycosis. J Clin Lab Anal. 1997;11:73–77. doi: 10.1002/(SICI)1098-2825(1997)11:2<73::AID-JCLA1>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz M B, Bernard E M, Edwards F F, Marrinan J A, Dropinski J, Douglas C M, Armstrong D. Aerosol and parenteral pneumocandins are effective in a rat model of pulmonary aspergillosis. Antimicrob Agents Chemother. 1995;39:1784–1789. doi: 10.1128/aac.39.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law D, Moore C B, Wardle H M, Ganguli L A, Keaney M G L, Denning D W. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–668. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 5.Maesaki S, Marichal P, Hossain M A, Sanglard D, Vanden Bossche H, Kohno S. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J Antimicrob Chemother. 1998;42:747–753. doi: 10.1093/jac/42.6.747. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K-I, Ishikawa N, Hara K. Plasma (1→3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 8.Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, Yamaguchi H, Shimada K, Kawai T. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/s0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller M A, Messer S A, Coffman S. In vitro susceptibilities of clinical yeast isolates to a new echinocandin derivative, LY303366, and other antifungal agents. Antimicrob Agents Chemother. 1997;41:763–766. doi: 10.1128/aac.41.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanglard D, Ischer F, Monod M, Bille J. Susceptibility of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternberg S. The emerging fungal threat. Science. 1994;266:1632–1634. doi: 10.1126/science.7702654. [DOI] [PubMed] [Google Scholar]
- 13.Vanden Bossche H, Marichal P, Odds F C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 1994;2:393–400. doi: 10.1016/0966-842x(94)90618-1. [DOI] [PubMed] [Google Scholar]